Back to Journals » Cancer Management and Research » Volume 14
Clinical Evaluation of Avelumab in the Treatment of Advanced Urothelial Carcinoma: Focus on Patient Selection and Outcomes
Authors Ten Eyck JE , Kahlon N, Masih S , Hamouda DM, Petros FG
Received 11 October 2021
Accepted for publication 27 January 2022
Published 22 February 2022 Volume 2022:14 Pages 729—738
DOI https://doi.org/10.2147/CMAR.S227323
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Jennifer E Ten Eyck,1 Navkirat Kahlon,2 Sonia Masih,1 Danae M Hamouda,2 Firas G Petros1
1Department of Urology, College of Medicine and Life Sciences, The University of Toledo, Toledo, OH, USA; 2Department of Hematology Oncology, College of Medicine and Life Sciences, The University of Toledo, Toledo, OH, USA
Correspondence: Firas G Petros, Department of Urology, The University of Toledo, College of Medicine and Life Sciences, 3000 Arlington Ave., Mail Stop 1091, Toledo, OH, 43614-2598, USA, Tel +1 419 383 3584, Fax +1 419 383 3785, Email [email protected]
Background: First-line therapy for treatment of advanced urothelial carcinoma includes combination platinum-based chemotherapies, though resistance and long-term toxicity concerns to these regimens cause limitations in progression-free survival and overall survival. Maintenance treatment with an alternative agent such as the PD-L1 inhibitor, avelumab (Bavencio®), after initial chemotherapy has been shown to prolong overall survival. The aim of this review is to provide a landscape clinical use of avelumab in the treatment of advanced urothelial carcinoma with a focus on patient selection and outcomes.
Methods: This review includes the most up to date phases and results from clinical trials published in peer-reviewed journals.
Results: Three studies were included, one phase 1B trial, one phase 1B trial with 2 year follow-up, and one phase 3 trial. Patients receiving avelumab maintenance therapy at 10 mg/kg IV every two weeks had an overall better performance status, though those with an increased ECOG-PS, increased Bellmunt risk score, or failure of ≥ 3 chemotherapies had poorer responses. Patients over the age of 65 had a higher ORR (18– 25%) compared to younger patients (13– 14%). Patients with PD-L1 positive tumors had a significantly increased CR median ORR (13.8%), median PFS (5.7 months), and median 12-month OS rate (79.1%) compared to control subjects receiving best supportive care (1.2%, 2.1 months, 60.4%, respectively). TRAEs were seen in 86.7% of patients, with 32.4% of patients experiencing a ≥grade 3 AE. The most common AE was IRR (32.4%, ≥grade 3 1.01%) and irAE 25.6% of any grade, including various rashes and pruritus AEs, immune-related thyroid disorders, and immune related hepatitis. There were 3 reported treatment-related deaths (0.05%). Ongoing phases of one of the trials is investigating the use of docetaxel and avelumab together after failure of one chemotherapy.
Conclusion: Avelumab as a maintenance therapy after platinum-based chemotherapy failure or in platinum-ineligible patients with advanced or metastatic urothelial carcinoma is an effective option with increased ORR, PFS, and OS with a similar safety profile to other chemotherapies. Ongoing studies currently in recruitment and active clinical trials will yield valuable insights into optimizing avelumab therapy in conjunction with chemotherapies and/or immunotherapies, better characterization of response for PD-L1 positive tumors, and a clearer insight into clinically validated prognostic factors to improve patient outcomes.
Keywords: avelumab, Bavencio®, metastatic, advanced, urothelial carcinoma, patient outcomes
Introduction
First-line therapy for treatment of advanced urothelial carcinoma includes combination platinum-based chemotherapy such as gemcitabine plus cisplatin or dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (ddMVAC) for cisplatin eligible patients.1 For cisplatin ineligible patients, therapy includes gemcitabine with carboplatin or checkpoint inhibitor (CPI) with atezolizumab and pembrolizumab.2 Resistance to these chemotherapy regimens, however, causes limitations in progression-free survival and overall survival. Furthermore, long-term platinum-based chemotherapy is not feasible due to the increasing risk of toxicity over time. Median survival for cisplatin-based regimens has been reported as 14–15 months, while carboplatin-based regimens have been reported as 9–10 months. Disease progression for most patients usually occurs within 9 months of treatment initiation.3 The use of some alternative agents for maintenance treatment following chemotherapy has been shown to prolong overall survival for a subset of tumor types, but in general the prolongation of overall survival with these modalities has not been shown.
