Back to Journals » International Journal of General Medicine » Volume 13
Clinical Evaluation for the Role of High-Sensitivity C-Reactive Protein in Combination with D-Dimer and Wells Score Probability Test to Predict the Incidence of Deep Vein Thrombosis Among Cancer Patients
Authors Setiawan B , Rosalina R , Pangarsa EA , Santosa D , Suharti C
Received 8 May 2020
Accepted for publication 7 August 2020
Published 9 September 2020 Volume 2020:13 Pages 587—594
DOI https://doi.org/10.2147/IJGM.S261718
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Budi Setiawan,1 Rosalina Rosalina,2 Eko Adhi Pangarsa,1 Damai Santosa,1 Catharina Suharti1
1Division of Hematology-Medical Oncology, Department of Internal Medicine, Faculty of Medicine, Diponegoro University/Dr. Kariadi Hospital, Semarang, Indonesia; 2Department of Internal Medicine, Faculty of Medicine, Diponegoro University/Dr. Kariadi Hospital, Semarang, Indonesia
Correspondence: Damai Santosa
Division of Hematology-Medical Oncology, Department of Internal Medicine, Faculty of Medicine, Diponegoro University/Dr. Kariadi Hospital, Semarang, Indonesia
Fax +622 48453201
Email [email protected]
Background: Deep vein thrombosis (DVT) is a frequent complication in cancer patients and is the second leading cause of death. The high level of C-reactive protein (CRP) is an acute phase reactant that induces tissue factor (TF) expression in monocytes, smooth muscle cells, and endothelial cells. The CRP level positively correlates with the incidence, extension, and volume of thrombus. TF expression triggers the coagulation system including the formation of thrombin and circulating fibrin such as prothrombin fragment 1+2 (F1 + 2) and D-dimer.
Objective: To determine the diagnostic value of high-sensitivity (hs)-CRP, D-dimer, and Wells score combination to predict the incidence of DVT on clinically suspected DVT (Wells score ≥ 2) cancer patients.
Subjects and Methods: This study was a cross-sectional study on a diagnostic test to determine the diagnostic value of hs-CRP and D-dimer for early detection of DVT on clinically suspected DVT (Wells score ≥ 2) cancer patients. It was conducted in Dr. Kariadi Hospital, Semarang Indonesia on 35 subjects. The diagnosis of DVT was confirmed by color duplex sonography. The diagnostic accuracy of combination of hs-CRP, D-dimer, and Wells score was analyzed by logistic regression.
Results: DVT was confirmed in 10 subjects (28,6%). The cut-off point of hs-CRP levels for probable DVT was ≥ 51.05 mg/L and for D-dimer was ≥ 5030 μg/L. The median levels of both variables were higher in the subjects with DVT compared with the subjects without DVT, but it was not statistically significant. The combination of hs-CRP (≥ 51.05 mg/L), D-dimer (≥ 5030 μg/L), and Wells score ≥ 3 had the high accuracy (94.1%) to predict the incidence of DVT compared with hs-CRP (65.0%), D-dimer (54.7%), and combination of hs-CRP and D-dimer (71.0%).
Conclusion: The combination of hs-CRP (≥ 51.05 mg/L), D-dimer (≥ 5030 μg/L), and Wells score ≥ 3 can predict the incidence of DVT in cancer.
