Back to Journals » Journal of Asthma and Allergy » Volume 13
Clinical Features and Disease Course of Primary Angioedema Patients in a Tertiary Care Hospital
Authors Pall AH , Lomholt AF, von Buchwald C , Bygum A , Rasmussen ER
Received 8 January 2020
Accepted for publication 5 May 2020
Published 17 July 2020 Volume 2020:13 Pages 225—236
DOI https://doi.org/10.2147/JAA.S245161
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Amrita Dosanjh
Amalie Hartvig Pall,1 Anne Fog Lomholt,1 Christian von Buchwald,1 Anette Bygum,2– 4 Eva Rye Rasmussen1,2
1Department of Otorhinolaryngology, Head and Neck Surgery and Audiology, Rigshospitalet, University Hospital of Copenhagen, Copenhagen, Denmark; 2OPEN – Open Patient Data Explorative Network, Odense University Hospital, Odense, Denmark; 3Department of Dermatology and Allergy Centre, Odense University Hospital, Odense 5000, Denmark; 4Department of Clinical Research, University of Southern Denmark, Odense 5000, Denmark
Correspondence: Amalie Hartvig Pall
Department of Otorhinolaryngology, Head and Neck Surgery and Audiology, Rigshospitalet, University Hospital of Copenhagen, Blegdamsvej 9B, Copenhagen 2100, Denmark
Email [email protected]
Purpose: To give a better understanding of primary AE, the clinical characteristics and the possible therapeutic approaches.
Background: Angioedema (AE) is a non-pitting, non-itching swelling of skin or mucosa. The symptom can become life-threatening if located in the airways. Primary (monosymptomatic) AE is a manifestation of several different diseases and the diagnosis is not always straight-forward. The aetiological and pathophysiological factors of primary AE are not completely clarified. There is a need for further investigation.
Patients and Methods: This was a retrospective cohort study of patients referred to an outpatient dermatology clinic in a tertiary care hospital for clinical assessment due to primary AE in the period from 1996 to 2014.
Results: A total of 315 patients were identified with primary AE. The most frequent subtype was idiopathic AE (42.5%) and the second most common was angiotensin-converting enzymeinhibitor (ACEi)-induced AE (31.1%). Three patients were diagnosed with hereditary AE and one patient was diagnosed with acquired C1-inhibitor deficiency. At least 107 (34.0%) patients had established histaminergic AE. More than 1/3 of the patients were treated in an emergency room or hospitalized due to AE. A 98.1% of patients had experienced AE in the head and neck area. Seven patients were in the need of acute airway intervention. Six of these had ACEi-induced AE. Female sex and smoking were found to be risk factors for developing AE.
Conclusion: The most frequent diagnoses were histaminergic-, non-histaminergic idiopathic AE and ACEi-induced AE, whereas complement C1-inhibitor deficiency was rare. Histaminergic AE made up a substantial group of patients with primary AE. Even though there are different pathophysiological causes of AE, many cases have overlapping clinical manifestations, which make diagnosis and treatment difficult.
Keywords: primary angioedema, urticaria, hereditary angioedema, angiotensin-converting enzyme inhibitors, bradykinin
Background
Angioedema (AE) is a non-pitting, self-limiting swelling of skin and mucosa, which can become life-threatening when located in the airways. The condition is caused by an increase in vascular permeability due to different mediators.1 More than one-third of AE cases are estimated to be associated with urticaria.2,3 AE and urticaria can be seen as different manifestations of common pathological processes. AE is located to the deeper layer of the skin and the maximum duration of the symptom is 7 days, whereas urticaria is located in the superficial dermis with a duration of 1–24 hours.4,5
AE can be defined as either acquired (AAE) or hereditary (HAE). AAE can be further sub-divided into several categories: idiopathic histaminergic AE, idiopathic non-histaminergic AE, ACEi-induced AE and acquired complement C1-inhibitor deficiency (C1-INH-AAE). An AAE subgroup associated with allergic reactions has been described by some studies.1,6 HAE is categorized into HAE with C1-INH deficiency (C1-INH-HAE) and HAE with normal C1-INH.7 The former is associated with mutations in the SERPING1 gene whilst the latter has been associated with mutations of the following genes: Factor XII, angiopoietin-1, plasminogen and kininogen 1.8,11
C1-INH-HAE is an autosomal dominant hereditary disease with a prevalence of 1:50.000–1:71.000.12,13 C1-INH-AAE has an even lower prevalence estimated as 1:10 of C1-INH-HAE patients.14,15 Histaminergic AE is caused by a release of histamine from mast cells and basophils and often presents with concomitant urticaria.1 Non-histaminergic AE is associated with bradykinin and substance P release from the contact system and sensory nerve endings.16 Bradykinin-mediated AE is not associated with urticaria and does not respond to anti-allergic therapy with antihistamines, corticosteroids or adrenaline.1 Even though there are different pathophysiological causes of AE, many cases have overlapping clinical manifestations, which makes diagnosis and treatment difficult.17,18 Overall the treatment of AE depends on the symptoms. AE attacks are self-limiting and last 1–7 days without medical treatment. Recurrent AE attacks are associated with a significant reduction in health-related quality of life due to limitations in daily activity, fear of suffocation and in some cases worries about heritability.19
The current treatment of acute AE in patients who have not been diagnosed with C1-INH deficiency consists of corticosteroids, antihistamines and in severe cases adrenalin. Histamine is the predominant cause of AE and the current acute first line of treatment thus favours patients with histaminergic AE.20 Patients with swelling attacks due to C1-INH deficiency can effectively be treated with C1-INH concentrate or a bradykinin receptor antagonist.21,23
The objective of this study is to describe the clinical characteristics of patients found to have primary AE in order to give a better understanding of the disease.
