Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Clinical Features and Vitreous Biomarkers of Early-Onset Type 2 Diabetes Mellitus Complicated with Proliferative Diabetic Retinopathy
Authors Ke D, Hong Y, Jiang X , Sun X
Received 11 February 2022
Accepted for publication 13 April 2022
Published 26 April 2022 Volume 2022:15 Pages 1293—1303
DOI https://doi.org/10.2147/DMSO.S362074
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
DanDan Ke,1 YiYi Hong,2 XinNan Jiang,1 XuFang Sun1
1Department of Ophthalmology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2Research Center of Ophthalmic Diseases, Guangxi Academy of Medical Sciences & Department of Ophthalmology, The People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, People’s Republic of China
Correspondence: XuFang Sun, Department of Ophthalmology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1095 Jie-Fang Road, Wuhan, People’s Republic of China, Email [email protected]
Purpose: To compare the clinical features and vitreous biomarkers of proliferative diabetic retinopathy (PDR) between patients with early-onset and late-onset type 2 diabetes mellitus (T2DM).
Materials and Methods: This case-control study analyzed the clinical data of 74 patients with PDR who underwent vitrectomy. The patients were divided into the early-onset (T2DM diagnosis age ≤ 40 years, n = 39) and late-onset (T2DM diagnosis age > 40 years, n = 35) groups. Thirty-six specimens were collected, and the liquid chip technology was used to detect the content of 27 types of cytokines in the vitreous. Differences in clinical features and cytokine levels between the two groups were evaluated. Bonferroni correction was applied for multiple comparisons.
Results: Compared with the late-onset group, the levels of hemoglobin A1c (HbA1c) and total cholesterol were significantly higher in the early-onset group (P < 0.001 and P = 0.009, respectively). Patients with early-onset T2DM PDR had worse visual prognoses and a higher rate of postoperative recurrent vitreous hemorrhage. The results of cytokine detection showed that the levels of interleukin-4 (IL-4), IL-6, IL-8, IL-9, granulocyte colony-stimulating factor, interferon-γ, interferon-inducible 10 kDa, monocyte chemotactic protein 1, macrophage inflammatory protein (MIP)-1α, and MIP-1β in the early-onset group were significantly higher than those in the late-onset group (p < 0.0026). Age at diabetes diagnosis and HbA1c, IL-4, and regulated upon activation, normal T cell expressed and secreted levels were independent risk factors for visual acuity after undergoing vitrectomy.
Conclusion: Early-onset T2DM PDR patients had poor blood glucose and lipid metabolism, higher levels of inflammatory factors, and worse visual prognosis. Stricter metabolic management and earlier anti-inflammatory interventions may be required for patients with early-onset T2DM.
Keywords: early-onset type 2 diabetes mellitus, proliferative diabetic retinopathy, vitrectomy, cytokines, hemoglobin A1c
Introduction
Proliferative diabetic retinopathy (PDR), a common complication of diabetes mellitus (DM), can lead to severe visual impairment and blindness. The age at onset of DM is considered a risk factor in the occurrence and development of diabetic retinopathy (DR).1,2 With the changes in people’s modern lifestyles and diet structure, the onset age of Type 2 DM (T2DM) is getting lower. Epidemiological investigations indicated that the incidence of early-onset T2DM (defined as a DM diagnosis age ≤ 40 years) in China increased nearly fourfold from 1997 to 2010.3 Patients with early-onset T2DM have a higher risk of progressive chronic kidney disease compared with late-onset patients.4,5 Similarly, the prevalence of DR and diabetic macular edema (DME) is significantly higher among patients with early-onset T2DM than among those with late-onset T2DM.3 However, no study has reported the difference in clinical manifestation and prognosis between patients with early-onset and late-onset T2DM complicated with PDR.