Keywords: hs-CRP, D-dimer, Wells score, deep vein thrombosis, cancer
Introduction
Venous thromboembolism (VTE) is a condition in which thrombus are forming in venous blood vessels, commonly found in legs and pelvis known as DVT. A thrombus may detach and circulate in blood vessels particularly to the pulmonary artery; such condition is known as pulmonary embolism (PE).1 Venous thromboembolism consists of DVT and PE.1–3
Cancer patients have 4 to 7 times higher risk of having VTE than those without cancer. Around 20–30% of DVT cases occur in cancer patients.4 Venous thromboembolism is the leading cause of death, morbidity, late and increase cost of treatment. The risk of death is also tripled in asymptomatic DVT.5,6
Cancer patients have exposure to pro-inflammatory stimuli in the cancer microenvironment,7 and chemotherapy causes endothelial cell damage which results in activating endothelial cells and eventually leads to inflammatory responses,8,9 increase coagulation activation among cancer patients.10,11 Inflammation on blood vessel wall initiates thrombus formation in the vein. Venous wall inflammation is very likely to be the initial cause of VTE formation. It has been confirmed by the study which established the relation between VTE and inflammation markers such as CRP, Interleukin 6 (IL-6), IL-8, and tumor necrosis factor-α (TNF-α).9,12,13
Pro-inflammatory cytokines play their part in promoting pro-coagulant status especially by inducing TF expression,12 which activates coagulant cascade to form thrombus marked by increased level of thrombin forming markers such as F1+2, thrombin-antithrombin complex (TAT), fibrinopeptide A (FpA) and D-dimer in the circulation.14 Nuclear factor kappa B (NF-κB) signaling in cancer patients also generates the production of adhesive molecules which cause thrombosis.15–17
IL-6 is a pro-inflammatory cytokine produced by various types of cells including inflammatory cells, keratinocytes, fibroblasts, and endothelial cells. Interleukin-6 is synthesized in the early phase of inflammation and induces several acute-phase proteins including CRP.18 A high level of CRP in venous thrombosis is a secondary acute-phase reaction and induces TF expression in monocytes, smooth muscle cells, and endothelial cells. CRP level positively correlates with the incidence of thrombosis, extension, and volume of thrombus. Some studies have shown a significant correlation between a high level of CRP and an extensive incidence of VTE. C-reactive protein level is an important part in assessing the risk of death and early complication of DVT.19
C-reactive protein has a molecular weight of 115 kDA. C-reactive protein has been assumed to act as an acute phase reactant and a neutralizing factor for endotoxin. C-reactive protein has been known to have a role in the opsonization of bacterial products. Earlier CRP assay methods were found to be less sensitive than a recent development of hs-CRP.20
D-dimer is a biomarker that globally indicates the activity of hemostasis and fibrinolysis. It is a degradation product of fibrin, produced by cross-linked fibrin degradation by plasmin-induced fibrinolytic activity. D-dimer is a very sensitive marker and has been used worldwide to predict a VTE event.21
This study is aimed to determine the hs-CRP, D-dimer, and Wells score diagnostic value for early detection of venous thrombosis on clinically suspected DVT cancer patients.
Methods
Study Setting
This study is a cross-sectional study to determine the combination of hs-CRP and D-dimer levels for early detection of DVT on clinically suspected DVT (Wells score ≥2) cancer patients. It was conducted in Dr. Kariadi Hospital, the university hospital of Diponegoro University, Semarang, Indonesia. Research proposal was approved by the Review Board of the Dr. Kariadi Hospital, No; 346/EC/FK-RSDK/2015. Written informed consent was obtained from the subjects. This study was conducted in accordance the Declaration of Helsinki.
Subjects and Data Collection
The study was conducted from April 2016 to March 2017, this study included 35 from the initial 49 eligible patients with cancer as subjects with various cancer stages. A total of 14 patients were excluded and diagram of patient’s selection and inclusion are shown in Figure 1. Prior to the study, the researchers explained regarding the goal, examination procedures, and the expected positive outcomes of the study to the respondents. The inclusion criteria for the study were (i) definitive cancer diagnosis from the pathology anatomy result (ii) aged >40 years old (iii) Wells Score value ≥2 (iv) willing to participate and (v) give written consent. Exclusion criteria were subjects who have to undergo surgery, pregnant, suffer from acute infections, sepsis, have antithrombotic therapy, and history of disturbed coagulation processes such as hemophilia, thrombocytopenia (<50.000/ul), antiphospholipid syndrome and von Willebrand’s disease.
 |
Figure 1 Diagram of patients selection and inclusion. |
Subjects were interviewed regarding their medical history, tumor site, and tumor histology. Wells score was determined by anamnesis and physical examination. A blood sample was drawn to determine the laboratory parameters.