Patients and Methods
Study Population
This was a retrospective observational cohort study of patients with primary AE. Seven hundred and thirty-four patients were referred to a dermatology department in a tertiary care hospital due to a suspicion of AE between 01-01-1996 and 31-12-2014. Patients were included if they had experienced AE at least once. The patients were selected based on International Classification of Diseases Version 10 (ICD-10) diagnostic codes possibly linked to the AE diagnosis: DT78.3 Quincke’s edema, DL50 Urticaria, or DD84.1A Hereditary angioedema. One hundred and thirty-two patients were excluded due to insufficient clinical information. Seventy-four patients were excluded due to missing concluding AE diagnosis by a dermatologist (defined as DT78.3 Quincke’s edema or DD84.1A Hereditary angioedema). Four patients were excluded due to other underlying factors of swellings such as Melkersson–Rosenthal syndrome or Panniculitis. Two hundred and nine patients were excluded due to concomitant urticaria (DL50.8A Chronic urticaria, DL50.8B Urticaria recidivans, DL50.8D Urticaria acuta, DL50.8E Urticaria due to pressure, DL50.1 Idiopathic urticaria, DL50.8 Other forms of urticaria). A total of 315 patients were diagnosed with primary AE (Figure 1).
 |
Figure 1 Patients with primary AE included in study. Seven hundred thirty-four patients referred to a dermatology department. Three hundred fifteen patients included in study. |
Data Extraction
Baseline characteristics such as sex, age, underlying cause of AE, ICD-10 codes, anatomical site of AE, daily drug intake, comorbidities, treatment, hospitalization and follow-up time were obtained from patient medical records in an electronic data system. The follow up time was calculated from the first visit to ended clinical investigation by a dermatologist.
Data Sources
The drug sales information for the Danish background population was acquired from the Register of Medicinal Product Statistics (Medstat.dk) for comparative statistical analysis.24 This registry dates back to 1999; hence, data from the background population was only to be found from 1999 to 2014. Eight patients in the cohort were referred before 1999 and were excluded in the analysis of daily drug intake only. Drug intake information for the patients was obtained for 308 patients. Comorbidity in the background population was extracted from Sundhed og Sygelighed 2010 and hjertesvigt.dk.25,26
Diagnosis
Idiopathic AE was used as a diagnosis of exclusion by a dermatologist when no specific underlying factors of AE attacks were identified after clinical and biochemical assessment. Patients were diagnosed with idiopathic histaminergic AE if symptoms resolved following the continuous administration of antihistamines. Patients were diagnosed with idiopathic non-histaminergic AE diagnosis if symptoms did not resolve following continuous administration of high dose antihistamines. Patients were diagnosed with allergic AE if symptoms clearly occurred after allergen exposure and they had a positive skin prick test and/or positive histamine-release test of allergen-specific IgE. In other cases, a specific causative factor was identified, as the AE stopped after the elimination of a specific drug or underlying infection.
Patients were diagnosed with ACEi-induced AE if they received ACEi during an AE attack and no other cause of AE was found. Patients were diagnosed with NSAID-induced AE if symptoms started after NSAID intake and improved after drug discontinuation. HAE and C1-INH-AAE were diagnosed according to established criteria.7
Statistical Analysis
Analyses and calculations were performed in SPSS-24. A calculation of frequencies between the AE group and the general population was performed using the binomial distribution. Multivariate logistic regression was used to calculate the risk of admission due to different factors. p values ≤ 0.05 were considered statistically significant.