Accumulating evidence has shown that the inflammatory process is implicated in the pathogenesis of DR.6,7 Multiple factors, such as vascular endothelial growth factor (VEGF) and inflammatory cytokines, have been identified in the intraocular fluid of patients with PDR.8–12 Some investigators speculate that young people with diabetes may respond more strongly to hypoxia and hyperglycaemia, resulting in large amounts of VEGF in the eyes, consequently, they are more likely to develop retinopathy. This suggests that the identification of cytokines may provide an important clue for explaining the differences in clinical features and prognosis between early-onset and late-onset T2DM PDR. In addition, compared with the aqueous humor, vitreous specimens are closer to the retina and can truly reflect the cytokine levels, which are associated with the pathogenesis of PDR.13
The current study aimed to investigate the clinical features, prognosis, and risk factors for early-onset and late-onset T2DM PDR among patients who underwent vitrectomy, and to compare the differences in cytokines in the vitreous between the two groups. Awareness of these will help in early patient management, and the differences in cytokines may help in the prediction of disease outcomes and formulation of more targeted treatment.
Materials and Methods
Calculation of Sample Size
Since no previous literature was found, the sample size was estimated based on postoperative visual acuity in the pre-experiment. The postoperative visual acuity of early-onset group and late-onset group were 0.95 ±0.11 and 0.78 ±0.18 respectively. Using an alpha value of 0.05 and a power of 90%, the sample size was calculated by PASS15 software, we got the required sample size for early-onset group = 16 eyes and late-onset group = 16 eyes, and considering 10% of the lost follow-up, at least 36 cases should be included in the study. To achieve this sample size in the vitreous study, we recruited 48 eyes of early-onset patients and 38 eyes of late-onset patients.
Inclusion and Exclusion Criteria
This study was approved by the Ethics Committee of Tongji Hospital and conducted in accordance with the tenets of the Declaration of Helsinki. All participants signed informed consent forms after full explanation of the nature and potential risks of the study. From August 2019 to March 2021, consecutive patients who were diagnosed with PDR and underwent pars plana vitrectomy (PPV) were enrolled for the study. Patients with T1DM (7 eyes), inflammatory and ischemic intraocular diseases (3 eyes) or previous vitrectomy (6 eyes) were excluded. The final analysis included 86 eyes of T2DM patients (Figure 1). The patients were aged 18–80 years and were operated on by the same ophthalmologist. Surgical indications include uncleared vitreous hemorrhage (VH), extensive fibrovascular proliferation (FVP), tractional retinal detachment (TRD) and tractional rhegmatogenous retinal detachment (TRRD). The patients were divided into two groups according to the age of onset of diabetes: early-onset T2DM group (diagnosis age ≤ 40 years) and late-onset T2DM group (diagnosis age > 40 years).
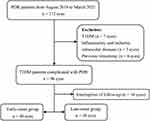 |
Figure 1 Flow chart of the study from recruiting participants to conducting analysis. |
The exclusion criteria were as follows: (I) Type 1 DM complicated with PDR, (II) the presence of other inflammatory and ischemic intraocular diseases, (III) previous vitrectomy, or other ocular surgery in the preceding three months.
Data and Sample Collection
Clinical characteristics of each patient were collected, including age, gender, duration of diabetes, age at diagnosis of diabetes, systemic conditions, body mass index (BMI), hypertension, and family history of DM. The hemoglobin A1c (HbA1c) levels, glomerular filtration rate (GFR), total cholesterol (TC), blood pressure, and blood glucose (FBG) were measured before operation. Preoperative ophthalmologic examinations included measurements of best-corrected visual acuity (BCVA) and intraocular pressure (IOP), slit lamp microscopy, ocular B-ultrasound, optical coherence tomography, and indirect ophthalmoscopic fundus examination. The abnormalities of eye examination before and during operation were recorded, including the existence of iris neovascularization, DME, VH, FVP, TRD and TRRD. If the FVP contained visible neovascularization tissue, it would be defined as active FVP. Combined intraoperative procedures, including cataract extraction, anti-VEGF surgery, and vitreous cavity packing, were also recorded. Regular follow-up was performed to record the BCVA, IOP, and fundus conditions at one, three, and six months after operation, as well as postoperative complications, such as recurrent VH, DME, neovascular glaucoma (NVG), cataract, and recurrent retinal detachment (RD).
Vitreous samples were collected from the eyes of the patients during the first vitrectomy. Before starting infusion, a 25G vitreous cutter was used to collect at least 1 mL undiluted vitreous samples. It is worth mentioning that we excluded patients who received anti-VEGF injection and photocoagulation within three months, while the rest of the patients who required anti-VEGF therapy underwent the operation after vitrectomy. No complication associated with sampling was observed. In total, 36 samples were collected and stored at −80 °C until analysis.
