Back to Journals » Infection and Drug Resistance » Volume 15
Clinical Epidemiology of Sporotrichosis in Jilin Province, China (1990–2019): A Series of 4969 Cases
Authors Lv S, Hu X , Liu Z, Lin Y, Wu H, Li F
Received 23 December 2021
Accepted for publication 26 March 2022
Published 11 April 2022 Volume 2022:15 Pages 1753—1765
DOI https://doi.org/10.2147/IDR.S354380
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Sha Lv,1,* Xin Hu,1,2,* Zhe Liu,1 Yitong Lin,1 Hanfei Wu,3 Fuqiu Li1
1Department of Dermatology, the Second Hospital of Jilin University, Changchun, 130041, People’s Republic of China; 2Department of Microbiology, Graduate School of Medicine, Kyoto University, Kyoto, 606-8510, Japan; 3Department of General Surgery, The First Clinical Hospital of Academy of Science of TCM in Jilin Province, Changchun, 130041, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fuqiu Li, Department of Dermatology, the Second Hospital of Jilin University, 218 Ziqiang Street, Changchun, 130041, People’s Republic of China, Email [email protected]
Introduction: This study was aimed to examine the clinical and epidemiological characteristics of sporotrichosis in China and specifically Jilin Province, which is one of the areas with the highest incidence worldwide, and to provide data support for the global prevalence of sporotrichosis.
Methods: A total of 4969 cases of sporotrichosis diagnosed at the Second Hospital of Jilin University from January 1, 1990 to December 31, 2019 were collected.
Results: In Jilin Province, the male-to-female ratio was 1:2, the average age at onset was 48 ± 1 years, and the average disease duration was 4.8 ± 2.7 months. The most susceptible individuals were farmers. Cases occurred more commonly in the winter and spring (71.5%) than in the summer and autumn (28.5%). The fixed type infection was more prevalent. Among the cases, 64.8% showed typical mycological changes, and 77.6% showed atypical pathological changes. Regarding the epidemiological characteristics of sporotrichosis in China, 6565 cases were retrieved from the literature from January 1, 2010 to December 31, 2019. Among them, the most affected area was Jilin Province, followed by Heilongjiang Province, and Liaoning Province. The male-to-female ratio was 1:1.46. The fixed type infection was the most common. A total of 241 strains were identified by molecular biotechnology; among these, 217 were identified as Sporothrix globosa and 24 were identified as S. schenckii sensu stricto.
Discussion: The results add clarity to the clinical epidemiology of sporotrichosis in China and specifically Jilin Province. We believe these data will help improve the epidemiology knowledge of sporotrichosis worldwide.
Keywords: clinical epidemiology, sporotrichosis, Jilin Province, China
Introduction
Sporotrichosis is a subacute and chronic endemic disease caused by pathogenetic Sporothrix.1 It can infect the skin and subcutaneous tissues, mucous membranes, and lymphatic and visceral systems.2,3 The incidence of sporotrichosis in China is among the highest in the world,2,3 it has been realized that it mostly occurs in northeastern areas of China, such as Jilin Province, Liaoning Province, and Heilongjiang Province.4 However, sporotrichosis is not a notifiable disease in China. The fungus survives in the natural environment, such as in soil, dead wood, moss, hay and corn stalks, and animal manure.2,5 The fungus has also been isolated from aquatic animals like fishes and dolphins; thus, their primary ecological habitat remains unclear.6,7 Currently, S. globosa accounts for the most of Sporothrix spp. in China, accompanied by a small amount of S. schenckii s. str. Besides, sporotrichosis has various non-specific manifestations in clinical practice, including papules, nodules, erythema, ulcers, plaques, etc., leading to misdiagnosis. The modes of transmission of sporotrichosis vary, and it can be spread through direct contact, close contact between human and human/animal, transmission between donors, and laboratory transmission. The main route of transmission of sporotrichosis is through scratches, bites, and close contact with animals, particularly cats in Brazil, whereas in China and other countries, it is predominantly caused by contact with substances contaminated by Sporothrix spp.7 Across English and Chinese literature, more than 6000 sporotrichosis cases have been described in China; however, there may be many more undiagnosed or unreported cases. This study was aimed at reviewing the clinical and epidemiological aspects of all sporotrichosis cases diagnosed at the Second Hospital of Jilin University from January 1, 1990, to December 31, 2019, so as to provide data support for the global prevalence of sporotrichosis. This hospital is the central and biggest department of dermatology in Jilin Province. It was also aimed to collect the published literature describing sporotrichosis cases and the epidemiology of sporotrichosis in China from January 1, 2010 to December 31, 2019.