Ethics
This study was approved by the Danish Data Protection Agency (jr. number 14/35206) and the Danish National Board of Health (jr. number 3–3013-805/1/) as appropriate.
Results
Of 734 referred patients 315 were found to have primary AE (Figure 1). There were significantly more females (59.0%) compared to males (p=0.001). The mean age at referral was 52.1 years. At AE onset the mean age was 48.2 years. The cohort mostly consisted of Caucasians (97.5%). Data regarding smoking was available in 199 patients and 64 (32.2%) were current smokers. Our study found significantly more current smokers amongst primary AE patients compared to the background population in a binomial distribution (p<0.001) (Table 1).
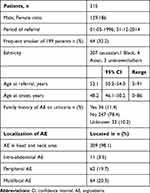 |
Table 1 Basic Data of 315 Patients with Primary Angioedema in the Cohort |
Idiopathic AE was most frequent (42.5%). Eighty-two of 134 patients continued using antihistamines and could be classified as histaminergic or non-histaminergic AE. Among these patients, 59 (72%) were idiopathic histaminergic AE and 23 (28%) were idiopathic non-histaminergic AE. For 52 patients the data was insufficient since the use of long-term antihistamines was not explicitly noted, or the follow-up time was too short to evaluate treatment response. Histaminergic AE was established in at least 107 (34.0%) cases (idiopathic histaminergic AE and AE associated with an allergic reaction). The second most frequent cause of primary AE was ACEi usage. A total of 10 patients experienced AE as a result of NSAID usage (Figure 2). Nineteen patients (6.0%) had AE due to a drug response associated with an allergic reaction caused by a drug that was not NSAID and ACEi (Figure 2). The drugs were penicillin,1 calcium-antagonists,3 female hormonal therapy,2 simvastatin,2 serotonin reuptake inhibitor,2 metformin,1 allopurinol,1 donepezil,1 metoprolol,1 fluconazole,1 and mefloquine.1 The rest of the patients experienced remission when several medications were discontinued, but the exact drug was not confirmed.3 Drug-related AE developed in 40.3% of the cases (NSAID, ACEi and drug response associated with an allergic reaction). For patients with infection-related AE, the infections were tooth infection, otitis, herpes zoster, helicobacter pylori, tuberculosis, and mononucleosis. AE was also seen together with the autoimmune disease cutaneous lupus erythematosus and cancer-related with T-cell lymphoma. The other underlying factors of AE are displayed in Figure 2.
 |
Figure 2 Underlying factors in 315 primary AE patients. |
The medical treatment of AE was mainly anti-allergic (antihistamines, corticosteroids and adrenaline). However, 24 (7.6%) patients were treated with other drugs for their AE attacks. Six patients were treated with tranexamic acid, six patients received a bradykinin receptor antagonist, and two patients were treated with C1-INH concentrate (Table 2).
 |
Table 2 Treatment of AE, Standard Therapy Treatment (Antihistamine, Corticosteroids and if Necessary Adrenaline/Epinephrine) and Second Line Drug Treatment of AE Incidents |
Hospitalizations, as a result of AE, were reported in 136 (43.2%) patients. The days of hospitalization ranged between 1 and 35 with a mean number of 3.2 days. One hundred and twenty-seven patients (40.3%) had been referred to the ER with a mean number of visits of 1.9. Seven patients needed acute airway management due to swelling of the airways. Four of them females, three males, a mean age at onset 72 years. Six of them had ACEi-induced AE, and one was AE associated with an allergic reaction. Four females and three males (Table 3). A parameter significantly associated with hospitalization was older age at referral. The odds ratio (OR) of hospitalization increased 3.0% for every year a patient increased in age. Comorbidities significantly associated with hospitalization were diabetes, hypertension, ischaemic heart disease, heart failure, allergy and asthma. Patients with ACEi-induced AE had a statistically significant greater risk of being admitted, whereas idiopathic AE patients had a statistically significant lower risk of being admitted (Table 4). The most frequent anatomical site of AE was the head and neck area (98.1%) whereas abdominal AE was the least frequent site of AE (3.5%). Patients with abdominal AE always manifested with AE in other locations as well. Sixty-two (19.7%) patients had experienced peripheral AE. One-fifth (20.9%) were reported with multifocal AE (Table 1).
 |
Table 3 Data Regarding ER Visits and Hospitalization |
 |
Table 4 Association Between Different Variables and the Need for Admission |
General practitioners (47.6%), internists (20.0%), otorhinolaryngologists (10.5%), and dermatologists (9.8%) were the physicians who most often referred the patients to the dermatology department.
