Back to Journals » Journal of Asthma and Allergy » Volume 15
Clinical Features and Efficacy of Benralizumab in Patients with Blood Eosinophil Count Between 300 and 450 Cells/mm3: A Post Hoc Analysis from the ANANKE Study
Authors Senna G, Aliani M, Altieri E, Bracciale P, Brussino L , Caiaffa MF, Cameli P , Canonica GW , Caruso C, D'Amato M, De Michele F, Del Giacco S, Di Marco F , Menzella F , Pelaia G , Rogliani P , Romagnoli M, Schino P, Schroeder JW, Vultaggio A, Rizzoli S, Zullo A, Boarino S, Palmisano M, Rossi A , Vitiello G , Centanni S
Received 25 July 2022
Accepted for publication 3 November 2022
Published 10 November 2022 Volume 2022:15 Pages 1593—1604
DOI https://doi.org/10.2147/JAA.S383012
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Amrita Dosanjh
Gianenrico Senna,1,2 Maria Aliani,3 Elena Altieri,4 Pietro Bracciale,5 Luisa Brussino,6 Maria Filomena Caiaffa,7 Paolo Cameli,8 Giorgio Walter Canonica,9,10 Cristiano Caruso,11 Maria D’Amato,12 Fausto De Michele,13 Stefano Del Giacco,14 Fabiano Di Marco,15 Francesco Menzella,16 Girolamo Pelaia,17 Paola Rogliani,18,19 Micaela Romagnoli,20 Pietro Schino,21 Jan Walter Schroeder,22 Alessandra Vultaggio,23 Sara Rizzoli,24 Alessandro Zullo,24 Silvia Boarino,25 Marilena Palmisano,26 Alessandra Rossi,26 Gianfranco Vitiello,26 Stefano Centanni27
1Department of Medicine, University of Verona, Verona, Italy; 2Allergy Unit and Asthma Center, Verona University Hospital, Verona, Italy; 3UO Pneumologia e Pneumologia Riabilitativa, ICS Maugeri, IRCCS Bari, Bari, Italy; 4Reparto di Pneumologia, P.O., Garbagnate Milanese, Italy; 5Reparto di Pneumologia, Ospedale Ostuni, Ostuni, BR, Italy; 6Dipartimento di Scienze Mediche, SSDDU Allergologia e Immunologia Clinica, Università degli Studi di Torino, AO Ordine Mauriziano Umberto I, Torino, Italy; 7Cattedra e Scuola di Allergologia e Immunologia Clinica, Dipartimento di Scienze Mediche, Università di Foggia, Foggia, Italy; 8Respiratory Diseases and Lung Transplantation, Department of Medical and Surgical Sciences & Neurosciences, Siena University Hospital, Siena, Italy; 9Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, MI, Italy; 10Personalized Medicine Center: Asthma and Allergology, Humanitas Research Hospital, Rozzano, MI, Italy; 11Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico A. Gemelli, IRCCS, Università Cattolica del Sacro Cuore, Roma, Italy; 12UOSD Malattie Respiratorie “Federico II”, Ospedale Monaldi, AO Dei Colli, Napoli, Italy; 13UOC Pneumologia e Fisiopatologia Respiratoria, AORN A. Cardarelli, Napoli, Italy; 14Department of Medical Sciences and Public Health, University of Cagliari, Cagliari, Italy; 15Department of Health Sciences, Università Degli Studi Di Milano, Pneumologia, ASST Papa Giovanni XXIII, Bergamo, Italy; 16UOC Pneumologia, Ospedale “S. Valentino”, AULSS 2 Marca Trevigiana, Montebelluna, TV, Italy; 17Dipartimento di Scienze della Salute, Università Magna Graecia, Catanzaro, Italy; 18Division of Respiratory Medicine, University Hospital “Tor Vergata”, Roma, Italy; 19Unit of Respiratory Medicine, Department of Experimental Medicine, University of Rome “Tor Vergata”, Roma, Italy; 20UOC Pneumologia, ULSS 2 Marca Trevigiana, Treviso, Italy; 21Fisiopatologia Respiratoria, Ospedale Generale Regionale, Ente Ecclesiastico “F. Miulli”, Acquaviva delle Fonti, BA, Italy; 22Allergy and Clinical Immunology, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy; 23Dipartimento di Medicina Sperimentale e Clinica, Università degli Studi di Firenze, Firenze, Italy; 24Medineos Observational Research - An IQVIA Company, Modena, Italy; 25Medical Evidence R&I, AstraZeneca, Milano, Italy; 26Medical Affairs R&I, AstraZeneca, Milano, Italy; 27Respiratory Unit, ASST Santi Paolo e Carlo, Department of Health Sciences, Università degli Studi di Milano, Milano, Italy
Correspondence: Marilena Palmisano, Medical Affairs R&I, AstraZeneca, Milano, Italy, Email [email protected]
Purpose: Benralizumab effectively reduces severe eosinophilic asthma (SEA) exacerbations in patients with a wide range of baseline blood eosinophil count (BEC). Patients included in real-world studies are often characterized by high mean/median BEC, while patients with BEC close to 300 cells/mm3 are poorly represented. This post hoc analysis from the Italian study ANANKE aims to define the clinical features and corroborate the efficacy of benralizumab in real world in the BEC 300– 450 cells/mm3 subset of patients.