Methods
Patients
The suspected patients diagnosed with sporotrichosis were collected from the Pathology unit of Department of Dermatology at the Second Hospital of Jilin University, over three decades (from 1/1990 to 12/2019) and screened out only if the pathological results were consistent with those of fungus testing. Jilin Province is located in mid-northeastern China, which is one of the areas with the highest incidence of sporotrichosis worldwide. The inclusion criteria were 1) outpatients and inpatients of our hospital, 2) patients diagnosed based on clinical manifestation, positive mycological culture, and pathological changes, 3) patients with available records in our hospital, and 4) patients from Jilin Province.
This study was approved by the regional ethics committee of the Second Hospital of Jilin University. Written informed consent was obtained from patients or their guardians, and they agreed to publish accompanying images.
Data Collection
The following data were obtained for patients: sex, age, occupation, time of onset (seasons), area of onset, trigger, disease course, location and type of lesion, mycological culture, and histopathological examination.
These data revealed information on two aspects of the type of lesion. The first was about the clinical type of sporotrichosis, which could be classified as fixed form, lymphocutaneous form, and disseminated cutaneous form. The second was that the lesion morphology varied in several aspects, such as nodules, erythema, plaques, papules, verrucous hyperplasia, ulceration, granuloma, and number of lesions.
The samples obtained via skin biopsy were divided into two tissues: one was for histopathological examination by hematoxylin and eosin (H&E) staining and the other was for fungal culture. The samples from biopsy were incubated on Sabouraud’s dextrose agar (SDA) at 25°C for 2 weeks, followed by slide culture. The isolated strains were identified using microscopy with lactophenol cotton blue staining.
A literature search was performed across “CNKI,” “WANFANG,” “VIP,” “Web of Science,” and “PubMed” using the keywords “sporotrichosis” and “epidemiology” for studies published from January 2010 to December 2019. Case reports, case series, clinical trials, and retrospective studies about sporotrichosis in China, including both English and Chinese articles were included. Articles about feline or canine sporotrichosis were excluded. We recorded terms including gender of patients, age of patients, region of patients, number of cases, clinical types of cases, and fungal types.
Statistical Analysis
Statistical analysis was performed using SPSS 26.0. Descriptive statistics are presented as counts and proportions for demographic data. Between-group differences in clinical presentations of sporotrichosis and its related factors were evaluated using the chi-square test as appropriate for data. Additionally, crude odds ratios (COR), adjusted odds ratios (AOR), and 95% confidence intervals were also evaluated in this study. A model approach to logistic regression was built after determining the factors causing the fixed type infection. Each variable was fed into the logistic regression model individually, and then, crude odds ratios and 95% confidence intervals were determined. All these factors were fed cumulatively into the logistic regression model to establish interaction and allow for these factors to adjust for another factors. Adjusted odds ratio and 95% confidence interval were used as interval estimates of the measure of the effects of these factors on the fixed type infection. P < 0.05 was considered statistically significant.
Results
Patient and Demographic Data
A total of 5005 patients suspected of sporotrichosis at the Second Hospital of Jilin University between 1 January 1990 and 31 December 2019. Based on the inclusion criteria, 36 patients were excluded, among which 5 lacking of the information on lesion locations, 12 without home addresses, 5 without the description of clinical manifestations, 14 not from Jilin Province. Finally, a total of 4969 patients were included in this study. All patients were diagnosed in the outpatient department and received treatments. One patient was initially admitted to the hospital with a diagnosis of vasculitis as there were no explicit changes noted in the first histopathological examination and fungal examination; however, subsequent histopathology and mycological culture confirmed the diagnosis of sporotrichosis. The patient was discharged for outpatient treatment.