Cytokine Measurements
Before measuring 27 cytokines, vitreous samples were centrifuged 10,000g for 10 min to remove cellular components. Vitreous samples were measured using a Bio-Plex Pro Human Cytokine 27-plex (Bio-Rad Cat# M500KCAF0Y, RRID:AB_2893118, California, USA), which included the following 27 cytokines: interleukin (IL)-1β, IL-1 receptor antagonist (IL-1ra), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, Eotaxin, basic fibroblast growth factor (b-FGF), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFN-γ), interferon-inducible 10 kDa protein (IP-10), monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein (MIP)-1α, MIP-1β, platelet-derived growth factor (PDGF), regulated upon activation, normal T cell expressed and secreted (RANTES), tumor necrosis factor alpha (TNF-α), and VEGF. The experimental procedure for the cytokine assay was in accordance with the instructions. When the measured values fell below the limit of detection, we set the recorded concentration at the limit of detection. A cytokine was excluded if more than 10% of the samples were below the detection limit.
Statistical Analysis
Statistical analysis was performed using IBM SPSS statistics software, version 22.0. (IBM corporation, Armonk, N.Y., USA). The missing data in this study was less than 5%, which was excluded from the statistical analysis. Mahalanobis distance method was used to identify multivariable outliers, the test standard was 0.005, and no outliers were found. A one-sample Shapiro–Wilk test was used to examine whether data were normally distributed. Normally distributed continuous variables are presented as mean ± standard deviation (SD) and were compared using the independent-samples t test. Categorical data are presented as percentages and were compared using the chi-square test. Non-normally distributed variables are presented as the median and interquartiles range (25th,75th percentile), and a non-parametric statistical analysis was employed. Intergroup comparisons were conducted using the Mann–Whitney U-test and Bonferroni correction, at a level of significance of P < 0.0026 (0.05/19). Spearman’s rank-order correlation coefficients were calculated to test the relationships between cytokine concentrations and clinical characteristics. R values < 0.30 were considered low or weak correlations; 0.30–0.70, modest or moderate correlations; and > 0.70, strong or high correlations. Simultaneously, multivariate stepwise linear regression analysis was used to explore the risk factors for postoperative visual outcomes. The independent variables included in the linear analysis were based on the variables associated with postoperative visual outcomes in the correlation analysis (R > 0.30). P < 0.05 was considered statistically significant.
Results
In total, 48 eyes (39 patients) were assessed in the early-onset T2DM PDR group, with a mean patient age of 44.69 ± 8.51 years. Further, 38 eyes (35 patients) were assessed in the late-onset T2DM PDR group (control), with a mean patient age of 57.76 ± 7.53 years. The mean ages at DM diagnosis were 32.33 ± 5.18 and 47.84 ± 4.77 years in the early-onset and late-onset groups, respectively, (P < 0.001). Moreover, mean HbA1c and TC levels in the early-onset group were significantly higher than those in the late-onset group (P < 0.001 and P = 0.009, respectively). There were no significant differences in other basic characteristics, except for the high incidence of family history of diabetes in the early-onset group. The detailed general conditions of patients with PDR are summarized in Table 1.
 |
Table 1 Clinical Characteristics and Surgical Methods in the Early- and Late-Onset Groups |
Preoperative and Intraoperative Outcomes
The median preoperative BCVAs were 1.85 (1.6, 2.3) and 1.85 (1.48, 2.3) in the early-onset and late-onset groups, respectively, (P = 0.620). In the early-onset group, the surgical indications were mainly TRD/TRRD (64.6%) and FVP (33.3%), while VH (28.9%) and FVP (47.4%) were the main reasons for undergoing PPV among patients in late-onset group. The rates of preoperative panretinal laser photocoagulation (PRP) and perioperative anti-VEGF were also significantly higher in the early-onset group (45.8% vs 21.1% and 70.8% vs 28.9%, respectively). Moreover, the early-onset group had a higher proportion of active FVP (77.1% vs 47.4%, P = 0.004). The proportions of preoperative DME, cataract extraction and vitreous tamponade were similar for the two groups (Table 1).