A 12.8% of patients did not have their follow-up time registered. A 9.1% of patients did not require any follow-up (follow-up = 0 days). A 72.5% of patients had one follow-up appointment in the out-patient department with a mean follow-up period of 139.1 days. A 5.9% attended the out-patient department at least twice with a mean follow up of 215 days. The mean follow-up time for all referrals was 146.7 days (95% CI 128.4–170.2). The minimum follow-up time was 1 day, and the maximum follow-up time was 1415 days, ie, almost 4 years.
Five comorbidities were significantly more prevalent in the cohort compared to the background population: diabetes, hypertension, ischemic heart disease, heart failure and allergy. Allergy was the only significant prevalent comorbidity in the cohort group when patients with ACEi-induced AE were not a part of the calculation. Drug intake and comorbidities in the cohort and comparison to the background population are shown in Table 5.
 |
Table 5 Comorbidity and Daily Drug Intake in the Cohort Compared to the Background Population |
Discussion
In this study of 315 primary AE patients referred to a outpatient clinic in a tertiary care hospital, we found idiopathic histaminergic and non-histaminergic AE and ACEi-induced AE to be the most frequent underlying factors of AE in a tertiary referral centre. Most of the idiopathic cases (72%) responded to antihistamines. The use of continuous antihistamines was not noted in the journals of 52 patients; hence, the number of histaminergic AE patients in the study population may be higher than noted. Thirty-four percent of patients were diagnosed with histaminergic AE, illustrating that histaminergic AE can also present without hives. Twenty-one (6.7%) patients developed AE related to specific external agents or concomitant diseases such as autoimmune diseases, cancer, infection, physical stimuli or Hypereosinophilic syndrome. This supports prior findings of how non-allergic external agents or concomitant diseases do sometimes manifest with primary AE.4 Only three patients were diagnosed with HAE and one patient with C1-INH-AAE. This supports the consensus of C1-INH-AAE and -HAE as very rare underlying factors of primary AE.7,27 ACEi-induced AE was seen in almost one-third of the cohort. Recent studies suggest an increase in ACEi-induced AE as a result of an increase in ACEi prescriptions.28 ACEi-induced AE was first reported in 1984 and 0.1–2.2% of all ACEi users are estimated to develop AE.29,31
Ten patients (3.2%) had AE due to NSAIDs. It has been hypothesized that NSAID-induced AE is not an allergic reaction per se, but is due to excessive leukotriene production as a result of COX1 blocking.6,32 Eighteen (5.6%) patients developed AE due to the use of another drug and were categorized as allergic.
Leeyaphan et al described NSAIDs and penicillin as drugs frequently associated with drug-induced AE.33,34 Calcium-antagonists, statins, metformin, metoprolol, female hormones, has also been reported in prior cases and are estimated as less frequent inducers of AE.33,35,38 This study confirms existing evidence in the area, that non-ACEi drug-induced AE does sometimes occur without concomitant hives.
The use of NSAIDs and female hormones in the background population was more frequent than seen in our study population. The underuse of female hormones is likely due to the higher mean age of the cohort group compared to the background population so especially birth control pills are less used in our cohort. Also, patients with AE are sometimes discouraged to use estrogens and often discouraged to use NSAIDs.