A continuous challenge with conventional chemotherapy is the development of tumor immunity. Gottesman et al proposed a mechanism of resistance for cisplatin, which functions by inducing apoptosis in cancer cells through DNA repair interference.4 These resistance mechanisms include changes in cellular uptake and efflux of the drug, increased biotransformation and detoxification in the liver, and an increase in DNA repair and anti-apoptotic mechanisms. Even so, tumor immunity continues to develop and contribute to limitation of survival prolongation. Maintenance treatment with an alternative agent after initial chemotherapy has been shown to prolong overall survival. Therefore, the aim of this review is to provide a landscape clinical use of avelumab in the treatment of advanced urothelial carcinoma with a focus on patient selection and outcomes.
Methods
For this review, literature was identified through PubMed and Embase databases with no limit on publication year or language. Search terms included “avelumab,” “Bavencio®,” “bladder cancer,” “malignant bladder cancer,” “advanced bladder cancer,” “urothelial,” “urothelial bladder cancer,” “advanced urothelial carcinoma,” “metastatic urothelial carcinoma,” “urothelial cancer cells,” “urothelial abnormalities,” “advanced urethra cancer,” “malignant urethra cancer,” “urethra carcinoma”, “urethra disease,” “urethra,” and “urethra neoplasms.” A total of 131 records were identified and screened for appropriate human trials as shown in Figure 1.5 Clinicaltrials.gov was cross-referenced using the terms “urothelial carcinoma” for disease and “avelumab” for other to ensure that no completed clinical trials were missed in the literature search.6
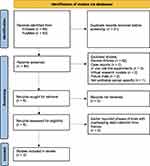 |
Figure 1 PRISMA flow diagram demonstrates identifications of studies via databases used for literature review, screening and final cohort of studies included in this review. |
Results
Studies of Avelumab in Advanced/Metastatic Urothelial Carcinoma
Eight studies evaluating avelumab, an anti-programmed cell death ligand 1 (PD-L1) antibody, in advanced metastatic urothelial carcinoma were retrieved. Five of the eight studies were previously published phases of clinical trials whose latest phases were available for review. Two of the trials assessed avelumab alone at a dose of 10 mg/kg IV every two weeks in patients who either did or did not have disease progression after first line platinum-based chemotherapy. The third study is investigating the use of docetaxel with avelumab after failure of one platinum-based chemotherapy. The tables in this review cover study design (Table 1) and significant outcomes (Table 2).
 |
Table 1 Overview of Peer-Review Avelumab Trials Reviewed |
 |
Table 2 Published Avelumab Patient Outcomes of Reviewed Papers |
Avelumab in Advanced Urothelial Carcinoma
Avelumab in recent studies has been found to improve outcomes in patients with locally advanced and metastatic urothelial carcinoma. Patients treated with an avelumab dosing schedule of 10 mg/kg every two weeks until disease progression or unacceptable toxicity after 4 to 10 weeks of completing four to six cycles of chemotherapy with response or stable disease. For maintenance, median overall survival (OS) has been reported to be 21 months in a phase 3 trial with absolute improvement in OS by 7.1 months as compared to placebo by Powles et al.9 Patients with previously treated advanced/metastatic urothelial carcinoma platinum-based chemotherapy with no previous immunotherapy were found to have a median OS of 7 months in a phase 1B trial by Apolo et al.7
FDA Approved Indications of Avelumab
In 2017, avelumab was granted an accelerated approval from the Food and Drug Administration (FDA) for the treatment of patients with locally advanced or metastatic urothelial carcinoma who experience disease progression during or following platinum-containing chemotherapy, or who have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.
In 2020, avelumab has been approved for patients with advanced bladder cancer (regardless of PD-L1 tumor status) who do not progress (ie, achieve an objective response or stable disease) following platinum-based chemotherapy and are eligible to receive checkpoint inhibitor immunotherapy. Maintenance avelumab, rather than best supportive care alone, improved overall survival and progression-free survival (PFS) in a phase III trial.9
Dosing
Maintenance avelumab was administered at 10 mg/kg every two weeks until disease progression or unacceptable toxicity after 4 to 10 weeks of completing four to six cycles of chemotherapy whether a response or stable disease has been confirmed on radiographic imaging. The same dose has also been studied as a second line or subsequent line of treatment. The FDA more recently approved avelumab dose is 800 mg administered as an intravenous infusion over 60 minutes every 2 weeks until disease progression or unacceptable toxicity as benefit:risk profiles for the two dosing regimens have been found to be similar.10
Avelumab in Advanced Urothelial Carcinoma – Age
The response of the elderly population to avelumab was overall very promising compared to that of younger populations. The study population in the JAVELIN solid tumor trial included two subgroups for age, less than 65 years (n = 50) and ≥65 years (n = 111). Patients in the older subgroup experienced an overall higher overall response rate (ORR) (18%, CI 11–26%) compared to the younger subgroup (14%, CI 6–27%).9 The Apolo et al study population included subjects <75 years old (n = 176, 70.7%) and ≥75 years old (n = 73, 29.2%). Similarly, to the JAVELIN solid tumor trial, there was a higher ORR to avelumab in the older subgroup (25.0%) compared to the younger subgroup (13.2%, CI 8.6–19.2%). The most common treatment-related adverse events in the elderly subgroup in this study was renal and urinary disorders (31.5%) and infusion-related reactions (28.8%).7 This data demonstrates improved response and survival in older patients.