Postoperative Visual Outcomes and Complications
There was no significant difference in the median of preoperative BCVA between the two groups (P = 0.620). Figure 2A shows the trend of BCVA changes at different times. Although the median postoperative BCVA was significantly increased in both groups (P < 0.05). The median BCVA at 1 month (1.0 vs 0.7 logMAR, P < 0.05), 3 months (0.9 vs 0.6 logMAR, P < 0.05) and 6 months (0.9 vs 0.5 logMAR, P < 0.05) after operation in the early-onset group was significantly higher than that in the late-onset group (Figure 2A).
Of the complications that occurred within 6 months after PPV, the incidence of recurrent VH in the early-onset group was significantly higher than that in the late-onset group (20.3% vs 5.8%, P = 0.039). The incidences of DME, NVG, cataract, and recurrent RD were also higher in the early-onset group than that in the late-onset group; however, there was no statistical difference between the two groups (Table 2, Figure 2B).
 |
Table 2 Postoperative Complications in the Two Groups |
Cytokine Expression Profiles
The vitreous cytokine levels in 36 PDR patients in the two groups are summarized in Table 3. The detection rates of IL-5, IL-7, IL-12, IL-15, b-FGF, Eotaxin, PDGF-BB, and GM-CSF were less than 90%, and were not included in subsequent analyses.
 |
Table 3 Vitreous Cytokine Levels in the Early- and Late-Onset Groups |
Among the 19 cytokines tested in the vitreous fluid, after Bonferroni correction, the early-onset group showed significantly higher levels of IL-4, IL-6, IL-8, IL-9, G-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, and MIP-1β compared to those in the late-onset group (P < 0.0026 as statistically significant).
Correlation Between Cytokine Concentrations and Clinical Features
Correlation between cytokine concentrations and clinical features are summarized in Figure 3. Significant positive correlations were observed among most cytokines. Likewise, the BCVA at 6 months after operation had significant positive correlations with the following cytokines: IL-1ra, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-9, G-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, RANTES, TNF-α, VEGF, and HbA1c. The level of HbA1c was positively correlated with some inflammatory factors and chemokines. However, the age at DM diagnosis showed significant negative correlations with many inflammatory factors and chemokines, as well as the BCVA at 6 months after operation.
Prognostic Factors for Visual Outcome
Stepwise multiple linear regression analysis was performed to identify potential risk factors for visual prognosis (Table 4, Supplementary data Tables S1–S3). Based on the correlation analysis between clinical characteristics and the cytokine concentration, variables significantly related to the BCVA at 6 months post-operation were included in the multiple linear regression analysis. After adjusting for preoperative BCVA, IL-1ra, IL-1β, IL-2, IL-6, IL-8, IL-9, G-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, TNF-α, and VEGF levels, the results revealed that the age at DM diagnosis (β =−0.028, P = 0.002), HbA1c (β = 0.110, P = 0.002), IL-4 (β = 0.355, P = 0.001), and RANTES level (β =0.004, P = 0.006) were independent predictors for visual outcomes at 6 months post-operation.
 |
Table 4 Stepwise Multivariate Linear Regression Analysis of Risk Factors for Visual Prognosis (the BCVA at 6 Months After Operation) of PDR |
Discussion
In the present study, PDR patients with early-onset T2DM showed poor blood glucose control and lipid metabolism, and a great likelihood of severe retinopathy. These findings are consistent with the clinical characteristics of early-onset T2DM patients reported in previous studies.14–16 Early-onset T2DM is often associated with genetic susceptibility and adolescent obesity.15,17 Compared with patients with late-onset T2DM, patients with early-onset T2DM had higher levels of HbA1c and TC, and a higher prevalence of DR.14 Moreover, the risk of DR and PDR increased significantly with the increase in HbA1c level.18 This suggests that more attention should be paid to the screening of complications in patients with early-onset T2DM, and that a strict diet control should be carried out.
Vitreoretinopathy progresses rapidly in patients with early-onset T2DM. A previous longitudinal study showed that nearly 1/3 patients with early-onset T2DM progressed from non-proliferative DR to PDR during an average follow-up of 7.1 years.19 In our study, we found that the incidence of simple VH was lower in the early-onset group, but the proportions of FVP and TRD/TRRD in the early-onset group were higher than those in the late-onset group. The proportions of gas and silicone oil tamponade were also higher in the early-onset group, this partly reflected the severity of retinopathy. However, the mechanism has not been fully clarified at present.