Patients and Methods: Post hoc analysis of the Italian, multicenter, observational, retrospective real-life study ANANKE (NCT04272463). Baseline clinical and laboratory characteristics were collected in the 12 months prior to benralizumab treatment and presented for a BEC 300– 450 cells/mm3 subgroup of patients. Change over time of BEC, annualized exacerbation rate (AER), asthma control (ACT), lung function and oral corticosteroid (OCS) use at 16, 24 and 48 weeks after benralizumab introduction were collected.
Results: A total of 164 patients were analyzed, 34 of whom with a BEC of 300– 450 cells/mm3. This subgroup was more likely to be female (64.7%), with lower rates of severe exacerbations at baseline when compared to the total population (0.69 vs 1.01). After 48 weeks of benralizumab treatment, the BEC 300– 450 subset showed similar reductions in AER (− 94.8% vs − 92.2%) and OCS use (median dose reduction of 100% in both groups), as well as improvement in ACT score (median scores 22.5 vs 22) and lung function (pre-BD FEV1: +200 mL vs +300 mL) when compared to the total population. No discontinuations for safety reasons were registered.
Conclusion: At baseline, apart from lower severe exacerbation rate, the BEC 300– 450 cells/mm3 subset of patients is comparable to the total population prescribed with benralizumab. In this real-life study, benralizumab is as effective in BEC 300– 450 patients as in the total population.
Keywords: severe eosinophilic asthma, blood eosinophil count, benralizumab, observational, real-world evidence, real-life
Introduction
Severe asthma (SA) is a serious chronic inflammatory condition associated with poor clinical outcomes which affects 5% to 10% of the total asthma population.1,2 About half of patients with severe asthma have an underlying eosinophilic phenotype3 which correlates with increased disease severity and exacerbation frequency as well as decreased lung function and symptom control.4–6 To control symptoms and prevent exacerbations of severe eosinophilic asthma (SEA), management often results in oral corticosteroid (OCS) use which can become excessive. In this scenario, not only chronic but also intermittent use of OCS has been associated with increased risk of developing severe adverse events and systemic conditions.7–11
A broadly accepted therapeutic alternative to OCS is represented by the treatment with biologic therapies. There are two monoclonal antibodies (mAb) (mepolizumab and reslizumab) approved for the treatment of SEA which target interleukin-5 (IL-5).12 Benralizumab represents an alternative therapeutic option, as it is the only mAb which targets the IL-5 receptor alpha (IL-5Rα) and ensures the near-complete depletion of eosinophils in blood and peripheral tissues, thanks to its unique Ab-dependent cell-mediated cytotoxicity (ADCC) mechanism of action.13 Benralizumab has been demonstrated to be highly effective in decreasing exacerbation frequency and improving asthma control in patients with a baseline blood eosinophil count (BEC) starting from 300 cells/mm3.14–16 More recently, the PONENTE study highlighted how the treatment with benralizumab successfully eliminated the use of OCS, with a parallel reduction in exacerbation frequency, in the majority of patients recruited, independently of BEC at baseline.17
Despite the body of data available confirming the efficacy of benralizumab in SEA patients with different ranges of BEC, several published Italian real-world evidence (RWE) studies included patients treated with benralizumab who were characterized by a high baseline BEC. Specifically, patients were often characterized by a mean or median value greater than 700 cells/mm3,18–22 with a minimum BEC equal or greater than 300 cells/mm3 required to be eligible for benralizumab treatment in Italy. As evident from those studies, patients with BEC close to 300 cells/mm3 have been poorly represented, hence the need to corroborate benralizumab real-world efficacy in this specific subset of patients.18–22
The ANANKE study (NCT0427246323,24) is a large observational, Italian multicenter, retrospective cohort study including 205 patients with SEA. In this study, patients showed a baseline median BEC of 580 cells/mm24 indicating a consistent presence of patients with BEC close to 300 cells/mm3. Thus, a post hoc analysis was conducted to describe the clinical characteristics and the effectiveness of benralizumab (exacerbation rate, asthma control, lung function parameters, reduction of OCS dosage) in the subset of patients having a BEC of 300–450 cells/mm3 and compare them to the total population. Of note, this post hoc analysis was conducted with data obtained from patients treated with benralizumab for 48 weeks.
Materials and Methods
Study Design
The ANANKE study design has been fully described elsewhere.24 In summary, ANANKE is an Italian multicenter, observational, retrospective study (ClinicalTrials.gov Identifier: NCT04272463) which included SEA patients who had started benralizumab as per clinical practice or within the Italian Sampling Program, activated following benralizumab approval in January 2018 and before reimbursement. Enrolment took place in an 8-month period between December 2019 and July 2020 in 21 Italian sites. Data collection regarded a period of at least 12 months of baseline features before benralizumab introduction into therapy and a follow-up of 48 weeks after benralizumab introduction. The study was performed following the regulation and guidelines of good medical practice in Italy.
