Back to Journals » OncoTargets and Therapy » Volume 13
Clinical Evaluation of the Safety and Efficacy of Trifluridine/Tipiracil in the Treatment of Advanced Gastric/Gastroesophageal Junction Adenocarcinoma: Evidence to Date
Authors Wheelden M , Yee NS
Received 31 March 2020
Accepted for publication 25 June 2020
Published 30 July 2020 Volume 2020:13 Pages 7459—7465
DOI https://doi.org/10.2147/OTT.S216598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Megan Wheelden,1 Nelson S Yee1– 3
1Division of Hematology-Oncology, Department of Medicine, Penn State Health Milton S. Hershey Medical Center, Hershey, PA, USA; 2Next-Generation Therapies Program, Penn State Cancer Institute, Hershey, PA, USA; 3Pennsylvania State University College of Medicine, Hershey, PA, USA
Correspondence: Nelson S Yee
Penn State Health Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033-0850, USA
Tel +1 717 531 8678
Fax +1 717 531 5076
Email [email protected]
Abstract: Trifluridine/tipiracil or TAS-102 (Taiho Oncology, Lonsurf®, Princeton, NJ, USA) is a combination tablet of trifluridine, a thymidine-based nucleoside analog, and tipiracil, a thymidine phosphorylase inhibitor, in a 1:0.5 molar ratio. This drug was first approved for use in metastatic colorectal cancer patients. Recently, the U S Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have granted approval of trifluridine/tipiracil for treatment of metastatic gastric and gastroesophageal junction adenocarcinoma in patients following at least two lines of chemotherapy including fluoropyrimidine and platinum chemotherapy agents, as well as taxanes or irinotecan. This approval was granted after the findings from first a Phase II trial (EPOC1201) investigating trifluridine/tipiracil, and later a global Phase III trial (TAGS trial) that compared trifluridine/tipiracil vs placebo with best supportive care. Both trials primarily utilized trifluridine/tipiracil at a dose of 35 mg/m2 twice daily. In the EPOC1201 trial, the primary end point of disease control rate was greater than 50% after eight weeks of therapy. The most common grade three or four adverse event was neutropenia; additional toxicities included leukopenia, anemia, and anorexia. In the TAGS trial, overall survival in patients treated with trifluridine/tipiracil (5.7 months) was significantly improved as compared to the placebo-controlled group (3.6 months). Treatment with trifluridine/tipiracil not only did not impair quality of life but also tended to reduce the risk of deterioration of quality of life. The results of these studies along with the subsequent FDA and EMA approval have generated an important breakthrough in regard to treatment options for patients with refractory metastatic gastric or gastroesophageal junction adenocarcinoma.
Keywords: gastroesophageal junction cancer, Lonsurf®, metastatic gastric cancer, quality of life, TAS-102, thymidylate synthase, thymidine phosphorylase
Introduction
Worldwide, gastric cancer is the fifth most common cancer—following lung, breast, colorectal, and prostate cancers.1 However, gastric cancer is the third most deadly malignancy and it represents approximately 8.3% of all cancer-related deaths. Both the incidence and mortality rate of gastric cancer are correlated to geographic area, due in part to variations in diet, smoking, and frequency of Helicobacter pylori infection.1 Gastric carcinoma is a relatively rare cancer in the US, with an estimated incidence of 27,510 patients in 2019. This represents 1.6% of all new cancer diagnoses, and it accounts for 1.8% of all cancer-related deaths based on data derived from trends in 2009–2015.2
Gastric cancer is typically diagnosed at advanced stages, including locally advanced unresectable or metastatic disease. For these patients, there are few options regarding palliative systemic treatment.3 Selection of an optimal regimen includes assessment of tumor expression of human epidermal growth factor receptor 2 (HER2) as well as patients’ performance status and medical comorbidities. Conventional regimens include combination of fluoropyrimidine (5-fluorouracil or capecitabine) and a platinum agent (oxaliplatin or cisplatin). For tumors with amplified expression of HER2, addition of trastuzumab has been demonstrated to produce clinical benefit.4 Second-line chemotherapy may involve the use of taxanes, irinotecan, or ramucirumab.5 Immunotherapy using the antiprogrammed death-1 (PD-1) antibodies, pembrolizumab, is indicated for treatment of patients with tumor expression of programmed death-ligand 1 (PD-L1) ≥1 as measured by combined positive score, or tumors with high microsatellite instability or DNA mismatch repair deficiency.6,7
Despite the currently available chemo- or immunotherapeutic agents, the median overall survival of patients with advanced or metastatic gastric cancer is 12 months. Multiplatform molecular analysis of gastric carcinoma may help identify biomarkers to guide selection of therapeutic agents.3 Various chemo- and targeted therapeutic agents have been investigated for treatment of advanced gastric cancer in pretreated patients with the goal of improving survival. One of these investigated agents involves a combination of trifluridine and tipiracil. On February 22, 2019, the US Food and Drug Administration (FDA) approved oral administration of trifluridine/tipiracil in patients with metastatic gastric or gastroesophageal junction adenocarcinoma who had progressed following at least two lines of prior chemotherapy. Approval of trifluridine/tipiracil for the same indication was granted by the European Medicines Agency (EMA) on September 6, 2019.