In this study, we proved that the levels of 10 cytokines including IL-4, IL-6, IL-8, IL-9, G-CSF, IFN- γ, IP-10, MCP-1, MIP-1α and MIP-1β in the early-onset group were significantly higher than those in the late-onset group. In previous studies, several cytokines have been identified in the eyes of patients with PDR; for example, MCP-1, IL-6, IL-8, and VEGF have been demonstrated to be the four main cytokines upregulated in eyes of patients with PDR.8,9,20,21 MCP-1 recruits mononuclear macrophages and has been shown to play a central role in the development of chronic fibroproliferative diseases.22 IL-6 is secreted by T cells and macrophages and causes inflammation in damaged tissue. Cohen et al23 confirmed that IL-6 may indirectly induce angiogenesis by inducing the expression of VEGF. IL-8 also plays a role in inducing inflammation and promoting angiogenesis in the eye.24,25 Yoshida et al26 believed that elevated levels of MCP-1, IL-6, and IL-8 were associated with postoperative fibrous proliferation in PDR. Our study shows that the expression of 10 cytokines, including IL-6, IL-8, MCP-1, were significantly increased in early-onset group. Moreover, there was a significant correlation between these cytokines. Interestingly, the level of VEGF was significantly higher in the early-onset group than that in the late-onset group before Bonferroni correction (P=0.036). These findings may partly explain why early-onset patients are more likely to develop postoperative recurrent VH. However, this statistical difference disappeared after Bonferroni correction, suggesting that the level of VEGF may not play a leading role in patients with early-onset T2DM.
IFN-γ is produced by T cells and is an immunomodulatory factor. IP-10 belongs to chemokine, which is mainly induced by INF-γ. Both of them cause inflammatory response. The concentrations of IFN- γ and IP-10 in intraocular fluid of patients with DR were increased.27,28 MIP-1α, MIP-1β, IL-9 and G-CSF in vitreous of patients with DR are rarely studied. MIP-1α and MIP-1β belong to the chemokine family and are involved in the process of local inflammatory injury. And MIP-1α can induce corneal neovascularization in mice,29 MIP-1β can also induce interstitial fibrosis.30 IL-9 can be used as a proinflammatory cytokine in autoimmunity and allergic reactions. G-CSF is a colony stimulating factor involved in regulating the proliferation and differentiation of inflammatory cells. Various chemokines can act as leukocyte attractants and angiogenesis inducers to act on endothelial cells together with pro-inflammatory cytokines, resulting in the destruction of blood-retinal barrier and neovascularization.31
The age at DM diagnosis and level of HbA1c are associated with multiple inflammatory factors and chemokines. The correlation analysis showed that the age at DM diagnosis was significantly negatively correlated with the above 10 cytokines, while HbA1c was was significantly positively correlated with IL-6, G-CSF, IP-10, MCP-1 and MIP-1 α. In addition, patients with early-onset T2DM had higher HbA1c level and more severe retinopathy than patients with late-onset T2DM. Therefore, we speculate that in the process of long-term chronic retinal inflammation caused by hyperglycemia, the combined action of inflammatory factors and chemokines leads to the rapid progression of retinopathy in patients with early-onset T2DM PDR. It also suggests that anti-inflammatory therapy, not just anti-VEGF therapy, can be performed in the early stage of the disease for patients with early-onset T2DM PDR. A recent clinical trial found that the combination of dexamethasone and silicone oil tamponade during PPV is a safe and effective treatment for PDR, and may reduce the incidence of DME and the need for anti-VEGF injections, which provides further support for anti-inflammatory therapies.32
The relationship between the age at DM diagnosis, cytokine levels, and visual prognosis after PPV in patients with PDR is poorly understood. Multiple linear regression analysis showed that the age at DM diagnosis was negatively correlated with postoperative LogMAR BCVA. However, HbA1c, IL-4, and RANTES were positively correlated with postoperative LogMAR BCVA. This suggests that the younger the age at T2DM onset, the worse the prognosis of visual acuity after vitrectomy. These results are consistent with the findings of Lv et al, who proved that early-onset diabetes is an independent risk factor for the development of PDR in T2DM patients with microalbuminuria.33 Many studies have demonstrated the relationship between the level of glycaemic control and progression of DR.34–37 Harris et al34 found that for every one point increase in the HbA1c level, the risk of progression to PDR increased by 14%, after adjusting for confounding factors. This suggests stricter blood glucose management should be performed in patients with early-onset diabetes. Blood glucose remains the most important modifiable measure to reduce the risk of progression of DR and vision loss. RANTES is a member of the chemokine family. It causes leukocyte infiltration, vascular injury, and neovascularization locally in the retina by chemotaxis of other inflammatory factors. A significant increase in RANTES has been proved to be associated with DR severity.38 IL-4 is considered an anti-inflammatory factor; however, its role remains controversial, with some studies finding elevated IL-4 levels in DR39,40 and others finding the opposite.41 In our study, the increased expression of the anti-inflammatory cytokine IL-4 could be interpreted as a compensatory response aimed at reducing PDR-related inflammation. The exact role of IL-4 in DR requires further investigation.
