Back to Journals » Clinical and Experimental Gastroenterology » Volume 16
Clinical Evaluation of Upadacitinib in the Treatment of Adults with Moderately to Severely Active Ulcerative Colitis (UC): Patient Selection and Reported Outcomes
Authors Irani M, Fan C, Glassner K, Abraham BP
Received 28 November 2022
Accepted for publication 28 February 2023
Published 7 March 2023 Volume 2023:16 Pages 21—28
DOI https://doi.org/10.2147/CEG.S367086
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Santosh Shenoy
Malcolm Irani,1 Christopher Fan,1 Kerri Glassner,1– 3 Bincy P Abraham1– 3
1Houston Methodist Gastroenterology Associates, Houston Methodist Hospital, Houston, TX, USA; 2Weill Cornell Medical College, New York, NY, USA; 3Houston Methodist Academic Institute, Houston, TX, USA
Correspondence: Bincy P Abraham, Houston Methodist Gastroenterology Associates, 6550 Fannin St, Suite 1201, Houston, TX, 77030, USA, Tel +1 713-441-8374, Email [email protected]
Abstract: This review addresses appropriate patient selection for upadacitinib, a Janus kinase inhibitor approved by the FDA and EMA for treatment of moderately to severely active ulcerative colitis (UC). Janus kinase molecules can contribute to the inflammatory pathway, so inhibiting certain of them may prove efficacious in treating UC and may reduce safety concerns. Upadacitinib is the newest Janus kinase inhibitor to be approved for UC, so it is timely and relevant to review patient selection and when to consider this medication. We will discuss efficacy and safety data from the pivotal clinical trials on upadacitinib. These data can be shared with patients and can inform the use of these agents in clinical practice.
Keywords: upadacitinib, ulcerative colitis, small molecule
Introduction
Upadacitinib has been approved by both the FDA and EMA for the treatment of adults with moderate to severe ulcerative colitis (UC), providing a daily oral option for patients who failed or were intolerant to anti-tumor necrosis factor (TNF) therapy.1 The pivotal trials for upadacitinib, as well as safety studies of other Janus kinase (JAK) inhibitors, highlight the importance of appropriate patient selection for this medication. Efficacy data—both short-term with onset of action and long-term regarding current guidelines emphasizing the importance of mucosal healing and potentially histological remission—can help providers in educating patients and deciding on whether to prescribe the medication. Because many medications have been introduced for the treatment of inflammatory bowel disease and especially ulcerative colitis, it is also useful to analyze data to assess appropriate positioning of therapies, including upadacitinib. With limited prospective studies on positioning medications for IBD, clinical parameters can be beneficial in determining the appropriate timing and placement of therapies. In this review, we will discuss safety and efficacy data and review patient selection criteria for appropriate timing to consider in the use of upadacitinib.
Indications
Upadacitinib was originally approved for the treatment of rheumatoid arthritis, but its indication has expanded to include ulcerative colitis, ankylosing spondylitis, psoriatic arthritis, and atopic dermatitis. For all of these indications, the patient must first have failed or been intolerant to anti-TNF therapy; the only exception is atopic dermatitis, as anti-TNFs are not indicated for its treatment. The dosing is generally higher for ulcerative colitis than for other indications: 45 mg daily induction dose for the first 8 weeks, then 30 mg daily. A lower dose of 15 mg (used for all other indications) is recommended in UC for those with renal or hepatic disease. Upadacitinib should be avoided in anyone with cirrhosis due to its hepatic metabolism.
