Back to Journals » International Journal of General Medicine » Volume 14
Clinical Evaluation of Active Tuberculosis-Related Deaths in Shenzhen, China: A Descriptive Study
Authors Zhang P , Xiong J , Zeng J, Zhan S, Chen T, Wang Y, Deng G
Received 10 November 2020
Accepted for publication 7 January 2021
Published 22 January 2021 Volume 2021:14 Pages 237—242
DOI https://doi.org/10.2147/IJGM.S291146
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Peize Zhang,1,* Juan Xiong,2,* Jianfeng Zeng,1,* Senlin Zhan,1 Tao Chen,1 Yuxiang Wang,1 Guofang Deng1
1Department of Pulmonary Medicine and Tuberculosis, The Third People’s Hospital of Shenzhen, Shenzhen, Guangdong, People’s Republic of China; 2School of Public Health, Health Science Center, Shenzhen University, Shenzhen, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guofang Deng
Department of Pulmonary Medicine and Tuberculosis, The Third People’s Hospital of Shenzhen, No. 29, Bulan Road, Longgang District, Shenzhen, Guangdong 518112, People’s Republic of China
Tel +86 135 300 27001
Fax +86 0755 6123 8928
Email [email protected]
Objective: The aim of this study was to assess active tuberculosis-related deaths in Shenzhen city of China to identify major causes of mortality in different age groups.
Patients and Methods: Medical records of mortality cases of patients with active TB diagnosed during 2013– 2018 were reviewed. All TB deaths were classified into two broad age groups (the young group: 18– 65 years old and the elderly group: > 65 years old). Causes of death were analyzed based on medical records.
Results: A total of 279 mortality cases of active TB were reviewed during the study period. Among them, mean age was 54.0± 20.5 years old; 80.6% (225/279) were male. There were 5.7% and 4.6% MDR/XDRTB patients in the young and elderly group. Newly treated TB accounted for 89.6% in the young group and 85.1% in the elderly group. Pulmonary TB was a major infection type in both groups (65.1% vs 77.0%). Advanced TB (23.4%) and HIV co-infection (20.8%) were the leading causes of deaths in the young group, but deaths in the elderly group were mostly associated with underlying diseases, including cardiovascular disease (52.9%), diabetes (33.3%), COPD (16.1%) and cancer (11.5%). Malnutrition was a significant condition in both groups (43.2% vs 35.6%). In terms of respiratory complications, bacterial infection was the leading comorbidity in both groups (27.1% vs 18.4%), followed by septic shock (18.2% vs 12.6%) and respiratory failure (12.0% vs 11.5%). There were no significant statistical differences between the two groups.
Conclusion: Our findings suggest that screening for HIV co-infection and early diagnosis of TB is vital in lowering TB-related deaths in young patients. Most deaths in elderly TB patients were caused by underlying health conditions or complications other than TB.
Keywords: active tuberculosis, TB-related death, tuberculosis complication, tuberculosis comorbidity, young death
Introduction
Tuberculosis (TB) is a pressing public health problem affecting millions of people globally and remains a major cause of mortality despite the availability of effective drugs and chemotherapy. World Health Organization (WHO) defines TB deaths as patients dying during TB treatment, irrespective of cause.1 The global mortality in HIV-negative TB was reported to be about 1.2 million in 2019. China is one of the high TB-burden countries. There were an estimated 833,000 TB cases, and an estimated 33,200 fatal cases in 2019.2
As a patient diagnosed of TB may not die directly from the disease itself, all-cause mortality was generally used for the description of TB-related deaths. Some studies had reported that human immunodeficiency virus (HIV) and multidrug-resistant and extensively drug-resistant TB (MDR/XDR-TB) attributed to risk factors for TB deaths.3–5 Other studies reported that malignant comorbidities and non-infectious diseases such as diabetes, renal failure, liver cirrhosis, cardiovascular disease were common risk factors of TB-related death.6–9 Aging was considered a natural hazard of mortality in many studies.10–12 It has been recognized that older people are more vulnerable to developing active tuberculosis and reactivation of latent tuberculosis. But comparison of causes of death in TB patients of different age groups was seldom made.