Diagnosis of Deep Vein Thrombosis
Color duplex sonography was performed at the Radiology Division of Dr. Kariadi Hospital, Semarang, Indonesia. The equipment used was Siemens sonography machine, Sonoline Omnia, serial number FBE 0322, model number GM-6801A2E00, made in Japan for Siemens Medical System, Inc. Ultrasonography Group, Issaquah, WA 98,029–7002 USA Part No. 5,931,030, distributed by Siemens Medical System, Issaquah, USA using a linear multi-frequency 7.5 MHz transducers.
Color duplex ultrasonography was performed to subjects with clinically suspected DVT and the probability DVT pretest (Wells score) ≥2. To avoid investigator-related variations of the results, color duplex sonography was performed in each patient by the same investigator. Diagnosis of DVT was established when the patient presented with symptoms of DVT and positive findings in color duplex sonography.
Blood Sampling and Laboratory Analysis
Complete blood count was measured by an automatic flow cytometry technique with automated hematology analyzer Sysmex XN 1000 (Sysmex Corporation, Japan). Plasma prothrombin time, activated partial thromboplastin time, fibrinogen, and D-dimer levels were measured by the turbidimetry chromogenic immunoassay method with automated blood coagulation analyzer Sysmex CS-2100i (Sysmex Corporation, Japan). Hs-CRP levels were measured using antihuman CRP mouse monoclonal antibody-coated latex (manufacturer Sekisui Medical Co., LTD, Japan) following the manufacturer’s instruction. D-dimer levels were measured using INNOVANCE D-dimer.
Statistical Analysis
To determine the diagnosis value of a combination of hs-CRP and D-dimer levels for early detection of DVT on cancer patients with Wells score ≥2 compared to color duplex sonography as a gold standard for DVT diagnosis. Prior to analysis, data were tabulated and processed. The data analysis consisted of a descriptive and hypothesis analysis. In the descriptive analysis, the data with categorical scales were written in frequency distributions and percentages. We used receiver operating characteristic (ROC) curve and descriptive analysis for hs-CRP, D-dimer levels and Wells score to find the best cut-off to predict DVT. Logistic regression is used to describe data and to explain the relationship between all of the three variables. We determine the accuracy of this combined variable to predict the incidence of DVT with ROC analysis.
Results
During the study period, 35 cancer patients diagnosed with Wells score ≥2 were enrolled in the study. DVT occurred in 10 (28.5%) subjects while the number of subjects without DVT was 25 (71.4%). Positive findings in color duplex sonography established the diagnosis of DVT. The most frequent type of cancer was lung cancer (34.2%), Wells score ≥2 was 51.4%, and D-dimer of all subjects was increased at >500 µg/L (100%) (Table 1). Table 2 shows various laboratory data, including hs-CRP and D-dimer, with their respective mean, median and 25th and 75th percentile value. In this study, the hs-CRP level in cancer patients is higher than the normal reference range (0–3.0 mg/L).
 |
Table 1 Clinical and Laboratory Characteristics of Subjects (n=35) |
 |
Table 2 Mean, Median, and Percentile Characteristics of Subjects |
Plasma Concentrations of hs-CRP and D-Dimer in Cancer Patients with and without DVT
All of our hs-CRP and D-dimer samples value were above the upper normal limit. The median of the hs-CRP level was 54.30 mg/L (min-max 3.30–301.00), the lowest level was 3.30 mg/L and the highest level was 301.00 mg/L. The median of hs-CRP with DVT was 62.90 mg/L (min-max 17.10–301.00). The median of hs-CRP without DVT was 46.25 mg/L (min-max 3.30–301.00) (Table 3). The cut-off value of hs-CRP was 51.05 mg/L, which was determined from ROC analysis. The median of D-dimer level was 5030 µg/L (min-mas 920–5030), for the 25th and 75th percentiles of 2850 µg/L and 5030 µg/L, respectively. We used 5030 µg/L as the best cut-off point to predict DVT incidence.
 |
Table 3 Median of hs-CRP and D-Dimer in Cancer Patients with DVT and Without DVT |
In the present study, the diagnostic test showed that the sensitivity, specificity, negative, and positive predictive value of the hs-CRP examination alone with a cut-off point ≥51.05 mg/L were 80%, 60%, 88%, and 44%, respectively. When we use D-dimer with cut-off point ≥5030 µg/L alone, the sensitivity, specificity, negative, and positive predictive value were 54.5%, 50%, 29.4%, and 33.3%, respectively.