Avelumab in Advanced Urothelial Carcinoma – Performance Status
Overall, studies showed that patients with a better performance status had better response to avelumab. In the JAVELIN solid tumor study, patients with Eastern Cooperative Oncology Group (ECOG) performance status 0 (n = 56) experienced an ORR of 23% (CI 13–36%) versus 13% (CI 8–21) in those with an ECOG ≥1 (n = 105). The Apolo et al study also demonstrated that patients with a better PS had greater benefit from avelumab therapy compared to those with a poorer PS. Those with an ECOG 0 had an ORR of 19.0% versus those with ECOG ≥1 who had an ORR of 15.2%. In the same study, for patients with a Bellmunt prognostic (or risk) score of 0, 1, 2 or 3, ORRs were 22.2% (CI 12.0–35.6%), 21.4% (14.2–30.2%), 6.9% (1.9–16.7%) and 0% (0–18.5%), respectively.7
Avelumab in Advanced Urothelial Carcinoma – PD-L1 Status
PD-L1 expression as a predictive biomarker for response to immunotherapy has its limitations. It has been measured in a variable fashion in tumor cells or tumor-infiltrating cells.11 Also, the thresholds are variable within and across tumor subtypes. In the context of avelumab and urothelial cancer, the two major studies ie, Phase 1B JAVELIN Solid Tumor by Apolo et al and JAVELIN Bladder 100 by Powles et al, included both PD-L1 positive and negative patients but the assays used and hence the definition of PD-L1 positivity was different.7,9
Apolo et al defined PDL1 positivity as >/= 5%, using Dako PD-L1 IHC 73–10 pharmDx PD-L1 assay. 34.1% patients were PD-L1 positive (+), 54.2% were PD-L1 negative (-) and 11.6% were not evaluable. Outcomes for subgroup analysis according to PD-L1 status were included in supplemental material. In the PD-L1+ group, the confirmed ORR (95% CI) was 23.8% (15.2–34.3%) and in the PD-L1- group was 12.3% (7.2–19.2%). Median PFS (95% CI) in PD-L1+ group was 2.2 months (1.4–4.11%) and 1.5 months (1.4–2.4%) in PD-L1- group. However, 12-month PFS rate (95% CI), in PD-L1+ patients was lower ie 14.6% (8.8–21.9%) as compared to 23.9% (14.2–35.0%) in PD-L1- patients. Median OS (95% CI) in months was 8.41 (6.0–11.3%) in PD-L1+ group and 6.5 (5.3–10.1%) in the PD-L1- group. 24-month OS rate (95% CI), % was 24.3% (15.6–34.0%) in PD-L1+ patients vs 17.9% (11.8–25.0%) in PD-L1- patients. Overall, patients with PD-L1+ had higher ORR, better median PFS and better OS as compared to PD-L1 negative patients. It is approved for use regardless of PD-L1 status.7
In JAVELIN Bladder 100 by Powles et al, PD-L1 expression was assessed in tumor samples by means of the Ventana PD-L1 assay (SP263, Ventana Medical Systems). Patients were classified as having PD-L1–positive status if at least one of the following three criteria were met: at least 25% of tumor cells stained for PD-L1, at least 25% of immune cells stained for PD-L1 if more than 1% of the tumor area contained immune cells, or 100% of immune cells stained for PD-L1 if no more than 1% of the tumor area contained immune cells. 51.1% had PD-L1–positive tumors in this study. The patient characteristics were balanced between the two treatment groups (placebo and avelumab maintenance group) for the PD-L1–positive population. This study allowed a crossover to receive immunotherapy as subsequent anticancer drug therapy. Subsequent treatment was given to 42.3% in the avelumab group and 61.7% in the control group, including an anti-PD-1 or anti-PD-L1 antibody in 6.3% in the avelumab group and 43.7% in the control group. Even with a significant number of patients receiving immunotherapy on progression in the placebo arm, avelumab in a maintenance setting, significantly prolonged overall survival in the PD-L1–positive population with overall survival at 1 year of 79.1% in the avelumab group and 60.4% in the control group (hazard ratio, 0.56; 95% CI, 0.40–0.79; P<0.001). The median progression-free survival in avelumab and placebo treated patients was 5.7 months and 2.1 months, respectively, in the PD-L1–positive population (hazard ratio, 0.56; 95% CI, 0.43–0.73).9
Avelumab in Advanced Urothelial Carcinoma – Patient Safety
Overall, no new toxicity signals were found in any of the studies included in the review.