Among the patients identified, there were more females than males (male: female, 1:2). The age at onset ranged from 4 months to 92 years (median [inter-quartile range]: 54[43, 64]), of which 68.8% were aged >40 years and 6.7% were children aged <10 years. Regarding the occupation, farmers accounted for 79.3% of the cases, followed by primary and secondary school students. Cases occurred more commonly in the winter and spring (71.5%) than in the summer and autumn (28.5%; Table 1).
 |
Table 1 Demographic and Epidemiological Data of Human Sporotrichosis Cases (n = 4969) Diagnosed in Jilin Province, China During 1990–2019 |
All 4969 patients were from Jilin Province. The distribution in different areas of Jilin Province was as shown in Figure 1. The number of sporotrichosis patients slowly increased from 1990 to 2004, dramatically increased in 2005, steadily declined from 2010 to 2016, and dramatically increased again from 2017 to 2018 (Figure 2).
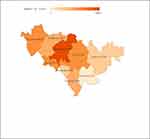 |
Figure 1 Distribution of sporotrichosis in Jilin Province, China, during1990–2019. The data from the Second Hospital of Jilin University diagnosed sporotrichosis, total 4969 cases. |
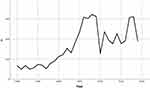 |
Figure 2 Changes in the visits of patients with sporotrichosis in Jilin Province, China, during 1990–2019. |
Clinical Presentation
Among these cases, 26.4% (1313/4969) had a clear history of trauma (including stab wounds from reeds, corn stalks, and wood). The disease course of 68.2% (3387/4969) individuals was primarily 2–6 months, with an average course of 4.8 ± 2.7 months. The cutaneous form of sporotrichosis includes three types: fixed, lymphocutaneous, and disseminated (Figure 3).
Fixed type infection was the most predominant type, accounting for 75.7% (3762/4969) of the cases, and the most affected site of infection was the face. Lymphocutaneous type infection accounted for 24.0% (1193/4969), mostly seen in limbs. Disseminated type infection accounted for only 0.34% (14/4969) of the cases. No case of disseminated infection with extracutaneous sporotrichosis was identified.
We found using the chi-square test that the lesion location significantly differed based on the clinical type (χ2 = 312.502, P < 0.05); however, no such differences were noted among different ages of onset (χ2 = 2.660, P = 0.850) or disease courses (χ2 = 2.0174, P = 0.3647; Table 2). In bivariate and multivariable analysis, facial lesions were strongly associated with incidence of fixed type (AOR 7.654, 95% CI: 4.297–13.632, P < 0.05), patients who had facial lesions were 7.654 times more likely to develop a fixed type infection than those who had lesions in trunk. In this study, there were no statistically significant associations between incidence of fixed type and course of disease, or age groups and other lesion location except face
 |
Table 2 Correlation of the Clinical Type with Age, Location, and Disease Course |
(P>0.05). (Table 3).
 |
Table 3 Bivariable and Multivariable Logistic Regression of Factors Influencing Sporotrichosis Types |
A single skin lesion was the most common observation (4330/4969, 87.1%), and among single lesions, nodules were the most prevalent (43.6%), followed by erythema (17.8%), plaques (11.4%), papules (11.3%), verrucous hyperplasia (1.4%), ulceration (1.2%), and granuloma (0.4%). Multiple lesions (639/4969, 12.9%) included two or more types of lesions.
Histopathology and Mycology
All 4969 cases underwent histopathological examination and mycological culture. Herein, 77.6% of diagnosed cases (3855/4969) were atypical pathological changes showing mixed-cell granuloma or nonspecific cellular inflammatory infiltration. The typical pathological manifestations accounted for 22.4% of lesions (1114/4969) and had the typical “three-layer inflammatory reaction”, wherein the lesion center is the purulent zone composed of neutrophils, which is surrounded by a caseous structure composed of tissue cells, multinucleated giant cells, and epithelial cells, and the outer layer is infiltrated by plasma cells and lymphocytes.