Mechanism of Action
Upadacitinib is a small molecule inhibitor that targets the Janus kinase (JAK) pathway, which is involved in many immune-mediated inflammatory diseases; hence, upadacitinib’s many indications. Four tyrosine kinases (JAK1, JAK2, JAK3, and TYK2) make up the JAK-STAT pathway and phosphorylate signal transducers once specific cytokines (including several interleukins and interferons) attach to their receptors on immune cells (Figure 1).2,3 This binding autophosphorylates the JAK and inputs signals into the nucleus of that immune cell, which activates transcription and regulates gene expression of multiple functions, including immune cell function, hematopoiesis, and antimicrobial and antiviral immunity.4–6 Upadacitinib inhibits the JAK pathway with higher selectivity against JAK1 than the other three subtypes.2 Specifically, upadacitinib is more than 100-fold more biochemically selective against JAK1 than JAK3 and TYK2, and 60-fold more selective in cellular assays against JAK1 than JAK2.7 Upadacitinib’s specific activity against JAK1 contrasts with tofacitinib, which inhibits JAK1 and JAK3 with some activity against JAK2 and very limited activity against TYK2.3–5
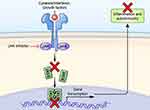 |
Figure 1 Mechanism of JAK inhibition. Adapted from Alexander M, Luo Y, Raimondi G, O’Shea JJ, Gadina M. Jakinibs of All Trades: Inhibiting Cytokine Signaling in Immune-Mediated Pathologies. Pharmaceuticals (Basel). 2021;15(1). Open Access.8 |
A study assessing the cytokine inhibition of various JAK inhibitors found that upadacitinib was more potent than tofacitinib against IL-2, IL-4, IL-15, IL-21, IL-3, GM-CSF, G-CSF, IFN-gamma stimulated monocytes, and IFN-alpha and had similar potency against IL6, IL10, and IFN-gamma stimulated B cells.9 When left unchecked, these proinflammatory cytokines can contribute to the inflammatory cascade, leading to mucosal inflammation, damage to the mucosal barrier, and dysregulation of the epithelial layer, and can contribute to further interaction with the immune cells.4 Inhibiting proinflammatory cytokines reduces the overall production of additional cytokines and thereby reduces additional recruitment of immune cells that cause inflammation. This reduction can prevent the chronic cycle of inflammation in immune conditions, including ulcerative colitis.
Key Clinical Trials
The key registration studies for upadacitinib stem from two induction trials—U-ACHIEVE (UC1) and U-ACCOMPLISH (UC2), which randomized patients to upadacitinib 45 mg vs placebo—and the accompanying maintenance trial, U-ACHIEVE (UC3), which re-randomized responders at week 8 in a 1:1:1 fashion to 30 mg dosing, 15 mg dosing, or placebo.10
Patient Selection
The induction and maintenance trials comprised a diverse group of patients across Europe, North and South America, Australasia, Africa and the Asia-Pacific region. This was reflected in the diversity of patient racial background of patients enrolled in the study: ~60% White, ~30% Asian and 2–5% Black or African American.10 Eligible patients were between the ages of 16–75 with a diagnosis of ulcerative colitis for at least 90 days and active disease, defined as an Adapted Mayo Score (Mayo score minus the Physician’s Global Assessment) of 5–9 with a Mayo endoscopic subscore (as determined by a central reader) of 2 or more. Patients had inadequate response, loss of response, or intolerance to at least one therapy, including corticosteroids, aminosalicylates, immunosuppressants, or previous biologic therapy (infliximab, adalimumab, golimumab, vedolizumab, or ustekinumab). Patients who had previously failed three or more biologic therapies comprised 30% of the total population. Exclusion criteria included a diagnosis of Crohn’s disease or indeterminate colitis, previous JAK exposure, disease limited to the rectum, active infection, and fulminant colitis or toxic megacolon.
For the induction studies, there was a slightly higher male population (63% and 61%) for UC1 and UC2, respectively.10 Patients who received upadacitinib had median disease duration of 6.6 and 5.6 years, respectively. A minority of patients were on steroids (39% and 35%) at baseline with an average dose of 20 mg daily. At week 0 of the maintenance study, there was a mandatory taper of prednisone according to a predefined schedule. Around half of the patients (53% and 50%) had previous biologic failures, with 20% having two previous treatments.10 Most patients had an adapted Mayo Score ≤7 (61% and 60%), and a majority had a Mayo Endoscopic Subscore of 3 (70% and 68%). Overall, this represented a heterogenous group of patients with regard to previous treatments and disease activity.