By using a new cut-off value of 51.05 mg/dL, high hs-CRP portend a 6-fold increase in risk for DVT (odds ratio, OR 6.3, 95% CI 1.11–35.67, p=0.037). To determine accuracy of the combination to predict the incidence of DVT, ROC analysis was used, with prior logistic regression analysis to explain the relationship between 3 variables (hs-CRP, D-dimer, and Wells score). The area under the ROC curve (AUC) for hs-CRP levels alone for DVT diagnosis was 0.65 (95% CI 0.46–0.83) and D-dimer levels alone was 0.547 (95% CI 0.35–0.75). When combined (hs-CRP, D-dimer, and Wells Score ≥2), the AUC was 0.71 (95% CI 0.51–0.91). The best accuracy was found in the combination of hs-CRP, D-dimer, and Wells Score ≥3, the AUC was 0.941 (95% CI 0.86–1.00). The ROC curve showing the AUC of three composite index (hs-CRP, D-dimer and Wells score probability test) are shown in Figure 2. Table 4 shows comparison of the combinations of the variables combinations to predict the incidence of DVT.
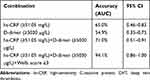 |
Table 4 Accuracy of the Combination of Variables in Predicting DVT Incidence |
 |
Figure 2 ROC curve of logistic regression (hs-CRP, D-dimer, and wells score ≥3) to DVT incidence. |
Discussion
In the present study, the diagnostic value of the hs-CRP or D-dimer examinations when used alone with cut-off points ≥51.05 mg/L and ≥5030 µg/L in clinically suspected DVT cancer patients were quite disappointing. A combination of these two variables has increased the accuracy to 71% (CI 0.51–0.91), but with the addition of Wells score ≥3, the accuracy has been increased to 94.1% (CI 0.86–1.00). These conclude that the combination of hs-CRP (≥51.05 mg/L), D-dimer (≥5030 µg/L) and Wells score ≥3 could effectively predict DVT incidence on clinically suspected DVT cancer patients.
In this study, it was found that the median of both hs-CRP and D-dimer level in cancer patients was high, both in subjects with DVT and without DVT but it was not statistically significant (p=0.160 and p=0.619, respectively). The median of both hs-CRP and D-dimer level was higher in cancer patients with DVT although it was not statistically significant. This suggests that the immune and inflammatory systems play an important role in the pathogenesis of VTE.11 Cancer and chemotherapy can cause inflammatory conditions,8 which trigger the NF-κB signaling pathway to produce pro-inflammatory cytokines.9 The role of pro-inflammatory cytokines such as CRP and IL-6 promote procoagulant status by inducing TF.12 TF expression triggers a coagulation system that is characterized by increased levels of biomarkers in the formation of thrombin and circulating fibrin such as F1 + 2 and D-dimer.14,22
Among proinflammatory cytokines, IL-6 has an important role in inducing coagulation.15 C-reactive protein genes transcriptional induction mainly occurs in the liver’s hepatocyte as a response to the rising level of inflammatory cytokine, especially IL-6.18 Plasma concentrations of IL-6 are increased in subjects with recurrent venous thrombosis. Increased levels of IL-6 are significantly associated with thrombus expansion. Elevated IL-6 levels are also associated with a high risk of first venous thromboembolism. CRP levels are directly proportional to the incidence of thrombosis, thrombus extension, and volume. Several studies have shown a significant correlation between high CRP levels and the incidence of DVT.19
The most frequent type of cancer was lung cancer (34.2%), this is consistent with previous research in which the primary location of the cancer is the strongest risk factor for DVT. Based on the VTE risk assessment scores in subjects with cancer (Khorana score) the very high risk of DVT is pancreatic cancer and gastric cancer, while the high risk of DVT among other is lung cancer, lymphoma, gynecological, bladder, and testicular cancer.23