Garje et al, in published abstract with combined chemoimmunotherapy, reported 1 dose limiting toxicity (neutropenic fever) in addition to the most common grade 3–4 adverse events (AE) of febrile neutropenia, urinary tract infection, and confusion.8
Adverse events from Apolo et al and Powles et al are summarized in Table 3. The most common treatment-related adverse events (TRAE) (>10%) of any grade from both trials include fatigue, various immune related rashes and pruritic AE, diarrhea, renal events (including increased creatinine and renal/urinary disorders), and immune-related thyroid disorders (including hypothyroidism, hyperthyroidism).7,9
 |
Table 3 Combined Summary of Adverse Events in Apolo et al and Powles et al for 593 Patients7,9 |
In the Apolo et al article 71.1% patients were found to have treatment-related adverse effects of any grade, 11.6% had TRAEs of grade 3 or higher with fatigue being most common. Treatment-related death occurred in one patient (0.4%), due to pneumonitis. The most common immune-related adverse events (irAE) (any grade) were immune-related rash (11.2%), including various rash/pruritus AE and immune related thyroid disorders (13, 5.2%), including hypothyroidism, hyperthyroidism and blood thyroid-stimulating hormone increase). In an exploratory post hoc analysis, frequency and extent of infusion related reactions (IRR) in high-risk patient subgroups (such as patients with renal insufficiency, upper tract disease, liver metastases) were similar to the overall population in study compared with the overall population. 15.7% of patients discontinued treatment due to adverse effects in this study.9
The Powles et al safety population, defined as any patient who received one or more doses of avelumab, also noted common TRAEs (>10%) of arthralgia, asthenia, constipation, back pain, nausea, pyrexia, anorexia, cough, vomiting, anemia, and hematuria. The incidence of adverse events from any cause was 98.0% in the avelumab group and 77.7% in the control group; the incidence of adverse events of grade 3 or higher was 47.4% and 25.2%, respectively. In the avelumab group, median duration of trial treatment was 24.9 weeks and adverse events led to treatment discontinuation in 11.9% patients. Death was attributed by the investigator to the toxicity of trial treatment in two patients (0.6%) in the avelumab group. 29% of patients had irAE 7.0% with grade 3 events. No grade 4 or fatal irAE occurred. The most frequent irAE was thyroid disorders (12.2%). 9% of patients required high-dose glucocorticoids (≥40 mg total daily dose of prednisone or equivalent) for irAE in avelumab-treated patients.9
Future Directions
Continued research is needed in the AVETAX and JAVELIN Bladder 100 clinicals trials as the research progresses through their respective clinical trial phases, including particular attention to the response of PD-L1 positive tumors versus controls. In June 2020, avelumab was granted the FDA approval to change the approved dosage from 10 mg/kg IV every two weeks to a flat-dose of 800 mg IV every two weeks per pharmacological models analyzing exposure-safety and exposure-efficacy models.10,12 The flat-rate dosage has yet to be implemented in published clinical trials and will need further verification. The prognostic model for survival in post-platinum patients with metastatic urothelial carcinoma (based on ECOG performance status, liver metastasis, platelet count, neutrophil-to-lymphocyte ratio, and lactate dehydrogenase) also needs further validation with avelumab based trials. It is also important to account for flat-rate versus body-weight dosing and comparing its prognostic outcomes versus the Bellmunt risk score.13
There are currently 19 active or actively recruiting trials on clinicaltrials.gov for studies involving avelumab and urothelial carcinoma. Many of the active trials are now focusing on the optimal duration of avelumab therapy, combining avelumab with other chemotherapy agents and/or immune therapies, and assessing the efficacy of avelumab therapy after other immune checkpoint inhibitor therapies have failed. Several of these trials have faced delays due to the SARS-CoV-19 pandemic and will hopefully be able to progress at an appropriate pace as the pandemic slows.6
Discussion
In 2017, avelumab was granted FDA approval for the treatment of patients with locally advanced or metastatic urothelial carcinoma who experience disease progression during or following platinum-containing chemotherapy, or who have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. In 2020, avelumab was approved for patients with advanced bladder cancer (regardless of PD-L1 tumor status) who do not progress (ie, achieve an objective response or stable disease) following platinum-based chemotherapy and are eligible to receive checkpoint inhibitor immunotherapy. It has been found to be both an efficacious and tolerable option in those settings. In maintenance settings, it improved ORRs, median PFS, and median OS even with subsequent crossover in the placebo arm to immunotherapy on progression. Benefit was seen in both PD-L1 positive and negative patients. No new toxicity signals were found with avelumab use in urothelial cancer.