Study Population
The subsequent inclusion criteria were considered for the inclusion of patients in the study:
- Adult patients (age at least 18 years) at the start of benralizumab treatment within the sampling program or per clinical practice (“index date”).
- Patients with severe eosinophilic asthma requiring a stable treatment of high doses of inhaled corticosteroids and a long-acting β2agonist ± additional asthma controller (according to a clinician’s judgment).
- Patients who started benralizumab and received at least one injection at least 3 months before enrollment, either within the sampling program or as per routine clinical practice.
- Patients who signed the informed consent and privacy form during the enrollment visit.
- Patients with hospital medical charts available from the start of benralizumab treatment within the sampling program or per clinical practice (“index date”).
The following exclusion criteria were considered:
- Patients who, during the observation period, received benralizumab during a clinical experimental trial.
- Patients who, during the observation period, participated in studies imposing a specific patient management strategy that does not correspond to the site’s normal clinical practice.
Patients with BEC 300–450 cells/mm3 were considered as a separate group and compared to the total population in study. The BEC range between 300 and 450 cells/mm3 was chosen as already used elsewhere to identify a specific BEC patient cohort.16
Outcomes and Variables
Data were collected from hospital medical charts according to clinical practice and entered the electronic case report form (eCRF) for patients who signed the informed consent and the privacy form.
The primary endpoint was to describe the clinical features of the total population and the population with BEC 300–450 cells/mm3 recorded at the index date (defined as the start date of benralizumab treatment). Data collection started 12 months before the index date. Demographics (age, sex, body mass index [BMI], comorbidities and smoking status), features of asthma (age at diagnosis and duration), laboratory results (BEC and total serum immunoglobulin E [IgE]), presence of atopy (defined as a positive skin prick test for a perennial allergen), lung function parameters, asthma control (defined by Asthma Control Test [ACT]), OCS use and dosage, annualized exacerbation rates (AER) for any exacerbation (defined as a physician-diagnosed clinically relevant asthma exacerbation), and severe exacerbation (defined as worsening of asthma that leads to one of the following: 1) use of systemic corticosteroids for 3 days or more or a temporary increase in a stable, background dosage of oral corticosteroids; 2) an emergency department or urgent care visit [<24 h] due to asthma that required systemic corticosteroids; or 3) an inpatient admission to hospital [≥24 h] due to asthma) were registered.
The secondary endpoint was the description of outcomes in the group of patients with BEC 300–450 cells/mm3 and in the total population during benralizumab treatment between the index date and 48 weeks, with specifics at baseline, 16, 24 and 48 weeks (when available) of the following parameters:
- Change over time of BEC.
- AER for any exacerbation and severe exacerbations during benralizumab treatment.
- Change over time of ACT.
- Change over time of forced expiratory volume in the first second (FEV1).
- Change over time of OCS use and dosage.
- Benralizumab discontinuation and reasons for discontinuation.
Statistical Analysis
Full statistical analysis specifics were described in the full manuscript.24 In brief, the analyses were descriptive only, using mean, standard deviation (SD), median, interquartile range (IQR), range and absolute and relative frequencies, when appropriate. In particular, median and IQR were preferred over mean (SD) in case of high variability in data distribution. No formal hypotheses were prespecified. The analyses were performed using SAS software v9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline Characteristics are Similar Between the BEC 300–450 Subset and the Total Population
A total of 218 SEA patients were enrolled between December 2019 and July 2020 from 21 Italian centers in the ANANKE study; among these, 169 patients were considered eligible for the analysis at 48 weeks. The reasons for the exclusion of non-eligible patients were as follows: benralizumab treatment was not started at least 3 months before enrollment, informed consent and/or amendment form was not signed, incomplete data entry and lack of attendance at the 48-week visit. A total of 164 patients who had a valid baseline value for BEC completed 48 weeks of treatment with benralizumab (median duration of treatment with benralizumab: 10.9 [IQR 7.4–14.9] months). Within this population, a post hoc analysis was conducted comparing the subset of patients showing a BEC between 300 and 450 cells/mm3 (median 400 [IQR 350–440], N=34) to the entire population (median 575 [IQR 415–848], N=164). In Table 1, the demographics and clinical characteristics of the two groups at index date are reported. In general, the total population and the BEC 300–450 subset had similar age (mean 56.5 ± 12.6 years vs 56.1 ± 13.0 years), age at diagnosis of asthma (mean 38.9 ± 16.1 years vs 39.1 ± 15.4 years), duration of asthma (median 13.4 [IQR 8.0–24.9] years vs 12.4 [IQR 8.0–24.0] years) and presence of atopy (N, 67/164 [40.9%] vs N, 13/34 [38.2%]), with a trend towards increased serum IgE levels in the total population (236 IU/mL [IQR 73.3–545] vs 138 [67–362] in the BEC 300–450 subset). Females were slightly more frequent among the BEC 300–450 subset (N=22/34 [64.7%] vs N=101/164 [55.8%] in the total population). Although patients with low and normal BMI were equally distributed in the two groups (N=57/164 [34.7%] in the total population vs N=11/34 [32.3%] in the BEC 300–450 subset), overweight patients prevailed in the BEC 300–450 subset (N=17/34 [50.0%] vs N=65/164 [39.6%] in the total population) and obese patients were slightly more frequent in the total population (N=27/164 [16.5%] vs N= 4/34 [11.8%] in the BEC 300–450 subset).