In addition, 64.8% (3219/4969) of mycological culture showed typical changes. It showed colonies with white yeast-like, moist and smooth surface, which gradually transitioned into brown-to-black wrinkled colonies within the first two weeks (Figure 4A). The samples were cultured on slides, and it was noted that the conidia stalks grew approximately perpendicular to the main hyphae, with plum-shaped conidia on the top, some of which were sleeve-like hyphae under the microscope (Figure 4B).
Chinese Epidemiological Characteristics
A literature search was performed across CNKI, WANFANG, VIP, Web of Science, and PubMed using the keywords “sporotrichosis” and “epidemiology” for studies published from January 2010 to December 2019. PRISMA guidelines were followed (Figure 5, 549 articles were discovered after duplicates removed by screening titles and abstracts of papers, then Studies were excluded: non-Chinese area, non-human infection, non-clinical origin, redundant content or irrelevant content, full-text articles assessed not in English or Chinese, then 115 articles were included in the study). The literature review identified 2928 cases through Chinese language databases and 3637 through English language databases, amounting to a total of 6565 cases. Among 5966 cases for which the data on the patient’s sex were available, 2429 were male, and 3537 were female, with a male-to-female ratio of 1:1.46. Among 4982 cases with known clinical type, 3465 were of the fixed type (69.5%), 1468 were of the lymphocutaneous type (29.4%), and 49 were of the skin disseminate type (1.0%). Regarding the distribution areas of sporotrichosis in China, Jilin Province had the highest number of reported cases, followed by Heilongjiang and Liaoning; 101 cases were reported in Chongqing, and 10–100 cases were reported in Beijing, Henan, Inner Mongolia, Xinjiang, Guangzhou, Jiangsu, and Shandong. (Table 4, Figure 6). A total of 241 strains were identified to the species level by molecular assays, 217 of which were identified as S. globosa, including 41 strains in Changchun, 37 in Chongqing, 21 in Liaoning, 19 in Sichuan, 15 in Jiangxi, 14 in Beijing, nine in Shanghai, nine in Shandong, seven in Hubei, six in Jiangsu, two in Guangzhou, two in Anhui, one in Inner Mongolia, and one in Hebei. The source area was unclear for 33 strains; 24 strains were identified as S. schenckii s. str, including 23 strains in Jiangxi and one in Hubei.
 |
Table 4 The Distribution of Sporotrichosis in China |
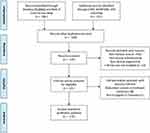 |
Figure 5 Flowchart of the literature search. |
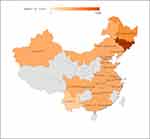 |
Figure 6 Distribution of sporotrichosis in China (2010–2019). The data are from the literature search depicted in Supplementary Table 1, including 17 studies in English and 98 in Chinese. |
Discussion
It is being increasingly recognized that prevention and control of sporotrichosis requires a “One Health” approach due to the interconnectedness among people, animals, plants, and their shared environment.8
Appropriate environmental conditions are essential for Sporothrix to survive at the proper temperature and humidity and on specific plants. Sporothrix grows in soils, plants, and contaminated organic matter at temperatures of 6°C–28.84°C and humidity of 37.5%–99.1%.2,9 Jilin Province is located in mid-northeastern China, has a dry and cold winter, a warm and humid summer. The annual average temperature is 6°C, and the average winter temperature is −15°C,4 which is not suitable for the survival of Sporothrix, particularly in winter. Nevertheless, the highest proportion of new cases of sporotrichosis are reported during winter. This may be because farmers burn a larger amount of straw during the cold season. The lower incidence rate in autumn may suggest that fresh corn stalks without rot are not a suitable living environment for the fungus. Notwithstanding, the ecological factors involved in Sporothrix survival need to be examined.