D-dimer levels in this study were all above normal (>500 µg/L). D-dimer, a global indicator of coagulation and fibrinolysis activation, is a fibrin degradation product which usually increases in acute thrombosis. There is a significant relationship between cancer development and coagulation system activation. Some studies have shown that D-dimer is related to VTE risk in cancer patients. Similarly, Vienna Cancer and Thrombosis Study (CATS) confirms that D-dimer is a vital biomarker in predicting VTE among cancer patients. D-dimer has been validated as a predictive parameter in CATS patient independent cohort research.24,25
Increased hs-CRP value has been seen in many diseases, including infectious disease, diabetes, autoimmune disorders, arthritis, cardiovascular disease, and cancers.26 Elevated hs-CRP mostly found in the lung, colorectal, breast, and ovarian cancers.27 Lee et al have shown that among 729 cancer patients with elevated hs-CRP levels, most of them are adenocarcinomas (83.4%), followed by squamous cell carcinomas (2.5%), and others (14.1%).26
This study found that hs-CRP and D-dimer alone could not be an effective additional diagnostic tool to help diagnosing DVT in clinically suspected one in cancer patients. When hs-CRP, D-dimer and Wells score combined together, these examinations could effectively predict the incidence of DVT with accuracy of 94.1% on clinically suspected DVT cancer patients.
The limitation of this study is the absence of regular color duplex sonography to the clinically suspected DVT (Wells score ≥2) cancer patients, as thrombus may turn positive in the repeated examination. Another limitation of this study is the small sample size. Further research with improvement of the above factors may be needed to obtain more accurate results.
Conclusion
In conclusion, our study demonstrated that (i) Combination of hs-CRP (≥51.05 mg/L), D-dimer (≥5030 µg/L), and Wells score ≥3 could effectively predict the incidence of DVT in clinically suspected DVT cancer patients (ii) hs-CRP and D-dimer plasma levels were all elevated in cancer patients and should not be used alone in predicting the incidence of DVT (iii) the median levels of hs-CRP and D-dimer were higher in subjects with DVT although it was not statistically significant. Further research is needed to understand the role of immune and inflammatory systems in the pathogenesis of VTE.
Acknowledgments
We sincerely thank Mika Lumban Tobing, MD and Suyono MD, from the Department of Internal Medicine, Dr. Kariadi Hospital, Diponegoro University, Semarang, Indonesia for the data support. We would also thank Darminto, MD from the Clinical Epidemiology Unit of Diponegoro University for the statistical support. Thanks to Ridho M Naibaho, MD; Daniel Rizky, MD; and Ridwan Angkasa. MD for finalizing the manuscript.
Disclosure
Part of the data in this study were taken from the Identia Registry that has been published by K. L. Tambunan et al in Acta Medica Indonesiana in January 2020. All authors declared that they have no potential conflicts of interest for this work.
References
1. NICE, National Institute for Health and Care Excellence. [Internet]. Venous thromboembolism in adults: diagnosis and management. 2013. Available from: https://www.nice.org.uk/guidance/qs29/resources/venous-thromboembolism-in-adults-diagnosis-and-management-pdf-2098554175429.
2. Wilbur J, Shian B. Diagnosis of deep venous thrombosis and pulmonary embolism. Am Fam Physician. 2012;86(10):913–919.
3. Konstantinides SV, Torbicki A. Management of pulmonary embolism: recent evidence and the new European guidelines. Eur Respir J. 2014;44(6):1385–1390. doi:10.1183/09031936.00180414
4. Elyamany G, Alzahrani AM, Bukhary E. Cancer-associated thrombosis: an overview. Clin Med Insights Oncol. 2014;8:129–137. doi:10.4137/CMO.S18991
5. Kalayci A, Gibson CM, Chi G, et al. Asymptomatic deep vein thrombosis is associated with an increased risk of death: insights from the APEX trial. Thromb Haemost. 2018;118(12): 2046–2052.