It is worth mentioning that our research has several advantages and limitations. One advantage is that, to our knowledge, this is the first study to evaluate the relationship between early-onset T2DM PDR and vitreous cytokines. Another advantage is that the effect of the age at onset of T2DM on the severity and prognosis of PDR has also been studied. The first limitation of this study is that the sample size was small and all patients were hospitalized in the same centre. This may have led to selection bias. The sample size of vitreous specimens was also small, which may lead to the omission of some predictive factors. Therefore, the results of this study should be verified with a larger sample size that includes a multicentre population. Second, since the onset of T2DM is often insidious, the exact onset age cannot be determined; therefore, the observed onset age may be higher than the actual onset age. In addition, the postoperative follow-up period was only six months; longer follow-ups and observations should be conducted in the future.
Conclusion
This study found that, compared with patients with late-onset T2DM PDR, patients with early-onset T2DM PDR had poorer blood glucose control and lipid metabolism, a more severe retinopathy, higher levels of inflammatory factors, and a poorer visual prognosis. Age at DM diagnosis, HbA1c, IL-4, and RANTES levels were independent predictors of visual acuity after PPV. These findings emphasize that attention should be paid to patients with early-onset Type 2 diabetes, with stricter metabolic management and earlier anti-inflammatory interventions.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Acknowledgments
We would like to thank all the staff supporting study delivery and all participants for their contributions to this study.
Funding
This work was supported by the National Natural Science Foundation of China [Grant numbers 81570868 and 81974136].
Disclosure
The authors report no conflicts of interest, financial or otherwise for this work.
References
1. Huo X, Gao L, Guo L, et al. Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: a cross-sectional study. Lancet Diabetes Endocrinol. 2016;4(2):115–124. doi:10.1016/S2213-8587(15)00508-2
2. Wong J, Molyneaux L, Constantino M, Twigg SM, Yue DK. Timing is everything: age of onset influences long-term retinopathy risk in type 2 diabetes, independent of traditional risk factors. Diabetes Care. 2008;31(10):1985–1990. doi:10.2337/dc08-0580
3. Wang Y, Lin Z, Zhai G, et al. Prevalence of and risk factors for diabetic retinopathy and diabetic macular edema in patients with early and late onset diabetes mellitus. Ophthalmic Res. 2020. doi:10.1159/000508335
4. Liu JJ, Liu S, Gurung RL, et al. Risk of progressive chronic kidney disease in individuals with early-onset type 2 diabetes: a prospective cohort study. Nephrol Dial Transplant. 2020;35(1):115–121. doi:10.1093/ndt/gfy211
5. Zheng L, Chen X, Luo T, et al. Early-onset type 2 diabetes as a risk factor for end-stage renal disease in patients with diabetic kidney disease. Prev Chronic Dis. 2020;17:E50. doi:10.5888/pcd17.200076
6. Capitao M, Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy. J Cell Biochem. 2016;117(11):2443–2453. doi:10.1002/jcb.25575
7. Forrester JV, Kuffova L, Delibegovic M. The role of inflammation in diabetic retinopathy. Front Immunol. 2020;11:583687. doi:10.3389/fimmu.2020.583687
8. Suzuki Y, Suzuki K, Kudo T, Metoki T, Nakazawa M. Level of vascular endothelial growth factor in the vitreous fluid of proliferative diabetic retinopathy patients and prognosis after vitrectomy. Ophthalmologica. 2016;236(3):133–138. doi:10.1159/000449261
9. Tsai T, Kuehn S, Tsiampalis N, et al. Anti-inflammatory cytokine and angiogenic factors levels in vitreous samples of diabetic retinopathy patients. PLoS One. 2018;13(3):e0194603. doi:10.1371/journal.pone.0194603
10. Sun C, Zhang H, Jiang J, et al. Angiogenic and inflammatory biomarker levels in aqueous humor and vitreous of neovascular glaucoma and proliferative diabetic retinopathy. Int Ophthalmol. 2020;40(2):467–475. doi:10.1007/s10792-019-01207-4