Floriculture, agriculture, mining, and woods exploitation are associated with sporotrichosis.2 Jilin Province is a nationally important commercial grain production base where various crops are cultivated. According to reports, the annual output of corn stalks from Jilin Province has increased from 2007 to 2014, and 58% of them are disposed of as household fuel.10 Jin et al11 isolated Sporothrix from corn stalks, suggesting that such a large amount of corn stalks as fuel would inevitably increase sporotrichosis infection. The middle of Jilin Province (Changchun, Jilin, Siping, Liaoyuan) accounts for 60% of the total corn straw use,12 and it also has a high proportion (3109, 62.6%) of sporotrichosis infections. In other areas of Jilin Province where corn stalks are used relatively less, the probability of exposure to stalks contaminated by Sporothrix is lower, thus translating into low proportion. Rice is the second most important crop in Jilin Province. Sporothrix can be isolated from rice straw as well; however, rice production in Jilin Province amounts to only a small proportion of the Chinese production. Taken together, high incidence of sporotrichosis in Jilin Province could be attributed to corn straw and its improper disposal.
Despite some initial fluctuation, patient visits for sporotrichosis showed an overall increase from 1990; this trend reflected the development of Chinese medical and health standards, and was also closely related to the improvement of personal health awareness. Upon investigating the ecological environment of Jilin Province, it was found that the annual average temperature has been increasing in the past 50 years. Furthermore, the climate change was found to be 0.299°C/10a, indicating that the annual average temperature of Jilin Province will increase by 0.299°C every 10 years,13 this rising trend in temperature creates more favorable conditions for Sporothrix survival. During 2006–2009 and 2017–2018, the number of cases showed small fluctuations; the number of cases in 2010–2016 and 2019 decreased compared with the previous period. There were some areas of floods in 2016, which may have increased the cases in 2017, and a large area of drought in 2018,14 which may be associated with the decline in cases in 2019. The risk may be higher in wetter days.15 Furthermore, besides ecological factors, the number of sporotrichosis cases may be associated with other factors, such as the increase in the number of hospitals that can be chosen, particularly with the improvements in the medical proficiency of primary hospitals.
The data indicated that there are considerably more female patients than male patients in Jilin Province as well as in China, probably because in an agricultural country, women were more exposed to corn stalks in the fields and in the house. In areas with more agricultural activities, such as, in consistent with the situation in India and Japan, the incidence is higher in women than in men.16,17 However, in South Africa, sporotrichosis is more likely to occur in men as it is an occupational hazard related to gold mining.18 In Uruguay, sporotrichosis has a higher incidence among male hunters.19
In this study, we collected demographic data, and to clarify the epidemiological characteristics, we further compared the variation in clinical epidemiology between 1990–2009 (n = 2836) and 2010–2019 (n = 2133). We found that the average age of onset had increased in recent decades (52 ± 1 years in 2010–2019; 45 ± 1 years in 1990–2009) probably due to faster urbanization, and the proportion of the left-behind elderly in Chinese rural areas has increased. The average disease course was shorter in 1990–2009 (4.2 ± 2.8 months) and longer in 2010–2019 (5.3 ± 2.5 months). This difference could be due to two factors. First, with the improved understanding and diagnosis of sporotrichosis in primary hospitals, many patients had taken oral antifungal drugs (itraconazole or terbinafine) before coming to our hospital; however, the treatment dose was insufficient and the treatment course was not standardized, thus resulting in longer treatment times. Second, the large-scale clinical application of broad-spectrum antifungal agents has resulted in the emergence of resistant strains. With the publication of clinical practice guidelines for sporotrichosis in China in 2016, the patient’s course of treatment has probably shortened with the administration of standard medications.20
The traditional classification of clinical manifestations includes cutaneous and extracutaneous sporotrichosis. The cutaneous forms include fixed, lymphocutaneous, and disseminated.3 The fixed cutaneous type was the predominant clinical type in Jilin Province and overall in China as well. Disseminated type infection accounted for only 0.34% (14/4969) of the cases in this study, characterized by scattered nodules and plaques of skin, with no immunosuppressive diseases. Conversely, in other countries, such as the United States and Europe, lymphocutaneous is the most common type. The prevalence of the extracutaneous type is higher Brazil and the United States, with relatively higher hospitalization and mortality rates.21 The differences in morbidity could be due to the virulence of pathogens, imbalance of immune response, and individual differences in the host immune response. Some studies believe that differences in morbidity may be related to the virulence of Sporothrix spp.5 The adaptive immune response mediates the imbalance of a Th1/Th17-based immune response after Sporothrix inoculation.22 Compared with the lymphocutaneous type, the fixed type with mild inflammatory infiltrates shows a more balanced immune response. Zhao et al23 argue that in sporotrichosis endemic areas, those who have obtained immunity after having been infected will systemically harbor future infections without letting the infection reach the lymph vessel; this may be a reason for the fixed cutaneous sporotrichosis accounting for a large proportion of infections in Jilin Province.
