Back to Journals » Chronic Wound Care Management and Research » Volume 2
Clinical efficacy of dressings for treatment of heavily exuding chronic wounds
Authors Wiegand C , Tittelbach J, Hipler U, Elsner P
Received 29 January 2015
Accepted for publication 13 April 2015
Published 10 June 2015 Volume 2015:2 Pages 101—111
DOI https://doi.org/10.2147/CWCMR.S60315
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Marco Romanelli
Cornelia Wiegand, Jörg Tittelbach, Uta-Christina Hipler, Peter Elsner
Department of Dermatology, University Hospital Jena, Jena, Germany
Abstract: The treatment of chronic ulcers is a complex issue and presents an increasing problem for caregivers everywhere. This is especially true in Germany, where more than 4 million chronic wounds are treated each year. Therapeutic decisions must be patient-centered and reflect wound etiology, localization, and healing status. The practice of using the same wound dressing during the entire healing period is no longer reasonable. Instead, multiple types of dressings may be needed for a single wound over its healing trajectory. Selection of the most appropriate dressing should be based on wound phase, depth, signs of infection, and level of exudate. Moisture balance is critical in wound care; dryness will hamper epithelial cell migration while excessive generation of fluid causes maceration at the wound margins. Hence, exudate management is a key issue in chronic wound therapy, particularly given that exudate from chronic wounds has a composition different from that of acute wound fluid. Several studies have shown that exudates from non-healing wounds contain significantly elevated levels of protease activity, increased formation of free radicals, and abundant amounts of proinflammatory cytokines, while concentrations of growth factors and protease inhibitors are markedly decreased. Application of dressings that remove and sequester excess amounts of wound fluid may not only help in restoring the correct balance of moisture, but also support the wound healing process by preventing tissue deterioration caused by abundant protease activity. Several types of dressings, such as hydrogels, hydrocolloids, alginates, hydrofibers, foams, and superabsorbent dressings, are reviewed here and evaluated with regard to their efficacy for highly exuding wounds.
Keywords: chronic wounds, exuding, dressings, clinical efficacy
Introduction
More than 4 million chronic wounds are treated in Germany every year.1 These include venous ulcers, ischemic wounds (mainly of atherosclerotic origin) diabetic foot ulcers, and decubitus ulcers.2 This clearly indicates that most chronic wounds are the expression of an underlying physiological condition or systemic disease, such as chronic venous insufficiency, increased mechanical pressure, and vascular, nervous, or metabolic tissue damage.1 Malignancy, persistent infection, poor primary treatment, and immunologic disease might also delay wound healing.1,3 Hence, a thorough medical history and physical examination are essential to every patient evaluation.4 Therefore, the patient history should include: a description of how the wound occurred; any past history of wounds, including previous diagnoses and response to treatment; family history of chronic wounds and/or poor healing; any dermatologic condition that predisposes to ulceration; assessment of edema; consideration of pain; evaluation of systemic conditions that may predispose to wound development or poor healing, including human immunodeficiency virus/acquired immune deficiency syndrome, sickle cell anemia, Raynaud’s syndrome, rheumatologic disease, chemotherapy, anemia, weight loss, viral hepatitis, illicit drug use, transfusions, or neurologic disorders; previous hospitalizations and surgeries; and all systemic and topical medications used by the patient.4 Consequently, the treatment of chronic ulcers is complex. After thorough wound diagnostics, therapy of the underlying disease (eg, metabolic control of diabetes, prevention of chronic venous insufficiency, slowing the progression of atherosclerosis) and general systemic treatment (eg, anticoagulant treatment, systemic antibiotic therapy, anti-inflammatory therapy, treatment inhibiting immunologic reactions, vasodilator treatment, rheology-improving drugs, and protein, vitamin, and microelement supplements) have to be initiated before local therapy can be effective.2
Preparation of the wound bed
Therapeutic decision-making must be patient-centered, and treatment goals need to reflect this,5 such as achieving a clean wound for skin grafting, containing odor or exudates, and reducing pain to improve the patient’s social life, or maintaining a clean wound bed to place the patient in another setting to continue care. Of course, the clinician’s overall aim is wound healing and prevention of relapses. Therefore, local wound treatment includes adequate preparation of the wound bed to accelerate endogenous healing and/or to increase the efficacy of other therapeutic interventions. Supported by the European Wound Management Association, Schultz et al developed a strategy called TIME, which suggests methods for reinforcing the natural healing process while eliminating aggressive and proliferation-inhibiting activities.6 The acronym stands for Tissue management (wound cleansing), Infection or inflammation (reduction of infection/inflammation), Moisture imbalance (humidification), and