11. Lei J, Ding G, Xie A, et al. Aqueous humor monocyte chemoattractant protein-1 predicted long-term visual outcome of proliferative diabetic retinopathy undergone intravitreal injection of bevacizumab and vitrectomy. PLoS One. 2021;16(3):e0248235. doi:10.1371/journal.pone.0248235
12. Wu F, Phone A, Lamy R, et al. Correlation of aqueous, vitreous, and plasma cytokine levels in patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2020;61(2):26. doi:10.1167/iovs.61.2.26
13. Nawaz IM, Rezzola S, Cancarini A, et al. Human vitreous in proliferative diabetic retinopathy: characterization and translational implications. Prog Retin Eye Res. 2019;72:100756. doi:10.1016/j.preteyeres.2019.03.002
14. Yeung RO, Zhang Y, Luk A, et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the jade programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol. 2014;2(12):935–943. doi:10.1016/S2213-8587(14)70137-8
15. Kong X, Xing X, Zhang X, Hong J, Yang W. Early-onset type 2 diabetes is associated with genetic variants of beta-cell function in the Chinese han population. Diabetes Metab Res Rev. 2020;36(2):e3214. doi:10.1002/dmrr.3214
16. Song SH, Gray TA. Early-onset type 2 diabetes: high risk for premature diabetic retinopathy. Diabetes Res Clin Pract. 2011;94(2):207–211. doi:10.1016/j.diabres.2011.07.030
17. Pan J, Jia W. Early-onset diabetes: an epidemic in China. Front Med. 2018;12(6):624–633. doi:10.1007/s11684-018-0669-1
18. Yuan J, Zhang L, Jia P, Xin Z, Yang JK. Early onset age increased the risk of diabetic retinopathy in type 2 diabetes patients with duration of 10–20 years and hba1c >/=7%: a hospital-based case-control study. Int J Endocrinol. 2021;2021:5539654. doi:10.1155/2021/5539654
19. Okudaira M, Yokoyama H, Otani T, Uchigata Y, Iwamoto Y. Slightly elevated blood pressure as well as poor metabolic control are risk factors for the progression of retinopathy in early-onset Japanese type 2 diabetes. J Diabetes Complications. 2000;14(5):281–287. doi:10.1016/S1056-8727(00)00114-8
20. Kovacs K, Marra KV, Yu G, et al. Angiogenic and inflammatory vitreous biomarkers associated with increasing levels of retinal ischemia. Invest Ophthalmol Vis Sci. 2015;56(11):6523–6530. doi:10.1167/iovs.15-16793
21. Yoshida S, Kubo Y, Kobayashi Y, et al. Increased vitreous concentrations of mcp-1 and il-6 after vitrectomy in patients with proliferative diabetic retinopathy: possible association with postoperative macular oedema. Br J Ophthalmol. 2015;99(7):960–966. doi:10.1136/bjophthalmol-2014-306366
22. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (mcp-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–326. doi:10.1089/jir.2008.0027
23. Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271(2):736–741. doi:10.1074/jbc.271.2.736
24. Koskela UE, Kuusisto SM, Nissinen AE, Savolainen MJ, Liinamaa MJ. High vitreous concentration of il-6 and il-8, but not of adhesion molecules in relation to plasma concentrations in proliferative diabetic retinopathy. Ophthalmic Res. 2013;49(2):108–114. doi:10.1159/000342977
25. Ghasemi H, Ghazanfari T, Yaraee R, Faghihzadeh S, Hassan ZM. Roles of il-8 in ocular inflammations: a review. Ocul Immunol Inflamm. 2011;19(6):401–412. doi:10.3109/09273948.2011.618902
26. Yoshida S, Kobayashi Y, Nakao S, et al. Differential association of elevated inflammatory cytokines with postoperative fibrous proliferation and neovascularization after unsuccessful vitrectomy in eyes with proliferative diabetic retinopathy. Clin Ophthalmol. 2017;11:1697–1705. doi:10.2147/OPTH.S141821
27. Cvitkovic K, Sesar A, Sesar I, et al. Concentrations of selected cytokines and vascular endothelial growth factor in aqueous humor and serum of diabetic patients. Semin Ophthalmol. 2020;35(2):126–133. doi:10.1080/08820538.2020.1755320
28. Abu El-Asrar AM, Struyf S, Kangave D, Geboes K, Van Damme J. Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur Cytokine Netw. 2006;17(3):155–165.