Different from China, lymphocutaneous type is the dominant of sporotrichosis in the high incidence area including Brazil, Mexico and Peru. Specifically, sporotrichosis is mainly caused by Sporotrichosis brasiliensis in Brazil,24 S. schenckii and S. globosa in Mexico,25 and S. schenckii in Peru.26,27 The onset age and gender were similar between Mexico and Peru. As reported, the males aged 0–15 years in Mexico and the males aged <14 years in Peru were mostly involved.25–27 However, sporotrichosis is more prevalent among the females aged 30–40 in Brazil.24 Among all the patients, 14.34% patients in Brazil and 3.43% patients in Mexico reportedly had extracutaneous sporotrichosis;24,25 however, no cases of extracutaneous sporotrichosis have been reported in China.
Yao et al28 analyzed the relationships among age, course of disease, site of onset and clinical type in 704 cases of sporotrichosis in children. The results showed that fixed clinical manifestations were more likely to occur at the age of 0–6 years; no correlation was identified between the lesion site and the clinical type or between the disease duration and the clinical type. We found a correlation between the site and the clinical type (p < 0.05) using the chi-square test. Furthermore, we found that having the lesion on the face was positively associated with the incidence of a fixed type infection (p < 0.05); this showed that the face lesion was more likely to be a fixed type infection of sporotrichosis. Attributing to the differences in disease characteristics between adults and children in this study, the results are different from those of previous analyses of pediatric patients.
Trauma is usually needed for fungus entry.2 Sharma et al29 reported 152 sporotrichosis cases, 48% of which had a history of trauma. Comparatively, among patients who visited our hospital, 26.4% reported a definite history of trauma (stab wounds by reed, corn stalk, or wood). In the clinic, it should be noted that a patient reporting a history of trauma does not suggest an indispensable condition for the diagnosis of sporotrichosis. With the low history of trauma in Jilin Province, the widespread infection may be due to the different transmission routes of Sporothrix spp. Even slight abrasions can cause an infection, and many farmers will ignore such minor trauma. Therefore, the real trauma injury rate is probably higher than what is suggested in our findings.
Currently, the diagnosis of sporotrichosis still relies on clinical manifestations, mycological cultures, and histopathologic examinations, with culture being the gold standard.2 Recently, the application of molecular biology techniques for diagnosing fungal infections has increased, thus allowing for rapid diagnosis and molecular typing. We selected 301 sporotrichosis strains diagnosed in the clinic and explicitly identified the species as S. globosa in recent research.30 In this study, molecular identification of 241 strains into species has been done. Among these strains, 217 strains were identified as S. globosa and 24 as S. schenckii s. str. were from Jiangxi Province and Hubei Province based on molecular biology techniques.31–33 With the development of molecular biology, the identification of strains is of great significance to obtain detailed molecular epidemiological information.
The key innovation of this study included: (1) It has been the largest series of sporotrichosis reports in China so far; (2) It was the first time to collect the cases of sporotrichosis reported in China in recent ten years, which helped to provide data support for the epidemiological characteristics of sporotrichosis in China.
There were several limitations in the present study. First, it was a retrospective study and there might be errors in the medical records, which would result in potential mistakes in the data. Secondly, the clinical data of some of the included studies were incomplete. Finally, the data derived from the literature review may be affected by publication bias.