Edge of the wound (epithelialization support). According to this concept, non-viable or deficient tissue needs to be debrided (by autolytic, surgical, enzymatic, mechanical, or biological means) to remove the defective matrix and cell debris that is impairing healing. This restores the wound base as well as functional extracellular matrix proteins and clinically leads to a viable wound base. Chronic wounds are characterized by prolonged inflammation and often high bacterial counts. The subsequent increase in inflammatory cytokines and abundant protease activity markedly reduces growth factor activity and tissue regeneration. Hence, infected foci have to be removed, and antimicrobials, anti-inflammatory agents, and protease inhibitors need to be administered to reduce bacterial counts and control inflammation. Moisture balance is critical in wound care, ie, while excessive fluid generation causes maceration at the wound margins, dryness will hamper the migration of epithelial cells. Application of moisture-balancing dressings, negative pressure wound therapy (NPWT), or other fluid removal methods, as well as compression, will control disproportionate release of exudate, avoid maceration, reduce edema, and prevent wound desiccation to enable optimal conditions for cell migration and proliferation. Clinically, the wound edge may be non-advancing or “undermined” as a result of non-responsive wound cells or abnormal protease activity. Debridement, skin grafting, biological agents, and other adjunctive treatment options have been suggested as corrective therapies to increase cellular migration and restoration of an appropriate protease profile. It is imperative that clinicians reassess wound status during dressing changes so that appropriate interventions can be implemented.5
Moist wound healing and exudate management
Wound exudate, which is essentially blood depleted of most of its red cells and platelets, is a key component in all stages of wound healing, irrigating the wound and keeping it moist, supplying nutrients, and providing favorable conditions for cell migration and proliferation.3 The wound tissue should be neither too dry nor too wet but physiologically humid.7 Since the work by Winter showing increased healing rates of wounds covered with occlusive dressings,8 the use of dressings that keep the wound moist has also been associated with improved cosmetic outcomes, less pain, lower infection rates, and decreased overall health care costs.7 If the wound tissues are adequately moist with minimal exudate production, then the applied dressing should maintain the tissue hydration status without too much absorption as this would desiccate the wound. Such moisture-retentive dressings retain moisture or have a low enough moisture vapor transmission rate (less than 35 g/m2/hour in partial thickness wounds), permitting moist wound healing.9 However, to clinically translate moist wound healing into practice remains difficult due to the lack of unified operational definitions.10 For instance, achieving or maintaining a moist environment does not mean that a wound should be covered in fluid. In certain conditions, such as venous leg ulcers or wounds associated with lymphedema, excessive amounts of exudate are present and may lead to complications, such as maceration of the surrounding skin, skin breakdown, wound enlargement, and increased pain.11,12 Increased exudate levels may further be the result of liquefying hard and eschar-like necrotic tissue producing a wet and sloughy mass.3
Exudate from chronic wounds has a composition that is considerably different from that of acute wound fluid. Several studies have shown that exudates from non-healing wounds contain significantly elevated levels of proteases, such as matrix metalloproteinases (MMPs) and polymorphonuclear (PMN) elastase.13–16 The continuous excessive production of proteases in chronic wounds and their persistently elevated activity lead to considerably reduced amounts of growth factors and proteinase inhibitors17 as well as successive degradation of extracellular matrix.18 So far, neutrophil-derived protease elastase and MMPs have received most of the attention in studies of chronic wounds. Acting in concert, they are capable of degrading every known constituent of soft connective tissue.19 MMPs mainly destroy extracellular matrix, and several studies showed that high levels of active MMP-9 are associated with lower wound closure rates.20–22 In addition, several MMPs are able to deactivate α1-protease inhibitor and α2-macroglobulin, which are two important inhibitors of the serine protease elastase. High MMP levels in wounds can therefore indirectly lead to increased amounts of this protease. Elastase, which mainly degrades elastin (a major constituent of elastic fibers), has been held responsible for degrading essential growth factors, such as platelet-derived growth factor and transforming growth factor-beta.15 In turn, elastase also leads to degradation of fibronectin, and the degradation products of fibronectin stimulate the release of MMPs.23,24 Effective management of exudate is therefore of crucial importance in chronic wound care. Application of dressings that remove and sequester excess amounts of wound fluid may not only help in restoring the right balance of moisture, but may also prevent the destruction of tissue by abundant protease activity to support the wound healing process.