Efficacy
The primary endpoint of the induction studies (U-ACHIEVE [UC1] and U-ACCOMPLISH [UC2]), was clinical remission at week 8, which was statistically significantly higher in the treatment groups (26% and 33% of patients in UC1 and UC2, respectively) than in the placebo groups (5% and 4%, respectively; p<0.001).10 In both induction studies, clinical remission was consistent across all subgroups, regardless of previous biologic failure. In addition, patients had clinical response as early as two weeks, with 60% of the pooled patients on upadacitinib responding vs 27% responding in the placebo arm. Secondary endpoints of the induction studies—including clinical response, endoscopic improvement, endoscopic remission, combined histologic-endoscopic mucosal improvement (HEMI), and resolution of abdominal pain and bowel urgency—were all statistically significantly higher in the treatment group than in the placebo (Table 1). Improvement in the HEMI score was seen at 8 weeks in 37% (UC1) and 30% (UC2) of patients in the treatment arms and in 6% and 7%, respectively, of patients in the placebo arms (p=<0.001). In the maintenance trial U-ACHIEVE (UC3), the primary endpoint of clinical remission was significantly higher in both the 15 mg dose (42% of patients) and 30 mg dose (52%) groups than in the placebo group (12%, p=<0.001). Both doses were superior to placebo for secondary endpoints, including endoscopic improvement, corticosteroid-free remission, endoscopic remission, mucosal healing, and symptom resolution in terms of urgency and abdominal pain (Table 2). At 52 weeks, HEMI improvement was seen in 50% of patients on 30 mg dosing and 35% of patients on 15 mg dosing, but only 12% in the placebo arm (p=<0.001).
 |
Table 1 Primary and Secondary Endpoints from Induction Studies |
 |
Table 2 Primary and Secondary Endpoints in Maintenance Study |
In the induction and maintenance phases, patient-reported outcomes were captured as secondary endpoints. After eight weeks of induction, 48% and 54% of patients on upadacitinib in UC1 and UC2, respectively, had no bowel urgency, compared to 21% and 26%, respectively, in the placebo arms (p<0.001). Patients also had resolution of abdominal pain, with 47% (UC1) and 54% (UC2) of patients in the treatment arms reporting no abdominal pain at eight weeks, compared to 23% and 24%, respectively, of patients on placebo (p=<0.001). Though not all patients completed the Inflammatory Bowel Disease Questionnaire (IBDQ), which includes factors such as bowel symptoms, systemic symptoms, emotional function and social function, patients who received upadacitinib 45 mg had a greater change from baseline in IBDQ total score than patients in the placebo group (55.3-point improvement vs 21.7, p < 0.0001). Change from baseline on FACIT-F, which measures fatigue and its impact on daily functions, showed an improvement of 9.5 vs 2.8 when comparing upadacitinib 45 mg to placebo (p < 0.0001). Similar findings were seen in the UC2 parallel study. The proportion of patients who achieved clinical response at two weeks with upadacitinib was significantly greater than with placebo in both UC1 and UC2 (UC1: 60% vs 27% and UC2: 63% vs 26%), which emphasizes the rapid onset of action for upadacitinib. Indeed, symptomatic improvement occurred as early as day 1 after upadacitinib 45 mg induction and was maintained over 14 days.11 The rapid onset of action could prove useful in treating acute severe ulcerative colitis, especially after failure with anti-TNF therapy. This would have to be studied prospectively, as data are currently lacking.
In an analysis of Phase 3 study findings,12 upadacitinib was found to significantly improve extra-intestinal manifestations (EIM) of ulcerative colitis compared to placebo. In the pooled induction studies, a higher proportion of participants in the upadacitinib group achieved resolution of any EIM at 8 weeks than in the placebo group (40% vs 33%). Participants in the upadacitinib group were more likely than those in the placebo group to have resolution of peripheral or axial arthropathies at 8 weeks (55% vs 42%) and resolution of anemia (38% vs 33%). Similar effects were observed in the maintenance study. Resolution of any EIM at 52 weeks was experienced by 66% of those in the 30 mg upadacitinib group, but only 42% in the 15 mg upadacitinib group and 24% in the placebo group. The 30 mg results were significantly different than placebo (p< 0.001).