Conclusion
Sporotrichosis is prevalent in Jilin Province, China, and is closely related to improper disposal of corn stalks. The population in which it is prevalent comprises middle-aged individuals and elderly women. The susceptible individuals are farmers, and the epidemic usually happens in the winter and spring. The clinical manifestation of sporotrichosis is mostly of the fixed type. There is no obvious correlation among different clinical types, the course of disease progression, and the age of onset in Jilin Province. Nevertheless, a correlation could be seen between different invasion sites and clinical types, with the limbs more likely to show the lymphocutaneous type. Sporotrichosis lesions are more frequent as nodules. Sporothrix spp. in China mainly include S. globosa and S. schenckii s. str. The latter was found in Jiangxi and Hubei areas, whereas S. globosa was the causative organism in the remaining areas. This is the largest case series published from China and highlights current knowledge and gaps in knowledge of the epidemiology of sporotrichosis in China. Data could also be applied to the global epidemiology of sporotrichosis.
Disclosure
The authors declare that we do not have any commercial interest that represents a conflict of interest in connection with the work submitted.
References
1. Rodrigues AM, de Hoog GS, de Camargo ZP. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016;12:e1005638. doi:10.1371/journal.ppat.1005638
2. Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633–654. doi:10.1128/CMR.00007-11
3. Kauffman CA, Bustamante B, Chapman SW, Pappas PG. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:1255–1265. doi:10.1086/522765
4. Song Y, Li SS, Zhong SX, Liu YY, Yao L, Huo SS. Report of 457 sporotrichosis cases from Jilin province, northeast China, a serious endemic region. J Eur Acad Dermatol Venereol. 2013;27:313–318. doi:10.1111/j.1468-3083.2011.04389.x
5. Mahlberg MJ, Patel R, Rosenman K, Cheung W, Wang N, Sanchez M. Fixed cutaneous sporotrichosis. Dermatol Online J. 2009;15:5. doi:10.5070/D330M45342
6. Zhang Y, Hagen F, Stielow B, et al. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14 000 human and animal case reports. Persoonia. 2015;35:1–20. doi:10.3767/003158515X687416
7. Rodrigues AM, de Melo Teixeira M, de Hoog GS, et al. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl Trop Dis. 2013;7:e2281. doi:10.1371/journal.pntd.0002281
8. Etchecopaz AN, Lanza N, Toscanini MA, et al. Sporotrichosis caused by Sporothrix brasiliensis in Argentina: case report, molecular identification and in vitro susceptibility pattern to antifungal drugs. J Mycol Med. 2020;30:100908. doi:10.1016/j.mycmed.2019.100908
9. Ramírez-Soto MC, Aguilar-Ancori EG, Tirado-Sánchez A, Bonifaz A. Ecological determinants of sporotrichosis etiological agents. J Fungi. 2018;4(3): 95
10. Dang YH, Li KX. The current situation and suggestions of corn stalk utilization in Jilin Province. Agric Technol. 2015;35:148–150.
11. Jin XZ. Analysis of 142 cases of sporotrichosis. Chin J Dermatol. 1997;1997:46.
12. Wang MX. Status and suggestions of maize straw comprehensive utilization of Jilin Province. Heilonjiang Agric Sci. 2017;2017:105–108.
13. Xu C, Guo L, Duan JY. Changes in temperature and precipitation in Jilin Province. Rural Pract Technol. 2021;150-151.
14. Zhang J. Main agricultural meteorological disasters and service research measures in Jilin Province. South Agric Mach. 2020;51:82-83.
15. Sivagnanam S, Bannan AM, Chen SC, Ralph AP. Sporotrichosis (Sporothrix schenckii infection) in the New South Wales mid-north coast, 2000–2010. Med J Aust. 2012;196:588–590. doi:10.5694/mja11.10755
16. Fukushiro R. Epidemiology and ecology of sporotrichosis in Japan. Zentralbl Bakteriol Mikrobiol Hyg A. 1984;257:228–233.