In this study, we analyzed all-cause mortality cases of active-TB patients in the only designated hospital for TB treatment in Shenzhen, China, to identify major causes of death in different age groups. We aim to alert clinicians and national Center of Disease Control the modifiable factors that may require enhancement to reduce TB-related mortality for different age groups.
Patients and Methods
Study Population
Shenzhen is one of the special administrative regions of China and is hosting a soaring population with high mobility due to its economic development and availability of job opportunities. The city’s population had grown from 10 million in 2013 to 13 million in 2019.13 The Third People’s hospital of Shenzhen is the only designated hospital for tuberculosis treatment in Shenzhen city. The medical records of 279 death cases of patients diagnosed of active tuberculosis over a 6-year period from January 1, 2013 to December 31, 2018 were retrieved. Sociodemographic characteristics including age, gender, living standard, poverty status, for example, homelessness were collected. Medical conditions were reviewed in the form of length of hospitalization, results of sputum smear, culture and drug sensitivity test (DST), comorbidities, and complications developed during the treatment period. The study was approved by the Ethic Committee of The Third People’s hospital of Shenzhen and requirement for informed consent of reviewing patient medical records was waived for public investigations related to infectious diseases under the National Key Project for Infectious Diseases. The hospital undertakes that in using these statistics, no personal information of any patients were involved and disclosed and confidentiality were compiled strictly. All data used in this study have been performed in accordance with the principles stated in the Declaration of Helsinki.
Study Design
To assess TB-related deaths in different age groups, patients were classified into two broad groups according to age. Those between 18 and 65 were classified as the young group and those >65 years as the elderly group. Sociodemographic characteristics, medical conditions and diagnosis during hospitalization were collected and compared between the two groups.
Statistics Analysis
Data analysis was conducted using the Statistical Analysis System (SAS 9.2 version, SAS Institute, Cary, NC. USA). Chi-square test was used to measure the difference between the two groups. P-values were reported to assess the statistical significance (p<0.05) of the difference.
Results
Gender and Age Distribution of the Study
Of the 279 TB death cases, 19.4% (54) were female, 80.6% (225) were male. The age distribution in the study ranged from 18 to 94 years. Mean age was 54.0 ± 20.5 years. The proportion of 18–20 years was 1.8%, the ratio of other age segments ranged from 12.1% to 15.8% (Figure 1).
 |
Figure 1 Age distribution of TB-related deaths in this study. |
Basic Characteristics and Type of TB in the Two Groups
There were 192 (68.8%) cases from the young group and 87 (31.2%) cases from the elderly group. Male accounted for 80.2% and 79.3% in these 2 groups (P = 0.862). Homelessness accounted for 7.8% in the young group and 0% in the elderly group. Median hospital stay was 8 days in both groups (P = 1.0). Moreover, 85.1–89.6% patients were newly diagnosed tuberculosis cases (P = 0.278). Only <6% MDR/XDR TB were observed in both groups (P = 0.9). As for the primary site of infection, miliary tuberculosis and tuberculous meningitis affected 23.4% and 8.0% (P = 0.002), while pulmonary tuberculosis affected 65.1% and 77% (P = 0.047) of the young group and elderly group, respectively (Table 1).
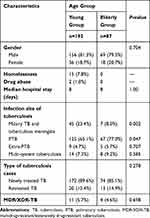 |
Table 1 Basic Characteristics of Patients in This Study |
Comorbidity Among Patients in Two Groups
Malnutrition was a widespread problem and represented for up to 35.6–43.2% in both groups, but no statistical difference was observed in the comparison. In the young group, HIV co-infection (20.8%) was the leading comorbidity, followed by cardiovascular disease (13.5%) and diabetes (12.5%). While in the elderly group, cardiovascular disease (52.9%), diabetes (33.3%), tumors, chronic liver and kidney failure (20%), COPD (13%) and cancer (11.5%) were the most commonly seen comorbidities. There was a significant statistical difference between both groups (Table 2).
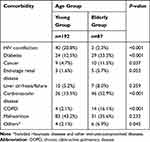 |
Table 2 Comorbidities Among Patients in This Study |
Respiratory Complications Among Patients in Two Groups
In terms of respiratory complications, bacterial infection was the most common in the young group and the elderly group (27.1% vs 18.4%), followed by septic shock (18.2% vs 12.6%), respiratory failure (12.0% vs 11.5%) and hemoptysis (6.8% vs 10.3%). But there were no significant statistical differences between the two groups (Table 3).