Similarly, secondary endpoints of the UC3 maintenance phase of the study included patient-reported outcomes for upadacitinib 15 mg and 30 mg daily dosing. Both maintenance dose groups saw improvements in bowel urgency and abdominal pain compared to placebo, as well as improvements in change from baseline in IBDQ and FACIT-F compared to placebo. Upadacitinib at the higher 30 mg dose showed greater improvement than the 15 mg dose in patient-reported outcomes that corresponded with clinical, endoscopic and histologic outcome measures. If the medication is well tolerated in the induction phase and the patient has no prior history of adverse events with IBD that could be exacerbated by upadacitinib, it would be reasonable to maintain the higher dose of upadacitinib 30 mg daily. Once endoscopic remission is achieved, it would be reasonable to consider decreasing the dose to 15 mg daily, but many patients may need to continue on 30 mg long-term.
Safety of Upadacitinib
Previous studies of upadacitinib in patients with atopic dermatitis, psoriatic arthritis or rheumatoid arthritis found a higher risk of serious infection with upadacitinib 30 mg than with lower dose upadacitinib, placebo and adalimumab.13–16 In UC1, reported adverse events were similar between the placebo (60%) and upadacitinib 45 mg (56%) groups. Interestingly, in UC2, reported adverse events were higher in the upadacitinib 45 mg group (53%) than the placebo group (40%). In UC1, the most common adverse events included nasopharyngitis, creatine phosphokinase elevation, and acne; UC2 reported frequent acne. Both UC1 and UC2 showed less frequent serious adverse events in the upadacitinib 45 mg than in the placebo group (UC1: 3% vs 6%, UC2: 3% vs 5%) and less frequent adverse events leading to discontinuation (UC1: 2% vs 9%, UC2: 2% vs 5%). Notably, no active tuberculosis, cancer, renal dysfunction, or adjudicated major adverse cardiovascular event (MACE) was reported in any treatment group. In UC2, gastrointestinal perforation and venous thromboembolism (VTE) were reported in the placebo group, with subsequent withdrawal. In UC1 and UC2, herpes zoster infection, cytomegalovirus (CMV) infection and colitis, oral fungal infection, increased creatine phosphokinase (one requiring discontinuation), neutropenia, and lymphopenia were reported.
In the maintenance UC3 study, upadacitinib dosing and placebo groups all reported adverse events including worsening of ulcerative colitis, nasopharyngitis, creatine phosphokinase elevation, arthralgia and upper respiratory infections. Serious adverse events were less frequent in the upadacitinib groups than in the placebo group. Serious infections included six events of herpes zoster, a known class risk in JAK inhibitors, with 4% incidence in both upadacitinib groups and a CMV infection in the upadacitinib 15 mg group. Gastrointestinal perforation and MACEs were reported in the placebo group; no tuberculosis was seen in any group. Two non-serious VTEs were reported in the upadacitinib 30 mg group (with one patient having serious COVID-19 pneumonia). Neutropenia, hepatic disorders and cholesterol concentration increases were more common in the upadacitinib groups than in the placebo group, but none led to discontinuation. Creatine phosphokinase elevations were reported more in the upadacitinib 15 mg and 30 mg groups than in the placebo group; one patient on 30 mg dose discontinued the medication due to muscle pain.
Recorded adverse events were similar to those reported previously with other JAK inhibitors and with upadacitinib use in rheumatoid arthritis, atopic dermatitis and psoriatic arthritis.13–16 These include serious infection, herpes zoster, VTE and malignancy. A recent network meta-analysis showed that only tofacitinib 10 mg BID and upadacitinib at 45 mg daily increased the risk of herpes zoster.17 Therefore, patients who receive JAK inhibitors should be counseled on herpes zoster vaccination to help mitigate these risks. Additionally, patients with ulcerative colitis have an increased risk of VTE. Furthermore, a recent study of the long-term risks of VTE and MACE in patients with ulcerative colitis on tofacitinib, another JAK inhibitor, in 7.8 years of safety data from a global clinical program found minimal additional risk to patients.18 The ongoing long-term extension study of upadacitinib will help determine the long-term safety profile of this medication.
Positioning
Upadacitinib is approved as a second line therapy for patients with moderate to severe UC who have previously not responded to, lost response to, or are intolerant to anti-TNF therapy. Although clinical trials showed upadacitinib to be effective in both biologic-naive patients and patients with prior anti-TNF exposure, the cardiovascular safety signals discussed previously account for upadacitinib’s second line positioning.19 This risk has not been seen in patients with UC treated with tofacitinib,18 but upadacitinib is a selective JAK 1 inhibitor, so it is unknown whether it poses the same risks as tofacitinib. Additional real-world data or head-to-head trials evaluating the safety of anti-TNF therapy compared to upadacitinib are needed.