 |
Table 1 Demographics, Clinical and Laboratory Features of the Patient Population |
Lung function was similar between the two groups at index date, with identical mean absolute values in pre-bronchodilator FEV1 (pre-BD FEV1) (1.9 L), post-bronchodilator FEV1 (post-BD FEV1) (2.1 L) and pre-BD FEV1/FCV ratio (0.7). The groups also had comparable ACT median scores (14 [IQR 12–18] in the total population vs 15 [IQR 12–17] in the BEC 300–450 subset), indicating the same degree of uncontrolled asthma. Interestingly, the annualized exacerbation rate (AER) (any kind) was similar between the two groups (3.87 [N=157] in the total population vs 3.66 [N=32] in the BEC 300–450 subset) but the severe AER was greater in the total population (1.01 [N=157] vs 0.69 [N=32] in the BEC 300–450 subset). The OCS usage was comparable between the two groups (N=43/164 [26.2%] in the overall population vs N=8/34 [23.5%] in the BEC 300–450 subset), with a median dosage (expressed in prednisone equivalent) of 10 mg/day (IQR 5–25) in the total population and 5 mg/day [IQR 5–25] in the BEC 300–450 subset (mean OCS dosages: 13 mg/day ± 9 in the total population, 13.2 mg/day ± 11.1 in the BEC 300–450 subset).
Table 2 shows the comorbidities diagnosed at the index date and their prevalence in the two groups. Overall, any relevant or asthma-related comorbidities were equally represented, including current or past nasal polyposis (N=89/164 [54.3%] in the total population vs N=19/34 [55.9%]), and OCS-related conditions (N=17/34 [50%] in the BEC 300–450 subset vs N=60/164 [40.2%] in the total population).
 |
Table 2 List and Percentage of Comorbidities Within the Study Population |
Benralizumab Induced a Rapid and Complete Depletion of Eosinophils Independently of BEC
Patients in both groups showed a complete depletion of blood eosinophils, which was independent of the BEC and was detected as soon as 16 weeks after starting the treatment with benralizumab (median: 0 cells/mm3 [IQR 0–0]) and persisted until 48 weeks (median: 0 cells/mm3 [IQR 0–0]).
Benralizumab Was Equally Effective in Reducing Exacerbations in Both BEC 300-450 Subset and Total Population
Treatment with benralizumab reduced exacerbations in both the total population and the BEC 300–450 subset. Specifically, any AER was reduced by 92.2% (from 3.87 to 0.3) and 94.8% (from 3.66 to 0.19) in the total population and the BEC 300–450 subset, respectively (Figure 1A). Similarly, the severe AER dropped by 90.1% (from 1.01 to 0.1) in the total population and 94.2% (from 0.69 to 0.04) in the BEC 300–450 subset (Figure 1B). The percentage of patients who did not experience any kind of exacerbations at 48 weeks was also similar between the two groups (N=105/135 [77.8%] in the total population vs N=23/28 [82.1%] in the BEC 300–450 subset).
Benralizumab Improved Asthma Control in Both BEC 300–450 Subset and Total Population
Consistently with the reduction of exacerbations, the treatment with benralizumab improved asthma control in both the total population and the BEC 300–450 subset, as indicated by the ACT score measured at index date, 16, 24 and 48 weeks. As shown in Figure 1C, the ACT score progressively increased over time in both groups, reaching median values of 22 (IQR 20–24) in the total population and 22.5 (IQR 20–24.5) in the BEC 300–450 subset. The percentage of patients who achieved an ACT score ≥20, indicative of a well-controlled asthma, at 48 weeks were 83.3% (N=75/90) and 85% (N=17/20) in the total population and the BEC 300–450 subset, respectively. And, 76.3% patients (N=61/80) in the total population and 83.3% (N=15/18) patients in the BEC 300–450 subset reached the minimum importance difference (MID).