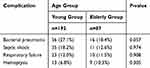 |
Table 3 Complications Among Patients in This Study |
Discussion
Tuberculosis is one of the leading causes of death among infectious disease worldwide. The World Health Organization (WHO) launched the “End TB Strategy” in 2014 aimed to reduce tuberculosis (TB) mortality by 95% (compared to 2015) by 2035.14 As an underlying cause of TB-related death varies, we analyzed the all-cause mortality in tuberculosis patients in our hospital to find out variance that constitutes fatality in different age groups. We hope the information can assist clinicians or policy makers in enhancing disease control programs to help reduce TB-related mortality.
WHO reported a higher global TB incident rate and mortality in male compared to female. Our study showed that most of the death cases were male patients (80.6%). This demonstrated a high consistency with reports from all over the world regarding gender distribution in TB mortality. But for age distribution, our study showed that a substantial proportion of TB deaths in Shenzhen was among people aged below 65 years. The mean age from our study was 54.0 years old, which was much younger than what was shown in other studies.6,8,15 People under 65 years old were quite actively participated in social activities, understanding the causes of their death may help dedicating appropriate intervention to improve treatment outcome.
MDR/XDR-TB has often been considered a refractory disease that made itself a leading cause of death for TB patients.16,17 Our study showed that only 5.4% of deceased TB patients, regardless of age, had MDR/XDR-TB, suggesting that MDR/XDR-TB had not been the leading cause of TB deaths in Shenzhen in the past 6 years. Therefore, we speculated that the cause of death for these patients may not be MDR/XDR-TB itself, but severe drug-susceptible tuberculosis, complications triggered by tuberculosis or worsening of an underlying disease.
In our study, there was a higher HIV co-infection proportion in the young group in comparison to the elderly group (20.8% vs 2.3%). And 23.4% of young patients suffered from miliary TB and central nervous TB, 43.2% were found to be malnourished on hospital admission, and 7.8% were homeless. It has been a widely recognized fact worldwide that HIV infection contributed to TB death to certain extent.8,10,18 And that incidence of TB, Hepatitis C and AIDS is correlated and often high among homeless people.19,20 HIV co-infection and sub-standard living environment can induce systematic and advanced tuberculosis, pulmonary bacterial or fungal co-infection, septic shock, respiratory failure, multi-organ dysfunction and severe malnutrition in these patients. Low income and education were also reported to be associated with treatment failure.17 An impaired immune state and vulnerable health conditions seen in the general homeless people can easily subject them to states of emergency at the time they have to seek medical help. Mortality rate of these patients being admitted to ICU with severe TB remains high.21–23 Even with active medical treatment, death was sometimes inevitable. As TB is a preventable and treatable infectious disease, early diagnosis and prompt treatment initiation are effective means to cure and reduce mortality. Even in patients with HIV co-infection, highly active antiretroviral therapy (HARRT) had been proved to be a life-saving method.4,24 Based on our findings, we believe enhanced social welfare support to needy TB patients with HIV co-infection may help improve treatment outcomes and reduce mortality.
We further analyzed the causes of death for patients over 65 years old. It had been reported that elderly people have an increased risk of low treatment adherence and consequent treatment failure.17 Also, unlike younger patients, more comorbidities were observed in this group of patients. More than half of the elderly had cardiovascular disease, one-third had diabetes, 20% had tumors, chronic liver and kidney failure, and 13% had COPD. Some of them had more than one of the above existing conditions. These chronic non-infectious diseases were the main causes of death among elderly TB patients. This basically is consistent with the findings of other research from around the world. Many advanced age TB patients did not die from TB, but from other underlying diseases. Only a few cases suggested TB being the direct cause of death.6,8,25 Malnutrition was also a common observation in elderly patients. But it was difficult to confirm if the cause is attributable to TB itself or other comorbidities.