In this review, the safety and efficacy of trifluridine/tipiracil are evaluated based on up-to-date evidence. An overview of the chemistry, pharmacodynamics, and pharmacokinetics of trifluridine/tipiracil is provided. Next, the data from the preclinical and clinical studies that investigated trifluridine/tipiracil in gastric adenocarcinoma and gastroesophageal junction (GEJ) adenocarcinoma are examined. Ongoing clinical studies to investigate trifluridine/tipiracil in combination with other chemotherapeutic or targeted agent for gastric/GEJ adenocarcinoma are described.
Trifluridine and Tipiracil: Chemistry, Pharmacodynamics, and Pharmacokinetics
Trifluridine is a nucleoside metabolic inhibitor, and it is used in combination with tipiracil, which is a thymidine phosphorylase inhibitor, at a molar ratio of 1:0.5. Trifluridine, chemically described as 2ʹ-deoxy-5(trifluoromethyl) uridine, is a thymidine-based nucleoside analog (Figure 1). Trifluridine is phosphorylated to its active monophosphate-derivative that subsequently inhibits thymidylate synthase. This enzyme is involved in the synthesis of pyrimidine deoxynucleotide, and therefore DNA. Additionally, trifluridine can be further phosphorylated to trifluorothymidine-triphosphate that can be incorporated into DNA. Through both of these mechanisms, trifluridine impairs DNA synthesis, leading to DNA damage and ultimately cell death (Figure 2). Although trifluridine is structurally and functionally similar to 5-fluorouracil, which is commonly used in gastrointestinal malignancies, the distinct mechanism of action of trifluridine contributes to its utility in patients with malignancies refractory to 5-fluorouracil.8,9
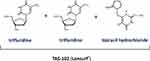 |
Figure 1 Chemical structure of trifluridine and tipiracil hydrochloride. One mole of TAS-102 is composed of two moles of trifluridine and one mole of tipiracil hydrochloride.The chemical structure of trifluridine and tipiracil shown in this figure is used with permission from Taiho Oncology.9 |
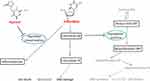 |
Figure 2 Mechanism of trifluridine/tipiracil-mediated cytotoxicity. By inhibition of thymidine phosphorylase, tipiracil hydrochloride blocks conversion of trifluridine into trifluorothymine. Trifluridine can be phosphorylated by thymidine kinase 1 to trifluridine monophosphate (MP), which reversibly inhibits thymidylate synthase by competing with deoxyuridine-MP. This results in depletion of deoxythymidine-MP and ultimately preventing DNA synthesis and cell division. Trifluridine-MP can be further phosphorylated to trifluridine triphosphate (TP), which becomes incorporated into DNA, leading to DNA damage and cell death. The chemical structure of trifluridine and tipiracil shown in this figure is used with permission from Taiho Oncology.9 |
Tipiracil, chemically described as 5 chloro-6-[(2-iminopyrrolidin-1-yl)methyl]pyrimidine-2,4-(1H,3H)-dione monohydrochloride (Figure 1), functions primarily to prevent the metabolism of trifluridine to trifluorothymine (Figure 2). In vivo testing demonstrated that addition of tipiracil to trifluridine significantly increased the bioavailability of trifluridine. Additional studies were performed to determine the optimal molar ratio of 1:0.5 for trifluridine and tipiracil. In addition to this function, tipiracil has postulated effects, including its ability to inhibit the degradation of thymidine to thymine. This process generates deoxyribose-1-phosphate and subsequently deoxyribose, which is thought to contribute to angiogenesis. It has been suggested that tipiracil can inhibit tumor angiogenesis for additional cytotoxicity.8,9
Pharmacokinetic studies with oral dosing of trifluridine at 35 mg/m2 twice daily yielded a mean half-life at a steady state of 2.1 and 2.4 hours for trifluridine and tipiracil, respectively. Metabolism of trifluridine is predominantly mediated by thymidine phosphorylase, and it is renally excreted. Administration of the combination increased the area under the curve (AUC) concentration of trifluridine by 37-fold compared to administration of trifluridine alone. Following administration of a single dose of trifluridine, the mean time to peak plasma concentration was approximately two hours. Additional studies to augment absorption of trifluridine-tipiracil showed that ingestion of a high-fat, high-calorie meal decreased the AUC of tipiracil but did not change that of trifluridine.8,9