In the U-ACHIEVE trials, about 50% of the study population had prior exposure to biologic therapy, which included anti-TNF’s, vedolizumab and ustekinumab.10 Among these patients, more than half had failed two or more biologics. Nonetheless, clinical remission in these studies was consistent across patient groups, regardless of previous biologic failure, which indicates that upadacitinib has efficacy in a refractory patient population. A meta-analysis conducted in 2021 identified 28 trials including 12,504 patients with moderate to severe UC treated with biologic or small-molecule therapy.20 Efficacy was judged using clinical remission, endoscopic improvement, or clinical response and according to exposure or non-exposure to prior anti-TNF therapy. Among the therapies represented in the meta-analysis, upadacitinib induced clinical remission best in all patients, including patients who previously had not responded to anti-TNF. For endoscopic improvement, upadacitinib ranked second behind infliximab. In patients with prior anti-TNF exposure, upadacitinib ranked first. Indeed, another systematic review and meta-analysis re-demonstrated the efficacy of upadacitinib; however, upadacitinib was more likely than others to have adverse events.21 Upadacitinib appears to be effective as a second-line therapy in UC, even in patients who have not responded to multiple prior biologics.
Patients previously exposed to tofacitinib were not included in the U-ACHIEVE trials.10 Therefore, additional studies are needed to evaluate the efficacy of upadacitinib in tofacitinib non-responders. In clinical practice, we may encounter patients who have not achieved remission with any of the currently available treatment options for UC. In this situation, it may be reasonable to use upadacitinib. Although upadacitinib is a JAK inhibitor like tofacitinib, upadacitinib targets JAK1 specifically, whereas tofacitinib targets JAK1, 2, and 3, so patients who did not respond to tofacitinib may still respond to upadacitinib.
Upadacitinib may be considered a first-line therapy for patients who have concomitant rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis or atopic dermatitis, for which it is also FDA approved. In addition, upadacitinib is an oral option, which is attractive for many patients, and it has a rapid effect, with a half-life of 9–14 hours, per patient-reported outcomes from the trials.22 There is also no risk of immunogenicity with upadacitinib, unlike with anti-TNFs. Upadacitinib should be avoided in patients with cardiovascular risk factors or cancer, or those who are pregnant, breastfeeding or over the age of 75. These patients were excluded from the clinical trials, so further data are needed to determine safety outcomes. In animal studies, upadacitinib was shown to cause fetal malformations in early pregnancy and was excreted in breast milk; however, it is unknown if it is secreted in human milk. Currently, it is contraindicated in pregnancy and while breastfeeding; however, more studies are needed for this patient population. For women of child-bearing age, effective contraception is recommended during and for at least 3 weeks after treatment with upadacitinib. See Table 3 for recommendations on when upadacitinib is and is not preferred, based on current evidence.
 |
Table 3 Upadacitinib Use in Moderate to Severe Ulcerative Colitis |
Prior to starting upadacitinib, it is important to screen patients for active infections, including tuberculosis and hepatitis B. Active tuberculosis and untreated hepatitis B are relative contraindications to starting upadacitinib. To reduce the risk of herpes zoster, it is also recommended to start the attenuated zoster vaccination upon initiation of upadacitinib. Age appropriate vaccinations should also be recommended and completed prior to or during initiation of upadacitinib.
Conclusions
Upadacitinib is a selective JAK1 small molecule inhibitor with oral route of administration, rapid effect, short half-life, and no risk of immunogenicity, that is approved for use in ulcerative colitis patients who have previously failed or were intolerant to anti-TNF therapy. The U-ACHIEVE clinical trials demonstrated the efficacy and safety of upadacitinib in moderate to severe UC. Although these studies comprised a diverse international cohort, it should be noted that only 2–5% of the study population were of African, American Indian or Alaska Native or Native Hawaiian and other Pacific Islander background. Upadacitinib is used in multiple other rheumatologic diseases, which makes it an appealing option for individuals with extra-intestinal manifestations or a combination of ulcerative colitis and concomitant diseases for which it is approved.