29. Yoshida S, Yoshida A, Ishibashi T, Elner SG, Elner VM. Role of mcp-1 and mip-1alpha in retinal neovascularization during postischemic inflammation in a mouse model of retinal neovascularization. J Leukoc Biol. 2003;73(1):137–144. doi:10.1189/jlb.0302117
30. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. doi:10.1002/path.2277
31. Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. Cxc chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25(4):201–209. doi:10.1016/j.it.2004.02.006
32. Altun A, Kanar HS, Aki SF, Arsan A, Hacisalihoglu A. Effectiveness and safety of coadministration of intravitreal dexamethasone implant and silicone oil endotamponade for proliferative diabetic retinopathy with tractional diabetic macular edema. J Ocul Pharmacol Ther. 2021;37(2):131–137. doi:10.1089/jop.2020.0079
33. Lv X, Ran X, Chen X, et al. Early-onset type 2 diabetes: a high-risk factor for proliferative diabetic retinopathy (pdr) in patients with microalbuminuria. Medicine. 2020;99(19):e20189. doi:10.1097/MD.0000000000020189
34. Harris nwanyanwu K, Talwar N, Gardner TW, et al. Predicting development of proliferative diabetic retinopathy. Diabetes Care. 2013;36(6):1562–1568. doi:10.2337/dc12-0790
35. Mitchell SL, Neininger AC, Bruce CN, et al. Mitochondrial haplogroups modify the effect of diabetes duration and hba1c on proliferative diabetic retinopathy risk in patients with type 2 diabetes. Invest Ophthalmol Vis Sci. 2017;58(14):6481–6488. doi:10.1167/iovs.17-22804
36. Hainsworth DP, Bebu I, Aiello LP, et al. Risk factors for retinopathy in type 1 diabetes: the DCCT/EDIC study. Diabetes Care. 2019;42(5):875–882. doi:10.2337/dc18-2308
37. Lind M, Pivodic A, Svensson AM, et al. Hba1c level as a risk factor for retinopathy and nephropathy in children and adults with type 1 diabetes: Swedish population based cohort study. BMJ. 2019;366:l4894. doi:10.1136/bmj.l4894
38. Tokarz A, Konkolewska M, Kusnierz-Cabala B, et al. Retinopathy severity correlates with rantes concentrations and CCR 5-positive microvesicles in diabetes. Folia Med Cracov. 2019;59(3):95–112.doi:10.24425/fmc.2019.131139
39. Chernykh VV, Varvarinsky EV, Smirnov EV, Chernykh DV, Trunov AN. Proliferative and inflammatory factors in the vitreous of patients with proliferative diabetic retinopathy. Indian J Ophthalmol. 2015;63(1):33–36. doi:10.4103/0301-4738.151464
40. Zeng Y, Cao D, Yu H, et al. Comprehensive analysis of vitreous humor chemokines in type 2 diabetic patients with and without diabetic retinopathy. Acta Diabetol. 2019;56(7):797–805. doi:10.1007/s00592-019-01317-6
41. Cao YL, Zhang FQ, Hao FQ. Th1/th2 cytokine expression in diabetic retinopathy. Genet Mol Res. 2016;15(3). doi:10.4238/gmr.15037311
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