Preclinical and Clinical Studies Using Trifluridine and Tipiracil
Trifluridine was initially synthesized as a potential chemotherapeutic drug, but investigations of the agent were subsequently halted due to side effects as well as its limited pharmacokinetic profile. In the mid-2000s, trifluridine and tipiracil were investigated in clinical trials for patients with a variety of malignancies—including breast and colon cancer. Over the years, trifluridine and tipiracil were studied for treatment of mostly gastrointestinal malignancies—specifically colorectal cancer. A phase III study demonstrated a significant improvement of overall survival of 7.1 months with trifluridine/tipiracil, compared to 5.3 months with placebo control.10 Based on the result of this study, approval of trifluridine/tipiracil for treatment of metastatic colorectal cancer that has progressed following second-line or beyond systemic therapy was granted by the US FDA in 2015.
To study this drug combination in patients with locally advanced or metastatic gastric cancer that had progressed following initial therapy containing 5-fluorouracil, an experiment was designed to explore their in vitro activity. The combination of trifluridine and tipiracil was demonstrated to produce growth inhibition of both 5-fluorouracil sensitive and resistant human gastric cancer cells. Additional testing in cultured cells overexpressing thymidylate synthase revealed decreased cell death to trifluridine suggesting cross-resistance. However, when trifluridine and tipiracil was administered in mouse xenograft models, again both sensitive and resistant to 5-fluorouracil, the tumors demonstrated decreased tumor volume. It was concluded that the primary antitumor mechanism of action was incorporation of the metabolite of trifluridine into DNA, as opposed to strictly relying on inhibition of thymidylate synthase.11
In phase I studies performed in the US, the recommended dose schedule of treatment on days one to five and eight to twelve of a 28-day cycle was developed. Initial studies in heavily pretreated breast cancer patients established a maximum tolerated dose of 25 mg/m2 twice daily. This dosing strategy was subsequently used in a phase II American study for advanced gastric patients.8 In the TAS102-9806 study, 18 patients with gastric cancer were enrolled for treatment using the drug combination with a dose of 25 mg/m2 twice daily. During the course of this study, one patient had stable disease, and after four cycles of therapy all patients had progressed.10 This study was subsequently closed early. However, later studies in Japan investigated the use of 35 mg/m2 twice daily and demonstrated that this was an acceptable dosing with grade 4 neutropenia as the primary dose-limiting toxicity.12
This increased dosing strategy was subsequently utilized in the Phase II EPOC1201 trial. In this study, a total of 29 patients with advanced or recurrent gastric or gastroesophageal junction adenocarcinoma after one or two prior lines of therapy was enrolled. These patients received trifluridine and tipiracil at 35 mg/m2 twice daily; there was also a cohort of six patients who were treated with trifluridine and tipiracil at a dose of 40 mg/m2 twice daily. The primary end point was a disease control rate (DCR) of greater than or equal to 50% after eight weeks of therapy. This was achieved in the trial, as the investigator-assessed DCR was 65.5% and the independent central review DCR was 51.9%. The median progression-free survival was 2.9 months, and overall survival was 8.7 months. Similar to prior trials, the most common grade three or four adverse event was neutropenia at 69%; additional grade three and four adverse events included leukopenia (41.4%), anemia (20.7%), and anorexia (10.3%).12