Current clinical trials are continuing to assess the efficacy of avelumab on its own as a maintenance therapy with and without disease progression and in combining avelumab with other chemotherapy and immunotherapy options. These trials will be critical in creating future guidelines for advanced or metastatic urothelial carcinoma and how to more effectively treat PD-L1 positive tumors.
Conclusion
In summary, avelumab is an effective and well-tolerated regimen for patients with advanced urothelial cancer who have not progressed on chemotherapy, as well as for patients with progression of disease after receiving platinum-containing chemotherapy. It is now the standard of care in these two settings.
Abbreviations
AE, adverse event; CI, confidence interval; CR, complete response; DOR, duration of response; ECOG, Eastern Cooperative Oncology Group; FDA, Food and Drug Administration; irAE, immune related adverse event; IRR, Infusion related reaction; IV, intravenous; MI, myocardial infarction; n/a, not applicable; NCT, national clinical trial; ORR, overall response rate; OS, overall survival; PD, progressive disease; PD-L1, programmed cell death ligand 1; PE, pulmonary embolism; PFS, progression free survival; PR, partial response; PS, performance status; Q2wk, every two weeks; SD, stable disease; s/p, status post; TRAE, treatment related adverse event; TSH, thyroid stimulating hormone; UTI, urinary tract infection; VT, venous thrombosis.
Acknowledgments
Thank you to Margaret Hoogland, MLS, AHIP at The University of Toledo Library for assistance in the literature search.
Disclosure
The authors report no conflicts of interest in this work.
References
1. NCCN. NCCN clinical practice guidelines in oncology (NCCN guidelines) for bladder cancer; 2021. Available from: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
2. Resch I, Shariat SF, Gust KM. PD-1 and PD-L1 inhibitors after platinum-based chemotherapy or in first-line therapy in cisplatin-ineligible patients: dramatic improvement of prognosis and overall survival after decades of hopelessness in patients with metastatic urothelial cancer. Memo. 2018;11(1):43–46. doi:10.1007/s12254-018-0396-y
3. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi:10.1200/JCO.2000.18.17.3068
4. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58.
5. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
6. ClinicalTrials.gov; 2021. Available from: https://clinicaltrials.gov.
7. Apolo AB, Ellerton JA, Infante JR, et al. Avelumab as second-line therapy for metastatic, platinum-treated urothelial carcinoma in the phase Ib JAVELIN solid tumor study: 2-year updated efficacy and safety analysis. J Immunother Cancer. 2020;8:e001246. doi:10.1136/jitc-2020-001246
8. Garje R, Ginader T, Laux DE, et al. A phase Ib study of combination of avelumab and taxane-based chemotherapy in platinum refractory or ineligible metastatic urothelial cancer (AVETAX study). J Clin Oncol. 2020;38(6_suppl):487. doi:10.1200/JCO.2020.38.6_suppl.487
9. Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–1230. doi:10.1056/NEJMoa2002788
10. Novakovic AM, Wilkins JJ, Dai H, et al. Changing body weight-based dosing to a flat dose for avelumab in metastatic Merkel cell and advanced urothelial carcinoma. Clin Pharmacol Ther. 2020;107(3):588–596. doi:10.1002/cpt.1645
11. Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:278. doi:10.1186/s40425-019-0768-9
12. U.S. Food and Drug Administration. FDA approves avelumab for urothelial carcinoma maintenance treatment. Silver Spring (MD): U.S. Food and Drug Administration; 2020. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-avelumab-urothelial-carcinoma-maintenance-treatment.
13. Sonpavde G, Manitz J, Gao C, et al. Five-factor prognostic model for survival of post-platinum patients with metastatic urothelial carcinoma receiving PD-L1 inhibitors. J Urol. 2020;204(6):1173–1179. doi:10.1097/JU.0000000000001199
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