17. Bhutia PY, Gurung S, Yegneswaran PP, et al. A case series and review of sporotrichosis in Sikkim. J Infect Dev Ctries. 2011;5:603–608. doi:10.3855/jidc.1305
18. Vismer HF, Hull PR. Prevalence, epidemiology and geographical distribution of Sporothrix schenckii infections in Gauteng, South Africa. Mycopathologia. 1997;137:137–143. doi:10.1023/A:1006830131173
19. Rodrigues AM, Bagagli E, de Camargo ZP, Bosco Sde M. Sporothrix schenckii sensu stricto isolated from soil in an armadillo’s burrow. Mycopathologia. 2014;177:199–206. doi:10.1007/s11046-014-9734-8
20. Li FQ, Wang AP, Ran YP, Li RY. Guidelines for the diagnosis and treatment of sporotrichosis. Chin J Dermatol. 2016;49:456–459.
21. Moreira JA, Freitas DF, Lamas CC. The impact of sporotrichosis in HIV-infected patients: a systematic review. Infection. 2015;43:267–276. doi:10.1007/s15010-015-0746-1
22. Conceição-Silva F, Morgado FN. Immunopathogenesis of human sporotrichosis: what we already know. J Fungi. 2018;4. doi:10.3390/jof4030089
23. Zhao B, Yang GL. Dermatology. Shanghai: Shanghai Medical University Press; 1992.
24. Rabello VBS, Almeida MA, Bernardes-Engemann AR, Almeida-Paes R, de Macedo PM, Zancopé-Oliveira RM. The historical burden of sporotrichosis in Brazil: a systematic review of cases reported from 1907 to 2020. Braz J Microbiol. 2022;53:231–244. doi:10.1007/s42770-021-00658-1
25. Toriello C, Brunner-Mendoza C, Ruiz-Baca E, Duarte-Escalante E, Pérez-Mejía A, Del Rocío Reyes-Montes M. Sporotrichosis in Mexico. Braz J Microbiol. 2021;52:49–62. doi:10.1007/s42770-020-00387-x
26. Ramírez Soto MC, Ratner AJ. Sporotrichosis: the story of an endemic region in Peru over 28 years (1985 to 2012). PLoS One. 2015;10:e0127924. doi:10.1371/journal.pone.0127924
27. Ramírez Soto MC. Is epidemic of sporotrichosis in Abancay, Peru, caused by zoonotic transmission of Sporothrix? Rev Iberoam Micol. 2016;33:256–258. doi:10.1016/j.riam.2016.03.006
28. Yao L, Song Y, Cui Y, et al. Pediatric sporotrichosis in Jilin Province of China (2010–2016): a retrospective study of 704 cases. J Pediatric Infect Dis Soc. 2020;9:342–348. doi:10.1093/jpids/piz052
29. Sharma R, Mahajan VK, Singh Chauhan P, Mehta KS, Sharma A, Sharma J. The clinico-epidemiological characteristics and therapeutic experience of 152 patients with cutaneous sporotrichosis: a 10-year retrospective study from India. Int J Dermatol. 2021;60:99–106. doi:10.1111/ijd.15299
30. Yan T, Li F, Chen F. Imbalance of Th1, Th17, and Treg cells in peripheral blood of patients with lymphocutaneous sporotrichosis: a comparative study. Eur J Dermatol. 2020;30:345–351. doi:10.1684/ejd.2020.3838
31. Jing DY. Phenotypic and molecular identification of Sporothrix 99 isolates of clinical origin. Chin J Dermatovenereol. 2015;39:231–234.
32. Li J, Zhan P, Jiang Q, et al. Prevalence and antifungal susceptibility of Sporothrix species in Jiangxi, central China. Med Mycol. 2019;57:954–961. doi:10.1093/mmy/myy163
33. Dong BL. “ITS/CAL” target molecular identification combined with phenotypic analysis to confirm a case of narrow-sense sporothrix schenckii-induced lymphangiogenic sporotrichosis. Chin Dermatol J. 2017;50:204–207.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.