Our study found female sex to be a risk factor of primary AE. These results support prior findings of female predominance in AE patients.4,39 Five hundred and twenty-four patients with AE were identified, but 39.9% presented with concomitant urticaria. The primary AE: AE with concomitant urticaria-ratio was 1.5, a ratio adjacent to 1.4 found by Mansi et al and 1.7 by Madsen et al.2,40 This study supports how histaminergic AE does not always manifest with urticaria, which makes it is difficult to differentiate between histaminergic and non-histaminergic AE based on clinical appearance. Why some histamine related AE patients present with urticaria and some do not are unknown. Eli et al have hypothesized how a greater activation of contact pathways and bradykinin production in primary AE leads to vasodilation of the deeper dermal layers, compared to AE patients with concomitant urticaria.41
Most AE attacks were located in the head and neck area (98.1%), these findings support other studies.4,40,42 Only seven patients were in the need of acute airway management. However, this should be taken seriously due to the high risk of asphyxiation. Six of these were ACEi-induced AE. This states a greater risk of airway intervention in ACEi-induced AE patients. Angioedema associated deaths are rare, but has been described as increasing due to the increased use of ACEi.43 Selection bias could play a role in the number of acute airway managements, since patients with head and neck AE are more likely to be referred to a tertiary care hospital, as it is potentially lethal. Patients with minor AE incidents in other locations are probably handled in general practice and thus never present in the hospital sector. Twelve patients presented with AE in the abdomen, but none of these patients presented with abdomen as the only afflicted area. This supports the assumption that single-area intra-abdominal AE is underreported due to the difficulty in diagnostics.17,44
Hospitalization and ER visit were observed in 43.2% and 40.3% of cases, respectively, establishing how AE is a patient group that requires acute resources in the medical care system. A study revealed increased annual number of admissions and costs due to AE during the period of 1998 to 2005.45
Prior studies reporting a great variation in admission rates of 25–84% for all AE types.39 These data do not indicate a dissimilarity in hospitalizations of primary AE compared to general AE. Older age, and ACEi-induced AE were reported as parameters significantly associated with admission.
Anti-allergic standard treatment (antihistamines, corticosteroids, adrenaline) was most frequently administered to patients in this cohort, despite the fact that these drugs are ineffective for patients with non-histaminergic AE.17 Treatment of AE is based on pathophysiology, but this is rarely possible in the acute setting to determine the underlying mediator of AE. Many of the patients in this study were reported with good effect of antihistamines and corticosteroids even though none showed clinical signs of urticaria. The plausible explanation is a combination of factors: some of these patients have histaminergic AE and respond to treatment, and for AE the swelling is self-limiting, and this can lead to the wrong conclusion of a response to anti-allergic therapy.
Today the first-line treatment of acute AE is antihistamines, corticosteroids and if necessary, adrenalin. This favours the larger group of patients with histaminergic AE. Crochet et al revealed a 45-fold higher mortality risk for non-histaminergic compared to histaminergic patients.18 This could be due to the first-line treatment targeting histaminergic- and not non-histaminergic patients. In this study, 24 patients were treated with other drugs than the anti-allergic drugs. Six ACEi-induced AE patients, three idiopathic AE, and two AE caused by physical stimuli were treated off the label with tranexamic acid or icatibant. One patient with AE caused by physical stimuli was treated with attenuated androgens and C1-INH concentrate. The efficacy was unclear from the medical record. C1-INH concentrate, icatibant, attenuated androgens and tranexamic acid approved for C1-INH-HAE, has shown promising results for other patients with non-histaminergic AE.2,41,46,48 Mansi et al found a reduction in symptoms and severity in patients with idiopathic non-histaminergic AE treated with tranexamic acid, especially in long-term prophylaxis for patients with recurrent severe AE attacks.2 Baş et al found a significant reduction in time to complete resolution in the acute phase for ACEi-induced AE patients treated with icatibant compared to standard therapy (from 27.1- to 8.0 hours).46 However, this was not confirmed in subsequent studies.49,50 Fresh frozen plasma has previously been used for patients with C1-INH-HAE, but in the western world, it is rarely used due to more effective medication being available.7 Greve et al found a positive effect of C1-INH concentrate for ACEi-induced AE compared to a control group and an ongoing randomized doubled blinded clinical trial is currently investigating the same.51,52
Our study revealed a significantly higher prevalence of comorbidities in the cohort compared to the background population. Comorbidities such as diabetes, hypertension, asthma, ischaemic heart disease, heart failure and allergies were also significantly associated with being admitted to a hospital due to AE. ACEi-induced AE and older age were also significantly associated with being admitted. Many patients receive ACEi due to the associated comorbidities.
The main limitation of the study is the retrospective design and the differences in evaluations by clinicians. One hundred and thirty-two (17%) patients were excluded due to insufficient data. The percentage might influence the scientific findings. The study has no control group, and comparison to the background population did not adjust for age and sex due to insufficient data.
Conclusion
In this study, more than one-third of patients had histaminergic AE. This establishes how histaminergic AE does often occur without hives. Our study found idiopathic AE as the most frequent type of primary AE. However, little is known about the pathophysiology of this heterogenic group of patients. Forty percent of patients had drug-induced AE, with ACEi being the most frequent causative agent. Drugs labelled for HAE are sparsely used off-label in selected cases in the clinical setting. AE of the head and neck area, which can become life-threatening, occurred in the majority of patients. Six of seven patients needing acute airway management had ACEi-induced AE as an underlying factor. Female sex and smoking were confirmed to be risk factors of primary AE. Patients with ACEi as a causative agent have a significant increased risk of admission compared to other types of primary AE.