Benralizumab Increased Pre-BD FEV1 in Both BEC 300–450 Subset and Total Population
Records on lung function parameters were limited, with an insufficient number of patients within the BEC 300–450 subset having data available at all time points. Therefore, the analysis was conducted only on pre-BD FEV1 using the values obtained from the most representative time points (index date and 16 weeks with N= 23 and N=15, respectively, in the BEC 300–450 subset). As shown in Figure 1D, the median pre-BD FEV1 increased from the index date to 16 weeks in both groups, from 1.8 (IQR 1.3–2.4) to 2.1 (IQR 1.5–2.9) in the total population and from 1.7 (IQR 1.1–2.4) to 1.9 (IQR 1.3–2.6) in the BEC 300–450 subset. Specifically, pre-BD FEV1 improved by +300 mL and +200 mL in the total population and BEC 300–450 subset, respectively. Such improvement was maintained over time until 48 weeks in the total population.
Benralizumab Eliminated OCS Usage in Both BEC 300–450 Subset and Total Population
At 48 weeks, data on OCS usage was available for 29 patients out of 43 OCS users in the total population and for 5 patients out of 8 OCS users in the BEC 300–450 subset. In both populations, the steroid-sparing efficacy of benralizumab was confirmed. Sixteen patients (55.2%) in the total population and 5 patients (100%) in the BEC 300–450 subset eliminated OCS usage (Table 3), with a median OCS dosage change from 10 mg/day (IQR 5–22.5) to 0 mg/day (IQR 0–5) in the total population and from 5 mg/day (IQR 5–25) to 0 mg/day (IQR 0−0) in the BEC 300–450 subset (Figure 2). Notably, the OCS dosage reduction was never lower than 100%, meaning that patients who could reduce OCS dosage could completely eliminate their usage.
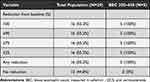 |
Table 3 OCS Reduction at End of Observation in the Patient Population |
Discontinuation and Safety
Patients who permanently discontinued the treatment with benralizumab at the end of observation were 6 out of 140 (4.3%) in the total population and 3 out of 28 (10.7%) in the BEC 300–450 subset. The reasons for discontinuation were lack of efficacy (N=3 in the total population and N=2 in the BEC 300–450 subset), patient decision (N=1 in the total population), unknown (N=1 in the total population) and other (N=1 in the total population and N=1 in the BEC 300–450 subset).
Discussion
This post hoc analysis of the Italian real-world study ANANKE24 highlighted the clinical features of 164 SEA patients in treatment with benralizumab for 48 weeks with a focus on patients with BEC 300–450 cells/mm3 and confirmed the efficacy and safety of benralizumab in this setting of patients. The specific range of BEC between 300 and 450 cells/mm3 was already used to identify a cohort of patients by FitzGerald et al, who conducted a pooled analysis of the SIROCCO and CALIMA Phase 3 studies to evaluate benralizumab effectiveness in patients stratified by BEC.16 In this analysis, benralizumab resulted to be effective in both the BEC cohorts of patients with BEC 300–450 cells/mm3 and BEC ≥450 cells/mm3; however, a trend towards a greater efficacy in association with greater BEC was noticed.16
In this study, the baseline clinical features of the BEC 300–450 subset were comparable with the characteristics shown by the total population. No substantial differences regarding age, age at onset of asthma, presence of atopy, nasal polyps prevalence, lung function and asthma control were found, although a slight prevalence of female sex and some differences in BMI were observed. Importantly, the percentage of patients treated with OCS was also similar between the groups, with the median value of OCS dosage in the BEC 300–450 subset resulting smaller when compared to the total population, indicating that the lower BEC in the 300–450 subset is not to be considered a consequence of a greater use of OCS. Interestingly, despite showing a similar rate of any AER in the previous year, the severe AER was lower in the BEC 300–450 subset when compared to the total population.
In keeping with our data, asthma control has been previously reported to be similar across different eosinophil cohorts, with a higher risk of severe exacerbations in patients with higher BEC.25,26 Previous works have also highlighted an association between lung function and BEC, both in terms of FEV1 decline27,28 and persistent airway obstruction,29 while in our study, the BEC 300–450 subset and the total population had analogous baseline lung function parameters. Similarly, Menzella et al reported comparable predicted pre-BDFEV1 values in patients with BEC with less or more than 500 cells/mm3, but worse values in the >500 cells/mm3 when FEV1 in liters was taken into consideration.30
The efficacy of benralizumab in the BEC 300–450 subset was confirmed and resulted to be consistent with the total population. As a result of benralizumab mechanism of action,13 eosinophil depletion was achieved in all patients irrespective of eosinophil count. More than 90% reduction in any and severe AER was reached after 48 weeks of treatment, with 77.8% and 82.1% of exacerbation-free patients in the total population and BEC 300–450 subset, respectively. Of note, these results are in agreement with the long-term data obtained from the MELTEMI study, where 87% of the patients did not have any exacerbation during the fifth year of treatment with benralizumab.31
The change in the ACT score obtained from the BEC 300–450 subset and the total population indicated a similar, progressive improvement in asthma control. In addition, benralizumab also resulted to be highly effective in improving lung function and reducing OCS use, although data regarding these two parameters were limited. Both the BEC 300–450 subset and the total population had a substantial increase in the pre-BD FEV1 after 16 weeks of treatment, with an improvement superior to what observed in previous studies.30 The FEV1 increase shown by the total population (300 mL) was greater when compared to the BEC 300–450 subset (200 mL) although it is not possible to exclude a further improvement of FEV1 in the BEC 300–450 subset during the following weeks of treatment. Although the number of patients receiving OCS was low, the OCS-sparing effect of benralizumab was reaffirmed, as OCS use was eliminated in 100% patients in the BEC 300–450 subset and in the majority of patients in the total population, with a reduction of OCS median dose reaching 0 mg/day prednisone equivalent in both groups.