The wound care market is consistently growing and new products seem to be introduced daily. Traditional classification into passive, inactive, and active dressings25 has therefore become difficult. Dressings can be categorized according to the materials used and important product groups according to the S3 guideline of the German Wound Healing Society as follows: pads (gauze/synthetic fibers), films, alginates, hydrogels, hydrocolloids, foams, microfibers, hydrofibers, and polyacrylates. Additionally, the guideline differentiates combinatory products (eg, foam dressings with hydrofibers).26
Products suitable for exuding wounds
There is no one dressing that is suitable for all wound types, so one of the most challenging aspects of wound care is choosing the right dressing, and that choice remains controversial. However, clear requirements for an optimal wound dressing exist, ie, it should: keep the wound moist; absorb excess exudate without leakage; eliminate dead space (a nidus for infection); protect against infection and external factors; provide optimal pH, thermoregulation, gas exchange, and humidity; cooperate with the wound healing process; avoid trauma and pain during dressing changes; minimize formation of scar tissue; have minimal toxicity to the surrounding skin/wound base and not be allergenic; be easy to use and comfortable for the patient; and have extended wear time, which directly translates into cost-effectiveness.2,27
Daily clinical routine shows that selection of dressing is based on local practice and empirical experience. That is not surprising, given that there are no large randomized controlled trials with definite conclusions (level A trials) for any type of dressing. Available systematic reviews on dressings for management of chronic wounds yield only weak levels of evidence for clinical efficacy,28 and no dressing can be recommended over another based on the results of these studies.1,26 In the absence of “hard” scientific evidence, selection of dressing should be guided by the type of wound, its appearance, the amount of exudate, the patient’s pain levels, and/or signs of infection.1,29
Dressings can be classified in different ways, according to their physical form (eg, gel, film, foam), chemical composition (eg, carboxymethylcellulose, alginate, collagen), material description (eg, hydrogel, hydrofiber, hydrocolloid), or function (debriding, antibacterial, absorbent). Unfortunately, these terms are often used interchangeably when it comes to sorting dressings into categories, eg, hydrogels and hydrocolloids (material description) are put alongside alginates (chemical composition), foams (physical form), and absorbent dressings (displaying function). However, it is difficult to avoid such pitfalls, given that some of these terms, eg, hydrogel, have become so popular that they are inseparable from daily practice. Who cares that most (but not all) of the hydrogels are polyacrylamide derivatives? Others, like hydrocolloids, may only be suitably rated according to their material description because their chemical composition would be too complex to describe and make them difficult to place. Where should they be put if one considers that they are a blend of polyurethane and swellable particles made from carboxymethylcellulose, pectin, or gelatin? All in all, different aspects and approaches have to be considered when classifying dressings. With regard to wound bed preparation and managing moisture imbalances, a suitable way would be sorting them according to their ability to manage exudate, employing the most commonly used and easiest product descriptions (Figure 1).
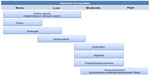  | Figure 1 Recommendations for types of wound dressing according to amount of exudate present. |
Gauze is often used in the clinical setting because it is inexpensive and easily accessible. However, there are also several limitations to its use, including its easy dehydration, its adherence, and often painful removal, as well as fiber shedding and poor barrier qualities.30,31 In the light of modern, active, and more suitable wound dressings, gauze is not recommended for management of chronic wounds. In these settings, films also may rather serve as secondary dressings than being placed directly on a chronic wound. Films are excellent for superficial lacerations and wounds producing small amounts of exudate like thin burn wounds, venous catheter sites, or split-thickness skin graft donor sites.32 However, fluid will accumulate underneath the film, and skin stripping could occur due to tight adherence and exertion of shearing forces.
Hydrogels consist of approximately 95% water inside a cross-linked hydrophilic polymer network comprised of polyacrylamides, polyethylene, polyvinyl alcohol, or others.32 Therefore, hydrogels are able to rehydrate a dry desiccated wound, promoting healing by creating a moist wound environment.5 These dressings are very comfortable to wear and provide a cool and soothing feeling to the patient accompanied by a certain amount of pain relief.4,32 However, although being slightly absorbent, use of hydrogels on wounds that are already highly moist may result in overhydration and cause maceration of the surrounding skin.