Besides systematic tuberculosis, miliary tuberculosis and central nervous tuberculous, also often lead to the critical emergency. Our study showed that the major type of tuberculosis most patients developed was pulmonary tuberculosis. Respiratory complications were observed in many patients, with bacterial infection being a typical complication in patients with advanced tuberculosis and HIV co-infection. About one-tenth patients had respiratory failure, 6.8% younger age and 10.3% elderly patients in our study group have hemoptysis. It showed that bacterial superinfections aggravated advanced tuberculosis, prolonged hospital stays and increased the risk of death.26 Chronic aspergillus infection was prone to occur in patients with structural lung damage caused by tuberculosis and this at times led to fatal hemoptysis.27 In our study, the proportion of patients with retreated TB was 11.8%. Among them, some had structural lung damage and COPD, which rendered the patient to increased risk for co-infection, respiratory failure and hemoptysis. In patients with HIV co-infection and advanced severe tuberculosis, bacterial superinfection and septic shock and acute respiratory failure resulted in the worst life-threatening complications which needed intensive care. Therefore, early diagnosis of bacterial superinfection and antibiotics administration may help reduce mortality in the future.
There were some limitations in our study. First, the study was a cross-sectional study. It failed to analyze the treatment outcome of all tuberculosis patients, and it was impossible to obtain the mortality of each age groups. Second, all patients did not undergo autopsy therefor a definite pathological cause of death was unable to be determined. The direct causes of death were evaluated from medical records. Thirdly, due to the relatively high percentage of mobility population, some tuberculosis patients were diagnosed in hospital and then left the city and lost follow-up. The lack of these data may lead to a lower number of death cases recorded in our hospital, resulting in an underestimation of tuberculosis mortality.
Conclusion
In summary, among the TB deaths in Shenzhen city, most patients aged 65 years and above died from another underlying diseases unrelated to TB. But in the young death group, HIV co-infection and advanced TB were the leading causes of death, indicating light for lowering mortality from controlling the disease at an early stage. Screening for HIV co-infection and early diagnosis and appropriate management of TB, especially in vulnerable patients with sub-standard living conditions should be the major focus to lower TB-related deaths.
Acknowledgment
The authors are grateful for the continuous academic support from The National Clinical Research Center for Infectious Diseases of China.
Funding
This study was funded by the 13th Five-Year National Key Project for Infectious Diseases in China (2018ZX10715004-002-007, 2018ZX10715004-002-012) which is a government fund for the research of tuberculosis treatment and control. Juan Xiong was supported by the Medical Scientific Research Foundation of Guangdong Province of China (A2020405).
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization: Global Tuberculosis Programme. A Framework for Effective Tuberculosis Control. WHO/TB/94.179. Geneva, Switzerland: WHO; 1994.
2. World Health Organization: global tuberculosis report 2020. 2020.
3. Abouzeid MS, Al RF, Memish ZA. Mortality among tuberculosis patients in Saudi Arabia (2001–2010). Ann Saudi Med. 2013;33(3):247–252. doi:10.5144/0256-4947.2013.247
4. Zheng Z, Lin J, Lu Z, et al. Mortality risk in the population of HIV-positive individuals in Southern China: a cohort study. PLoS One. 2019;14(2):e0210856–e0210856. doi:10.1371/journal.pone.0210856
5. Henegar C, Behets F, Vanden Driessche K, et al. Mortality among tuberculosis patients in the Democratic Republic of Congo. Int J Tuberc Lung Dis. 2012;16(9):1199–1204. doi:10.5588/ijtld.11.0613
6. Aljadani R, Ahmed AE, Al-Jahdali H. Tuberculosis mortality and associated factors at King Abdulaziz Medical City Hospital. BMC Infect Dis. 2019;19(1):427. doi:10.1186/s12879-019-4063-7
7. Karthika M, Philip S, Prathibha MT, Varghese A, Rakesh PS. Why are people dying due to tuberculosis? A study from Alappuzha District, Kerala, India. Indian J Tuberc. 2019;66(4):443–447. doi:10.1016/j.ijtb.2018.05.001
8. Lin CH, Lin CJ, Kuo YW, et al. Tuberculosis mortality: patient characteristics and causes. BMC Infect Dis. 2014;14(1):5. doi:10.1186/1471-2334-14-5
9. Romanowski K, Baumann B, Basham CA, Ahmad Khan F, Fox GJ, Johnston JC. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2019;19(10):1129–1137. doi:10.1016/S1473-3099(19)30309-3
10. Pedrazzoli D, Kranzer K, Thomas HL, Lalor MK. Trends and risk factors for death and excess all-cause mortality among notified tuberculosis patients in the UK: an analysis of surveillance data. ERJ Open Res. 2019;5(4):4. doi:10.1183/23120541.00125-2019
11. Horne DJ, Hubbard R, Narita M, Exarchos A, Park DR, Goss CH. Factors associated with mortality in patients with tuberculosis. BMC Infect Dis. 2010;10(1):258. doi:10.1186/1471-2334-10-258
12. Di Gennaro F, Vittozzi P, Gualano G, et al. Active pulmonary tuberculosis in elderly patients: a 2016–2019 retrospective analysis from an Italian Referral Hospital. Antibiotics (Basel). 2020;9(8):8. doi:10.3390/antibiotics9080489
13. The number of permanent residents of Shenzhen at the end of year. Available from: http://tjj.sz.gov.cn/ztzl/zt/sjfb/.