6. Vaitkus PT, Leizorovicz A, Cohen AT, et al. Mortality rates and risk factors for asymptomatic deep vein thrombosis in medical subjects. Thromb Haemost. 2005;93:76–79.
7. Karin M. NF-KB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2773649/pdf/cshperspect-NFK-a000141.pdf.
8. Boccaccio C, Paolo M. Comoglio. Oncogenesis, cancer, and hemostasis. In: Khorana AA, Francis CW, editors. Cancer-Associated Thrombosis. New York: Informa Healthcare USA, Inc; 2008:1–15.
9. Chen L, Deng H, Cui H, Fang J, Zuo Z. Inflammatory responses and inflammation-associated diseases in organs. Ontarget. 2018;9(6):7204–7218.
10. Kirwan CC, Mccollum CN, Mcdowell G, Byrne GJ. Investigation of proposed mechanisms of chemotherapy-induced venous thromboembolism: endothelial cell activation and procoagulant release due to apoptosis. Clin Appl Thromb. 2015;2(5):420–427.
11. Budnik I, Brill A. Immune factors in deep vein thrombosis initiation. Trends Immunol. 2018;39(8):610–623. doi:10.1016/j.it.2018.04.010
12. Branchford BR, Carpenter SL. The role of in flammation in venous thromboembolism. Front Pediatr. 2018;6(May):142. doi: 10.3389/fped.2018.00142
13. Park MH, Hong JT. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells. 2016;5:15.
14. Gorp ECM, Suharti C, Cate H, et al. Review: infectious diseases and coagulation disorders. J Infect Dis. 1999;180:176–186. doi:10.1086/314829
15. Saghazadeh A, Ha S, Rezaei N. Inflammation in venous thromboembolism: cause or consequence? Int Immunopharmacol. 2015;28:655–665. doi:10.1016/j.intimp.2015.07.044
16. Liu T, Zhang L, Joo D, Sun S. NF- κ B signaling in inflammation. Signal Transduct Target Ther. 2017;2:e17023.
17. Granger DN, Senchenkova E. Inflammation and the Microcirculation. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. [Internet]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK53380/#ch8.
18. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9(April):1–11. doi:10.3389/fimmu.2018.00754
19. Cocoi AF, Pop D, Cocoi M, Serban AM, Vida-simiti LA. Involvement of inflammatory markers in pathogenesis of venous thromboembolism. Rev Rom Med Lab. 2017;25(3):227–236.
20. Helal I, Zerelli L, Krid M, ElYounsi F, Maiz HB, Zouari B. Comparison of C-reactive protein and high-sensitivity C-reactive protein levels in patients on hemodialysis. Saudi J Kidney Dis Transpl. 2012;23(3):477–483.
21. Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci. 2014;6(10):491–499. doi:10.4103/1947-2714.143278
22. Haddad TC, Greeno EW. Chemotherapy-induced thrombosis. Thromb Res. 2006;118:555–568.
23. Thaler J, Ay C, Pabinger I. Venous thromboembolism in cancer patients – risk scores and recent randomized controlled trials. Thromb Haemost. 2012;16:1042–1048. Available at https://www.thieme-connect.com/products/ejournals/abstract/10.1160/TH12-04-0241.
24. Pabinger I, Thaler J, Ay C, Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood. 2013;122(12):2011–2018.
25. Schaefer JK, Jacobs B, Wakefield TW, Sood SL. New biomarkers and imaging approach for the diagnosis of deep venous thrombosis. Curr Opin Hematol. 2017;24(3):274–281.
26. Lee S, Choe J, Kim H, Sung J. High-sensitivity C-reactive protein and cancer. J Epidemiol. 2011;21(3):161–168. doi:10.2188/jea.JE20100128
27. Heikkila K, Harris R, Lowe G, Rumley A, Yarnell J, Gallacher J. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20:15–26. doi:10.1007/s10552-008-9212-z
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