Acknowledgments
We thank our scientific writer, Jonathan Feinberg, PhD for reviewing this manuscript.
Funding
No financial support or sponsorship was used for this review.
Disclosure
Bincy P Abraham has received research support from Takeda and consulting/honoraria from Abbvie, Bristol Myers Squibb, Fresenius Kabi, Lilly, Janssen, Pfizer, Samsung Bioepis, and Takeda. Kerri Glassner is a consultant for Lilly. Malcolm Irani and Christopher Fan have no conflicts of interest to report for this work.
References
1. Abbvie I. RINVOQ. North Chicago, IL: Abbvie, Inc.
2. Lin CM, Cooles FA, Isaacs JD. Basic Mechanisms of JAK Inhibition. Mediterr J Rheumatol. 2020;31(Suppl 1):100–104. doi:10.31138/mjr.31.1.100
3. Lahita RG. Lahita’s Systemic Lupus Erythematosus. Walthum: Elsevier; 2021.
4. Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. Erratum to: JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77(8):939. doi:10.1007/s40265-017-0736-y
5. Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: current and Future Prospects. Drugs. 2017;77(5):521–546. doi:10.1007/s40265-017-0701-9
6. Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann Rheum Dis. 2018;77(2):175–187. doi:10.1136/annrheumdis-2017-211555
7. Parmentier JM, Voss J, Graff C, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2018;2(1):23. doi:10.1186/s41927-018-0031-x
8. Alexander M, Luo Y, Raimondi G, O’Shea JJ, Gadina M. Jakinibs of all trades: inhibiting cytokine signaling in immune-mediated pathologies. Pharmaceuticals. 2021;15(1):48. doi:10.3390/ph15010048
9. McInnes IB, Byers NL, Higgs RE, et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther. 2019;21(1):183. doi:10.1186/s13075-019-1964-1
10. Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113–2128. doi:10.1016/S0140-6736(22)00581-5
11. Vermeire S, Colombel JF, Takeuchi K. DOP38 upadacitinib therapy reduces ulcerative colitis symptoms as early as day 1. J Crohns Colitis. 2022;16:087–088. doi:10.1093/ecco-jcc/jjab232.077
12. Colombel JF. OP33 Effect of upadacitinib (UPA) treatment on extraintestinal manifestations (EIMs) in patients with moderate-to-severe Ulcerative Colitis (UC): results from the UPA Phase 3 programme. J Crohns Colitis. 2022;16:i036–7
13. Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet. 2019;393(10188):2303–2311. doi:10.1016/S0140-6736(19)30419-2
14. Mease PJ, Lertratanakul A, Anderson JK, et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann Rheum Dis. 2021;80(3):312–320. doi:10.1136/annrheumdis-2020-218870
15. Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168. doi:10.1016/S0140-6736(21)00588-2
16. Cohen SB, van Vollenhoven RF, Winthrop KL, et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT Phase III clinical programme. Ann Rheum Dis. 2021;80(3):304–311. doi:10.1136/annrheumdis-2020-218510
17. Din S, Selinger CP, Black CJ, Ford AC. Systematic review with network meta-analysis: risk of Herpes zoster with biological therapies and small molecules in inflammatory bowel disease. Aliment Pharmacol Ther. 2023;57(6):666–675. doi:10.1111/apt.17379
18. Sandborn WJ, D’Haens GR, Sands BE, et al. Tofacitinib for the treatment of ulcerative colitis: an integrated summary of up to 7.8 years of safety data from the global clinical program. J Crohns Colitis. 2022. doi:10.1093/ecco-jcc/jjac141
19. Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. doi:10.1056/NEJMoa2109927
20. Burr NE, Gracie DJ, Black CJ, Ford AC. Efficacy of biological therapies and small molecules in moderate to severe ulcerative colitis: systematic review and network meta-analysis. Gut. 2021;71(10):1976–87
21. Lasa JS, Olivera PA, Danese S, Peyrin-Biroulet L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(2):161–170. doi:10.1016/S2468-1253(21)00377-0
22. Mohamed MF, Klunder B, Othman AA. Clinical pharmacokinetics of upadacitinib: review of data relevant to the rheumatoid arthritis indication. Clin Pharmacokinet. 2020;59(5):531–544. doi:10.1007/s40262-019-00855-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