Abbreviations
AE, angioedema; HAE, hereditary angioedema; AAE, acquired angioedema; C1-INH-HAE, hereditary angioedema with complement C1-inhibitor deficiency; C1-INH-AAE, acquired angioedema with complement C1-inhibitor deficiency; ACEi, angiotensin-converting enzyme inhibitor; ER, emergency room; CI, confidence interval; OR, odds ratio.
Data Sharing Statement
The dataset generated and analysed during the current study is available from the corresponding author on reasonable request.
Ethics Approval
This study was approved by the local ethics committee as applicable under Danish law (record S-20140165) and by the Danish Data Protection Agency (journal 2008-580035).
Acknowledgments
Institutions where the work was performed are as follows: collection of data: Department of Dermatology and Allergy Centre, Odense University Hospital, J.B. Winsloevsvej 4, Entrance 142, 5000 Odense C, Denmark, with associated physicians: Georg Authried, Sumangali Chandra Prasad, Kristine Appel U. Pallesen, Kawa Khaled Ajgeiy, Shailajah Kamaleswaran, and professor Anette Bygum; analysis and statistics: Department of Otorhinolaryngology, Head & Neck Surgery and Audiology, Rigshospitalet, University of Copenhagen, with associated physicians: MBBS Amalie Hartvig Pall and Dr. Eva Rye Rasmussen, MD. PhD.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
A. Pall has received one travel grant from CSL Behring. A. Bygum has been involved in clinical research and educational events involving CSL-Behring, Dyax, BioCryst and Shire/Takeda, reports payment for educational activities from Novartis, and grants and personal fees from CSL Behring and Shire (now part of Takeda), and personal fees from Novartis, outside the submitted work. E. Rasmussen has received research funding, travel grants and speakers fees from Shire, CSL Behring and Viropharma, reports personal fees from Shire-Takeda, outside the submitted work, and served on an advisory board on hereditary angioedema treatment for Shire-Takeda. The authors report no other possible conflicts of interest in this work.
References
1. Rasmussen E, Bindslev-Jensen C, Bygum A. Angioedema – assessment and treatment. Tidsskr nor Legeforen. 2012;20(20):2391–2395. doi:10.4045/tidsskr.12.0470
2. Mansi M, Zanichelli A, Coerezza A, et al. Presentation, diagnosis and treatment of angioedema without wheals: a retrospective analysis of a cohort of 1058 patients. J Intern Med. 2015;277(5):585–593. doi:10.1111/joim.12304
3. Champion RH, Roberts SOB, Carpenter RG, Roger JH. Urticaria and angio-oedema. Br J Dermatol. 1969;81(81):588–597. doi:10.1111/j.1365-2133.1969.tb16041.x
4. Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175(9):1065–1070. doi:10.1503/cmaj.060535
5. Kibsgaard L, Lefevre AC, Deleuran M, Vestergaard C. A case series study of eighty-five chronic spontaneous urticaria patients referred to a tertiary care center. Ann Dermatol. 2014;26(1):73–78. doi:10.5021/ad.2014.26.1.73
6. Depetri F, Tedeschi A, Cugno M. Angioedema and emergency medicine: from pathophysiology to diagnosis and treatment. Eur J Intern Med. 2019;59(May):8–13. doi:10.1016/j.ejim.2018.09.004
7. Cicardi M, Aberer W, Banerji A, et al. Classification, diagnosis, and approach to treatment for angioedema: consensus report from the hereditary angioedema international working group. Eur J Allergy Clin Immunol. 2014;69(5):602–616. doi:10.1111/all.12380
8. Bafunno V, Firinu D, D’Apolito M, et al. Mutation of the angiopoietin-1 gene (ANGPT1) associates with a new type of hereditary angioedema. J Allergy Clin Immunol. 2018;141(3):1009–1017. doi:10.1016/j.jaci.2017.05.020
9. Bork K, Wulff K, Steinmüller-Magin L, et al. Hereditary angioedema with a mutation in the plasminogen gene. Eur J Allergy Clin Immunol. 2018;73(2):442–450. doi:10.1111/all.13270
10. Dewald G, Bork K. Missense mutations in the coagulation factor XII (Hageman factor) gene in hereditary angioedema with normal C1 inhibitor. Biochem Biophys Res Commun. 2006;343(4):1286–1289. doi:10.1016/j.bbrc.2006.03.092
11. Bork K, Wulff K, Rossmann H, et al. Hereditary angioedema cosegregating with a novel kininogen 1 gene mutation changing the N-terminal cleavage site of bradykinin. Allergy Eur J Allergy Clin Immunol. 2019;74(12):2479–2481. doi:10.1111/all.13869