The data obtained in this study confirm the efficacy of benralizumab in SEA patients with BEC starting from 300 cells/mm3 and largely agree with data obtained from other RWE studies. In particular, Miralles López et al recently confirmed the efficacy of benralizumab in reducing exacerbation and eliminating the use of OCS in patients having eosinophils ≥300 cells/mm3 regardless of BEC stratification.32 A slightly greater reduction in exacerbations was seen in patients with BEC higher than 400 cells/mm3 when compared with patients showing BEC between 300 and 400 cells/mm3 in the Benralizumab Patient Access Programme (BPAP) study.33 With regard to lung function, benralizumab was found to be more effective in improving FEV1 in patients with BEC greater than 500 cells/mm3, in line with the results from this post hoc analysis.30 Potential differences between the results of this and other studies could be due to discrepancies in the number of patients enrolled as well as the baseline characteristics shown by the BEC cohorts in the various RWE.
The study presents some limitations, some of which are already discussed above. The retrospective nature of the study, the low number of OCS-using patients in the BEC 300–450 subset, and the absence of a comparator arm may be limiting, as well as missing lung function data at 24 and 48 weeks’ follow-up in the BEC 300–450 cells/mm3 subset. Moreover, the observational and retrospective nature of the ANANKE study did not allow to make any formal statistical hypotheses and the results are descriptive only.24 However, even though specific treatment recommendation could not be made based on this post hoc analysis, we are confident that this real-world experience is representative of the population being treated with benralizumab, and we believe it might be informative for the daily clinical practice of physicians.34
Conclusions
This post hoc analysis conducted from the data collected in the ANANKE RWE study confirms that the baseline characteristics presented in the BEC 300–450 subset and the total population are comparable. Importantly, the efficacy of benralizumab in improving clinical outcomes is also analogous between the BEC 300–450 subset and the total population. Those data suggest that the BEC 300–450 subset is a fair representation of the total population treated with benralizumab in a real-life setting, hence SEA patients having a BEC starting from 300 cells/mm3 should benefit from benralizumab treatment as much as patients with higher BEC.
Data Sharing Statement
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at
https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure
Ethical Approval and Informed Consent
The study was conducted in accordance with the principle of the Declaration of Helsinki and written informed consent was obtained from all participants. Ethical approval was provided by ethics committees at all the participating sites: Comitato Etico Milano Area 3, ASST GOM Niguarda; Comitato Etico Interaziendale, AOU Città della Salute e della Scienza Torino; Comitato Etico Interregionale, c/o A.O.U. Policlinico Consorziale Bari; Comitato Etico Indipendente, ASL di Brindisi; Comitato Etico Indipendente, Fondazione PTV Policlinico Tor Vergata Roma; Comitato Etico dell’Area Vasta Emilia Nord, c/o Policlinico Modena; Comitato Etico Area Vasta Sud Est, c/o A.O.U. Senese; Comitato Etico Regione Calabria Area Centro, c/o A.O.U. Mater Domini Catanzaro; Comitato Etico, Fondazione Policlinico Universitario Agostino Gemelli Roma; Comitato Etico Milano Area 1, ASST Fatebenefratelli Sacco; Comitato Etico Indipendente, A.O.U. Cagliari; Comitato Etico IRCCS, Istituto Tumori “Giovanni Paolo II” Bari; Comitato Etico, Università Federico II - AORN Cardarelli Napoli; Comitato Etico di Bergamo, c/o ASST Papa Giovanni XXIII; Comitato Etico per la Sperimentazione Clinica, Ospedale Ca’ Foncello Treviso; CESC delle Province di Verona e Rovigo, c/o Ospedale Borgo Trento Verona; Comitato Etico Area 1, A.O.U. Riuniti di Foggia; Comitato Etico Area Vasta Centro, c/o A.O.U. Careggi Firenze; Comitato Etico Indipendente, IRCCS Istituto Clinico Humanitas Rozzano; Comitato Etico Università Vanvitelli, AOU Vanvitelli – AORN Ospedali dei Colli Napoli.
Acknowledgments
We thank all the patients and physicians who participated in this study. We are grateful to Fabio Ferri, Claudio Marchese, Barbara Roncari and the entire Medineos team for their support during the design, management and statistical analysis of the data.
Funding
AstraZeneca funded the study, contributed to the study design, collection and analysis of data. AstraZeneca reviewed the publication, without influencing the opinions of the authors, to ensure medical and scientific accuracy and the protection of intellectual property. The corresponding author had access to all data in the study and the final responsibility for the decision to submit the manuscript for publication.