14. WHO documentation for World Health Assembly 67.Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA67/A67_11-en.pdf.
15. Shen X, Deriemer K, Yuan Z, et al. Deaths among tuberculosis cases in Shanghai, China: who is at risk? BMC Infect Dis. 2009;9(1):95. doi:10.1186/1471-2334-9-95
16. Bhering M, Duarte R, Kritski A. Predictive factors for unfavourable treatment in MDR-TB and XDR-TB patients in Rio de Janeiro State, Brazil, 2000–2016. PLoS One. 2019;14(11):e0218299. doi:10.1371/journal.pone.0218299
17. Di Gennaro F, Pizzol D, Cebola B, et al. Social determinants of therapy failure and multi drug resistance among people with tuberculosis: a review. Tuberculosis (Edinb). 2017;103:44–51. doi:10.1016/j.tube.2017.01.002
18. Nliwasa M, MacPherson P, Gupta-Wright A, et al. High HIV and active tuberculosis prevalence and increased mortality risk in adults with symptoms of TB: a systematic review and meta-analyses. J Int AIDS Soc. 2018;21(7):e25162. doi:10.1002/jia2.25162
19. Beijer U, Wolf A, Fazel S. Prevalence of tuberculosis, hepatitis C virus, and HIV in homeless people: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(11):859–870. doi:10.1016/S1473-3099(12)70177-9
20. Gao J, Zheng P, Fu H. Prevalence of TB/HIV co-infection in countries except China: a systematic review and meta-analysis. PLoS One. 2013;8(5):e64915. doi:10.1371/journal.pone.0064915
21. Hagan G, Nathani N. Clinical review: tuberculosis on the intensive care unit. Crit Care. 2013;17(5):240. doi:10.1186/cc12760
22. Duro RP, Figueiredo Dias P, Ferreira AA, et al. Severe tuberculosis requiring intensive care: a descriptive analysis. Crit Care Res Pract. 2017;2017:9535463. doi:10.1155/2017/9535463
23. Deng W, Yu M, Ma H, et al. Predictors and outcome of patients with acute respiratory distress syndrome caused by miliary tuberculosis: a retrospective study in Chongqing, China. BMC Infect Dis. 2012;12(1):121. doi:10.1186/1471-2334-12-121
24. Onyango DO, Yuen CM, Cain KP, Ngari F, Masini EO, Borgdorff MW. Reduction of HIV-associated excess mortality by antiretroviral treatment among tuberculosis patients in Kenya. PLoS One. 2017;12(11):e0188235. doi:10.1371/journal.pone.0188235
25. Liu Y, Zheng Y, Chen J, et al. Tuberculosis-associated mortality and its risk factors in a district of Shanghai, China: a retrospective cohort study. Int J Tuberc Lung Dis. 2018;22(6):655–660. doi:10.5588/ijtld.17.0726
26. Kobashi Y, Matsushima T, Okimoto N, Hara Y. [Clinical evaluation of the cause of death in patients with active pulmonary tuberculosis]. Kekkaku: [Tuberculosis]. 2002;77(12):771–775. Japanese.
27. Barac A, Kosmidis C, Alastruey-Izquierdo A, Salzer HJF. Chronic pulmonary aspergillosis update: a year in review. Med Mycol. 2019;57(Supplement_2):S104–S109. doi:10.1093/mmy/myy070
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