12. Zanichelli A, Arcoleo F, Barca MP, et al. A nationwide survey of hereditary angioedema due to C1 inhibitor deficiency in Italy. Orphanet J Rare Dis. 2015;10(1):11. doi:10.1186/s13023-015-0233-x
13. Bygum A. Hereditary angio-oedema in Denmark: a nationwide survey. Br J Dermatol. 2009;161(5):1153–1158. doi:10.1111/j.1365-2133.2009.09366.x
14. Gobert D, Paule R, Ponard D, et al. A nationwide study of acquired C1-inhibitor deficiency in France. Medicine (Baltimore). 2016;95(33):e4363. doi:10.1097/MD.0000000000004363
15. Bygum A, Vestergaard H. Acquired angioedema – Occurrence, clinical features and associated disorders in a Danish nationwide patient cohort. Int Arch Allergy Immunol. 2013;162(2):149–155. doi:10.1159/000351452
16. Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. Survey review the kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;38(1):6–38. doi:10.1254/jphs.SRJ05001X
17. Hirschy RA, Shah T, Davis T, Rech MA. Treatment of life-threatening ACE-inhibitor-induced angioedema. Adv Emerg Nurs J. 2018;40(4):267–277. doi:10.1097/TME.0000000000000211
18. Crochet J, Lepelley M, Yahiaoui N, et al. Bradykinin mechanism is the main responsible for death by isolated asphyxiating angioedema in France. Clin Exp Allergy. 2018;49(September 2018):252–254. doi:10.1111/cea.13297
19. Prior N, Remor E, Pérez-Fernández E, et al. Psychometric field study of hereditary angioedema quality of life questionnaire for adults: HAE-QoL. J Allergy Clin Immunol Pract. 2016;4(3):464–473. doi:10.1016/j.jaip.2015.12.010
20. Nedelea I, Deleanu D. Isolated angioedema: an overview of clinical features and etiology (review). Exp Ther Med. 2018;17(4):1068–1072. doi:10.3892/etm.2018.6982
21. Bork K, Staubach-Renz P, Hardt J. Angioedema due to acquired C1-inhibitor deficiency: spectrum and treatment with C1-inhibitor concentrate. Orphanet J Rare Dis. 2019;143(2):AB42.
22. Craig T, Zuraw B, Longhurst H, et al. Long-term outcomes with subcutaneous C1-inhibitor replacement therapy for prevention of hereditary angioedema attacks. J Allergy Clin Immunol Pract. 2019;7(6):1793–1802.e2. doi:10.1016/j.jaip.2019.01.054
23. Cicardi M, Banerji ABF, Malbrán A, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med. 2010;363(6):532–541. doi:10.1056/NEJMoa0906393
24. Sundhedsdatastyrelsen. Medstat.dk [Internet]. [
25. Christensen AI, Ekholm O, Davidsen M, Juel K. Sundhed Og Sygelighed 2010. 2010.
26. Kragh-Jakobsen AS, Haun Jesper B. Hjertesvigt [Internet]. [
27. Cicardi M, Zanichelli A. Acquired angioedema. Allergy Asthma Clin Immunol. 2010;6(1):1–5. doi:10.1186/1710-1492-6-14
28. Holm JPY, Ovesen T. Increasing rate of angiotensin-converting enzyme inhibitor-related upper airway angio-oedema. Dan Med J. 2012;59(6):A4449.
29. Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005;165(14):1637–1642. doi:10.1001/archinte.165.14.1637
30. Banerji A, Blumenthal KG, Lai KH, Zhou L. Epidemiology of ACE inhibitor angioedema utilizing a large electronic health record. J Allergy Clin Immunol Pract. 2017;5(3):744–749. doi:10.1016/j.jaip.2017.02.018
31. Rasmussen ER, Pottegård A, Bygum A, von Buchwald C, Homøe P, Hallas J. Angiotensin II receptor blockers are safe in patients with prior angioedema related to angiotensin-converting enzyme inhibitors – a nationwide registry-based cohort study. J Intern Med. 2019;285(5):1–9. doi:10.1111/joim.12867
32. Kaplan AP. Angioedema. World Allergy Organ J. 2008;1(6):103–113. doi:10.1097/WOX.0b013e31817aecbe
33. Leeyaphan C, Kulthanan K, Jongjarearnprasert K, Dhana N. Drug-induced angioedema without urticaria: prevalence and clinical features. J Eur Acad Dermatol Venereol. 2010;24(6):685–691. doi:10.1111/j.1468-3083.2009.03489.x
34. Kulthanan K, Jiamton S, Boochangkool K, Jongjarearnprasert K. Angioedema: clinical and etiological aspects. Clin Dev Immunol. 2007;2007:6–11. doi:10.1155/2007/26438
35. El Mekki AB, Chaib A, Kombich J. Drug induced angioedema: a rare side effect of simvastatin. Pan Afr Med J. 2017;26(7):213. doi:10.11604/pamj.2017.26.38.10312
36. Kuruvilla ME, Sanan N. Amlodipine-induced angioedema: an unusual complication of a common medication. Allergy Rhinol. 2018;6(9):1–2.