Disclosure
GS has nothing to declare; MA has nothing to declare; EA has nothing to declare; PB has nothing to declare; LB has nothing to declare; MFC has nothing to declare; PC reports having received in the last 3 years research grants and fees as speaker from AstraZeneca-MedImmune, Guidotti-Malesci and GlaxoSmithKline; GWC reports having received in the last 3 years research grants as well as lecture or advisory board fees from A. Menarini, Allergy – Therapeutics, AstraZeneca-MedImmune, Boehringer Ingelheim, Chiesi, Faes, Genentech, Guidotti-Malesci, GlaxoSmithKline, HAL Allergy, Novartis, Sanofi-Aventis, Sanofi-Genzyme/Regeneron, Stallergenes-Greer, Thermo Fisher, Valeas, Vifor Pharma; CC has nothing to declare; MDA has nothing to declare; FDMa has received lectures fees at national and international meetings and consultancy fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Dompé, Guidotti/Malesci, GlaxoSmithKline, Menarini, Novartis, and Zambon; SDG received grants and/or personal fees from AstraZeneca, Chiesi, Glaxo Smith Kline, Menarini, Novartis, Sanofi; FDMi has nothing to declare; FM declares research funding as Principal investigator by AstraZeneca, Chiesi Farmaceutici, Novartis, Sanofi; fees as speaker/lecturer by AstraZeneca, Chiesi Farmaceutici, GlaxoSmithKline, Novartis, Sanofi; GP has received lecture fees and consultancy fees from AlfaSigma, AstraZeneca, Chiesi, GlaxoSmithKline, Guidotti-Malesci, Menarini, Mundipharma, Novartis, Sanofi, Zambon; PR has participated as a lecturer, speaker, and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini Group, Mundipharma, and Novartis, her department has received funding from Almirall, Boehringer Ingelheim, Chiesi, Novartis, and Zambon; MR declares grants and personal fees from Boehringer Ingelheim, Roche, AstraZeneca, Novartis, Chiesi, GSK, Menarini, Guidotti, AlfaSigma, Zambon; PS has nothing to declare; JWS has nothing to declare; AV participated as a lecturer/speaker in scientific meetings under the sponsorship of AstraZeneca, Chiesi, GlaxoSmithKline, Novartis; SR and AZ are employees of Medineos Observational Research – an IQVIA company, Modena Italy contracted by AstraZeneca, Italy; SB, MP and AR are AstraZeneca employees; GV was an AstraZeneca employee at the time of manuscript preparation; SC declares grants and/or personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Glaxo Smith Kline, Guidotti, Menarini, Novartis, Valeas.
References
1. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi:10.1183/09031936.00202013
2. Hekking PPW, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135:896–902. doi:10.1016/j.jaci.2014.08.042
3. Perez-de-Llano L, Tran TN, Al-ahmad M, et al. Characterization of eosinophilic and non-eosinophilic severe asthma phenotypes and proportion of patients with these phenotypes in the international severe asthma registry (ISAR). Am J Respir Crit Care Med Am Thorac Soc. 2020;201:A4525.
4. de Groot JC, ten Brinke A, Bel EHD. Management of the patient with eosinophilic asthma: a new era begins. ERJ Open Res. 2015;1:24. doi:10.1183/23120541.00024-2015
5. Heaney LG, Perez de Llano L, Al-Ahmad M, et al. Eosinophilic and Noneosinophilic Asthma. Chest. 2021;160:814–830. doi:10.1016/j.chest.2021.04.013
6. Bakakos A, Loukides S, Bakakos P. Severe Eosinophilic Asthma. J Clin Med. 2019;8:1375. doi:10.3390/jcm8091375
7. Sweeney J, Patterson CC, Menzies-Gow A, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the optimum patient care research database and the British thoracic difficult asthma registry. Thorax. 2016;71:339–346. doi:10.1136/thoraxjnl-2015-207630
8. Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141:110–116.e7. doi:10.1016/j.jaci.2017.04.009
9. Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204. doi:10.2147/JAA.S176026
10. Lee H, Ryu J, Nam E, et al. Increased mortality in patients with corticosteroid-dependent asthma: a nationwide population-based study. Eur Respir J. 2019;54:1900804. doi:10.1183/13993003.00804-2019
11. Bourdin A, Molinari N, Vachier I, et al. Mortality: a neglected outcome in OCS-treated severe asthma. Eur Respir J. 2017;50:1701486. doi:10.1183/13993003.01486-2017
12. Brusselle GG, Koppelman GH. Biologic therapies for severe asthma. N Engl J Med. 2022;386:157–171. doi:10.1056/NEJMra2032506
13. Dagher R, Kumar V, Copenhaver AM, et al. Novel mechanisms of action contributing to benralizumab’s potent anti-eosinophilic activity. Eur Respir J. 2022;59:2004306. doi:10.1183/13993003.04306-2020
14. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–2127. doi:10.1016/S0140-6736(16)31324-1
15. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–2141. doi:10.1016/S0140-6736(16)31322-8
16. FitzGerald JM, Bleecker ER, Menzies-Gow A, et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med. 2018;6:51–64. doi:10.1016/S2213-2600(17)30344-2
17. Menzies-Gow A, Gurnell M, Heaney LG, et al. Oral corticosteroid elimination via a personalised reduction algorithm in adults with severe, eosinophilic asthma treated with benralizumab (PONENTE): a multicentre, open-label, single-arm study. Lancet Respir Med. 2022;10:47–58. doi:10.1016/S2213-2600(21)00352-0
18. Pelaia C, Busceti MT, Vatrella A, et al. Real-life rapidity of benralizumab effects in patients with severe allergic eosinophilic asthma: assessment of blood eosinophils, symptom control, lung function and oral corticosteroid intake after the first drug dose. Pulm Pharmacol Ther. 2019;58:101830. doi:10.1016/j.pupt.2019.101830
19. Pelaia C, Busceti MT, Crimi C, et al. Real-Life effects of benralizumab on exacerbation number and lung hyperinflation in atopic patients with severe eosinophilic asthma. Biomed Pharmacother. 2020;129:110444. doi:10.1016/j.biopha.2020.110444
20. Nolasco S, Crimi C, Pelaia C, et al. Benralizumab effectiveness in severe eosinophilic asthma with and without chronic rhinosinusitis with nasal polyps: a real-world multicenter study. J Allergy Clin Immunol Pract. 2021;9:4371–4380. doi:10.1016/j.jaip.2021.08.004
21. Scioscia G, Carpagnano GE, Quarato CMI, et al. Effectiveness of benralizumab in improving the quality of life of severe eosinophilic asthmatic patients: our real-life experience. Front Pharmacol. 2021;12:631660. doi:10.3389/fphar.2021.631660
22. Pelaia C, Crimi C, Benfante A, et al. Therapeutic effects of benralizumab assessed in patients with severe eosinophilic asthma: real-life evaluation correlated with allergic and non-allergic phenotype expression. J Asthma Allergy. 2021;14:163–173. doi:10.2147/JAA.S297273
23. ChAracterisation of Italian severe uncontrolled asthmatic patieNts key features when receiving benralizumab (ANANKE). Available from: https://clinicaltrials.gov/ct2/show/NCT04272463.
24. Menzella F, Bargagli E, Aliani M, et al. ChAracterization of Italian severe uncontrolled asthmatic patients key features when receiving benralizumab in a real-life setting: the observational rEtrospective ANANKE study. Respir Res. 2022;23:36. doi:10.1186/s12931-022-01952-8
25. Kraft M, Brusselle G, FitzGerald JM, et al. Patient characteristics, biomarkers and exacerbation risk in severe, uncontrolled asthma. Eur Respir J. 2021;58:2100413. doi:10.1183/13993003.00413-2021
26. Price DB, Rigazio A, Campbell JD, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med. 2015;3:849–858. doi:10.1016/S2213-2600(15)00367-7
27. Backman H, Lindberg A, Hedman L, et al. FEV1 decline in relation to blood eosinophils and neutrophils in a population-based asthma cohort. World Allergy Organ J. 2020;13:100110. doi:10.1016/j.waojou.2020.100110
28. Tan WC, Bourbeau J, Nadeau G, et al. High eosinophil counts predict decline in FEV 1: results from the CanCOLD study. Eur Respir J. 2021;57:2000838. doi:10.1183/13993003.00838-2020
29. Bumbacea D, Campbell D, Nguyen L, et al. Parameters associated with persistent airflow obstruction in chronic severe asthma. Eur Respir J. 2004;24:122–128. doi:10.1183/09031936.04.00077803
30. Menzella F, Fontana M, Galeone C, et al. Real world effectiveness of benralizumab on respiratory function and asthma control. Multidiscip Respir Med. 2021;16(1):785. doi:10.4081/mrm.2021.785
31. Korn S, Bourdin A, Chupp G, et al. Integrated safety and efficacy among patients receiving benralizumab for up to 5 years. J Allergy Clin Immunol Pract. 2021;9:4381–4392. doi:10.1016/j.jaip.2021.07.058
32. Miralles López J, Andújar-Espinosa R, Bravo-Gutiérrez F, et al. Effectiveness of benralizumab in severe eosinophilic asthma under routine clinical practice. J Investig Allergy Clin Immunol. 2022;32:220–223.
33. Jackson DJ, Burhan H, Menzies-Gow A, et al. Benralizumab effectiveness in severe asthma is independent of previous biologic use. J Allergy Clin Immunol Pract. 2022;S2213–2198(22):141.
34. Paoletti G, Di Bona D, Chu DK. Allergen immunotherapy: the growing role of observational and randomized trial “Real‐World Evidence”. Allergy. 2021;76:2663–2672. doi:10.1111/all.14773
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