Results of the Phase III TAGS trial that investigated trifluridine/tipiracil vs placebo in patients with heavily pretreated metastatic gastric cancer led to FDA and EMA approval of the drug combination. In this study, a total of 507 patients with metastatic gastric adenocarcinoma who progressed after two or more prior therapies was enrolled at 110 academic hospitals in 17 countries. Patients were randomized to trifluridine and tipiracil vs placebo in a 2:1 ratio; therefore 337 patients received therapy with the combination therapy at an initial dose of 35 mg/m2 twice daily and 170 received the placebo. The primary end point was overall survival, and efficacy as well as safety were monitored. The median overall survival in the experimental group was 5.7 months compared to 3.6 months in the placebo-controlled group with a two-sided P-value of 0.00058. Similar to prior studies, the most common grade three or higher adverse event was neutropenia (34%); the second most common was anemia at 19%. During the course of the trial, there was one fatal event in the patients receiving trifluridine and tipiracil due to cardiopulmonary arrest. Dose modification with dosing delays or dose reduction due to adverse events was required in 58% of the patients receiving trifluridine and tipiracil compared to 22% of patients receiving placebo.13
A subgroup analysis in the TAGS trial was conducted to examine efficacy and safety of trifluridine/tipiracil in patients who had undergone gastrectomy and those who had not undergone gastrectomy. The overall survival hazard ratios (trifluridine/tipiracil vs placebo) in the gastrectomy subgroup and no gastrectomy subgroup were 0.57 and 0.8, respectively. The progression-free survival hazard ratios (trifluridine/tipiracil vs placebo) in the gastrectomy subgroup and no gastrectomy subgroup were 0.48 and 0.65, respectively. Among patients treated with trifluridine/tipiracil, grade three or higher adverse events occurred in 84.1% and 76.3% of patients in the gastrectomy subgroup and no gastrectomy subgroup, respectively. Those adverse events were primarily neutropenia, anemia, and leukopenia. Result of this subgroup analysis indicates that treatment of patients with trifluridine/tipiracil was similarly tolerable in both subgroups, and the efficacy benefits may be greater in patients of the gastrectomy subgroup than the no gastrectomy subgroup.14
The health-related quality of life (QoL) outcomes in the TAGS trial were analyzed and compared among patients treated with trifluridine/tipiracil and compared with placebo. Using the EORTC QLQ-30 and EORTC QLQ-ST022 questionnaires, QoL was evaluated at baseline and at each treatment cycle. In both groups of patients who had been treated with trifluridine/tipiracil and those with placebo, no clinically significant deteriorations in the mean QLQ-C30 Global Health Status score were detected. A trend towards reduction of the risk of deterioration of QoL scores was found in the trifluridine/tipiracil-treated patients as compared with the placebo-controlled group. Results of this analysis indicates that treatment with trifluridine/tipiracil not only did not impair QoL, but also tended to reduce the risk of deterioration of QoL.15
Summary and Future Perspectives
In summary, the combination of trifluridine and tipiracil offers a new treatment option for patients with advanced metastatic gastric or gastroesophageal junction cancer who have progressed following at least two lines of prior chemotherapy. As demonstrated in the TAGS trial, this combination therapy was found to improve median overall survival and progression-free survival compared to placebo regardless of gastrectomy. These survival improvements outweigh the adverse events that primarily involved hematologic toxicity without causing deterioration of QoL. The survival outcomes of the TAGS trial and selected phase III clinical trials for advanced, unresectable or metastatic gastric adenocarcinoma or GEJ adenocarcinoma are described in Table 1. Currently, three clinical trials are ongoing for further investigation of trifluridine/tipiracil in locally advanced or metastatic gastric cancer (Table 2). Hopefully, these clinical studies will generate the supporting data to expand the use of trifluridine/tipiracil for improving the clinical outcomes for patients with gastric and GEJ adenocarcinoma.