37. Atik D, Büyükcam F, Yılmaz D, Işık B, Demir ÖF. Angioedema after the first dose of metformin. Am J Emerg Med. 2013;31(3):
38. Krikorian RK, Quick A, Tal A. Angioedema following the intravenous administration of metoprolol. Chest. 1994;106(6):1922–1923. doi:10.1378/chest.106.6.1922
39. Tai S, Mascaro M, Goldstein NA. Angioedema: a review of 367 episodes presenting to three tertiary care hospitals. Ann Otol Rhinol Laryngol. 2010;119(12):836–841. doi:10.1177/000348941011901208
40. Madsen F, Attermann J, Linneberg A. Epidemiology of non-hereditary angioedema. Acta Derm Venereol. 2012;92(5):475–479. doi:10.2340/00015555-1389
41. Eli M, Joseph M, Kuznik B, Menachem S. Chronic idiopathic angioedema: a single center experience. Int J Dermatol. 2014;53(10):e421–7. doi:10.1111/ijd.12601
42. Megerian CA, Arnold JE, Berger M. Angioedema: 5 years’ experience, with a review of the disorder’s presentation and treatment. Laryngoscope. 1992;(102):256–260.
43. Kim SJ, Brooks JC, Sheikh J, Kaplan MS, Goldberg BJ. Angioedema deaths in the United States, 1979–2010. Ann Allergy Asthma Immunol. 2014;113(6):630–634. doi:10.1016/j.anai.2014.09.003
44. Byrne TJ, Douglas DD, Landis ME, Heppell JP. Isolated visceral angioedema: an underdiagnosed complication of ACE inhibitors? Mayo Clin Proc. 2000;75(11):1201–1204. doi:10.4065/75.11.1201
45. Lin RY, Shah SN. Increasing hospitalizations due to angioedema in the United States. Ann Allergy Asthma Immunol. 2008;101(2):185–192. doi:10.1016/S1081-1206(10)60208-6
46. Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor–induced angioedema. N Engl J Med. 2015;372(5):418–425. doi:10.1056/NEJMoa1312524
47. Cicardi M, Bergamaschini L, Zingale LC, Gioffré D, Agostoni A. Idiopathic nonhistaminergic angioedema. Am J Med. 1999;106(6):650–654. doi:10.1016/S0002-9343(99)00123-0
48. Craig TJ, Bernstein JA, Farkas H, Bouillet L, Boccon-Gibod I. Diagnosis and treatment of bradykinin-mediated angioedema: outcomes from an angioedema expert consensus meeting. Int Arch Allergy Immunol. 2014;165(2):119–127. doi:10.1159/000368404
49. Sinert R, Levy P, Bernstein JA, et al. Randomized trial of icatibant for angiotensin-converting enzyme inhibitor–induced upper airway angioedema. J Allergy Clin Immunol Pract. 2017;5(5):1402–1409.e3. doi:10.1016/j.jaip.2017.03.003
50. Straka B, Ramirez C, Byrd J, et al. Effect of bradykinin receptor antagonism on ACE inhibitor-associated angioedema. J Allergy Clin Immunol. 2017;140(1):242–248.e2. doi:10.1016/j.jaci.2016.09.051
51. Greve J, Bas M, Hoffmann TK, et al. Effect of C1-esterase-inhibitor in angiotensin-converting enzyme inhibitor-induced angioedema. Laryngoscope. 2015;125(6):E198–202. doi:10.1002/lary.25113
52. Bas M. Randomized, double-blind, two arms, multicenter, phase III study of berinert for treatment of ACE induced angioedema [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT01843530?term=complement+C1&cond=Angioedema&cntry=DE&rank=3.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