 |
Table 1 Selected Phase III Clinical Studies of Systemic Therapies in Advanced and Metastatic Gastric Carcinoma |
 |
Table 2 Ongoing Clinical Studies to Investigate Trifluridine/Tipiracil in Gastric Cancer |
Disclosure
Dr Nelson S Yee reports nonfinancial support from Astra Seneca, Daichii Sankyo, Novartis, Caris Life Sciences, Eli Lilly, Regeneron, Abbvie, and Genentech; grants from Eli Lilly, Ipsen, Onxeo, Boston Biomedical, EMD Serono, Pharmacyclics, Merck, outside the submitted work. Taiho provided grant support for the symposium “Multi-disciplinary Patient Care in Gastrointestinal Oncology” organized by Penn State College of Medicine. The authors report no other conflicts of interest in this work.
References
1. Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–35. doi:10.5114/pg.2018.80001
2. National Cancer Institute. Surveillance, epidemiology, and end results program. Cancer Stat Facts: stomach Cancer; 2020. Available from: https://seer.cancer.gov/statfacts/html/stomach.html.
3. Kankeu Fonkoua L, Yee NS. Molecular characterization of gastric carcinoma: therapeutic implications for biomarkers and targets. Biomedicines. 2018;6:32. doi:10.3390/biomedicines6010032
4. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a Phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi:10.1016/S0140-6736(10)61121-X
5. Bilici A. Treatment options in patients with metastatic gastric cancer: current status and future perspectives. World J Gastroenterol. 2014;20(14):3905–3915. doi:10.3748/wjg.v20.i14.3905
6. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;10(4):e180013. doi:10.1001/jamaoncol.2018.0013
7. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi:10.1126/science.aan6733
8. Peters GJ. Therapeutic potential of TAS-102 in the treatment of gastrointestinal malignancies. Ther Adv Med Oncol. 2015;98(6):779–789. doi:10.1177/1758834015603313
9. US Food and Drug Administration. Lonsurf: highlights of prescribing information; 2019. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207981s008lbl.pdf.
10. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–1919. doi:10.1056/NEJMoa1414325
11. Matsuoka K, Nakagawa F, Kobunai T, Takechi T. Trifluridine/tipiracil overcomes the resistance of human gastric 5-fluorouracil-refractory cells with high thymidylate synthase expression. Oncotarget. 2018;9(17):13438–13450. doi:10.18632/oncotarget.24412
12. Bando H, Doi T, Muro K, et al. A multicenter phase II study of TAS-102 monotherapy in patients with pre-treated advanced gastric cancer (EPOC1201). Eur J Cancer. 2016;62:46–53. doi:10.1016/j.ejca.2016.04.009
13. Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437–1448. doi:10.1016/S1470-2045(18)30739-3
14. Ilson DH, Tabernero J, Prokharau A, et al. Efficacy and safety of trifluridine/tipiracil treatment in patients with metastatic gastric cancer who had undergone gastrectomy: subgroup analyses of a randomized clinical trial. JAMA Oncol. 2020;6(1):e193531. doi:10.1001/jamaoncol.2019.3531
15. Tabernero J, Alsina M, Shitara K, et al. Health-related quality of life associated with trifluridine/tipiracil in heavily pretreated metastatic gastric cancer: results from TAGS. Gastric Cancer. 2020;23(4):689–698. doi:10.1007/s10120-020-01053-9
16. Carmona-Bayonas A, Jimenez-Fonseca P, Custodio A, et al. Anthracycline-based triplets do not improve the efficacy of platinum-fluoropyrimidine doublets in first-line treatment of advanced gastric cancer: real-world data from the AGAMEMON National Cancer Registry. Gastric Cancer. 2018;21(1):96–105. doi:10.1007/s10120-017-0718-5
17. Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi:10.1200/JCO.2006.06.8429
18. Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus wither oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26(9):1435–1442. doi:10.1200/JCO.2007.13.9378
19. Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi:10.1016/S0140-6736(13)61719-5
20. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi:10.1016/S1470-2045(14)70420-6
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