Hydrocolloids contain hydrophilic colloidal particles made from carboxymethyl cellulose, pectin, or gelatin in an adhesive polyurethane matrix.5 They provide an occlusive environment as well as absorption and maintain a moist milieu.4 It is thought that they promote debridement of slough and necrosis and can reduce pain through hydration of nerve endings, similar to hydrogels.27 Hydrocolloids are best used in low to moderately exuding wounds because large amounts of exudate may cause peri-wound maceration and off-floating of the dressing. An offensive odor, skin stripping,4,32 and allergic reactions12,33,34 have also been reported.
Whenever the wound is generating moderate to high levels of exudate, an absorbent dressing is required. Absorbent dressings include alginates, hydrofibers, foams, and hydropolymers, as well as superabsorbent materials, which have a high capacity to capture and hold fluid. They require fewer dressing changes within a set period compared with dressings that are not as absorbent, so enable undisturbed wound healing and less time spent on the part of the caregiver (eg, nurse or clinician).7
Alginates are derived from seaweed and can be manufactured into highly absorptive, fibrous dressings that can hold up 10–20 times their own dry weight and in and have hemostatic properties.35 They form a soft gel upon contact with wound fluid, thereby effectively filling dead space and maintaining a moist wound environment.35 Because alginates require moisture to function, they are not indicated for dry wounds or wounds covered with hard necrotic tissue unless they are first moistened with saline.4 Furthermore, care has to be taken to cut alginates into the shape of the wound bed and avoid overlaps with normal skin, as peri-wound maceration may occur because of the distribution of fluid over the entire surface of the alginate dressing (“lateral wicking”).4
Hydrofiber dressings are moisture retention dressings that consist of sodium carboxymethyl cellulose fibers. Like alginates, they gel on contact with wound fluid, which promotes a moist wound healing environment yet retains wound exudates by vertical absorption. This has been found to be beneficial for both caregivers and patients in terms of ease of application and removal, as well as reduction of pain at dressing changes.28,36
Foam dressings were introduced into clinical practice to facilitate maintenance of a moist wound environment and thermal insulation.4 These products offered important advantages over traditional gauze dressings because they did not shed particles/fibers or adhere to the wound bed.5 Foams can manage light to moderate amounts of exudate,4,12 and might even be recommended for highly exuding wounds if combined with certain hydropolymers.11,35 The latter are thought to be appropriate for wounds with excess slough and to support autolytic debridement.35 However, foam dressings cannot prevent surrounding skin maceration in heavily exuding wounds, and formation of a malodorous discharge has been reported.4
Polyacrylate-containing wound dressings, also known as superabsorbent polymer (SAP)-containing dressings or just superabsorbent dressings, have been shown to be particularly effective in the treatment of heavily exuding wounds.37–39 The majority of SAPs is of synthetic/petrochemical origin, and most commonly acrylic acid (and its sodium or potassium salts) and acrylamide are used as the starting monomers. There are also polysaccharide-based and poly(amino acid)-based SAPs available that have different properties when compared with synthetic SAPs.40 Other dressings feature hydrokinetic fibers produced from hydrophilic cellulose and sodium polyacrylate particles using a special mechanical process without bonding agents or adhesives.41 Some studies suggest that such dressings also enhance selective autolytic debridement by attracting and retaining proteins from necrotic tissue as well as toxins and bacteria.38
Direct and impartial comparison of ultimate performance of these dressing types (Table 1) in vivo is difficult. Not only are clinical studies and randomized controlled trials lacking, but trials are also complicated by the fact that no two patients are identical. Hence, standard tests for characterizing wound dressings are usually employed to determine properties and potential functioning. These tests include fluid handling properties, absorbency, moisture vapor permeability, fluid affinity, water uptake, and gelling properties.3 Absorbency (the dressing’s ability to absorb and retain wound fluid) and moisture vapor loss (evaporation of water through the outer dressing surface) are crucial mechanisms in the management of exudate. They are measured as the fluid handling capacity of a dressing. Exudate handling properties are also related to the gel-forming characteristics of alginate, hydrofiber, and hydrocolloid dressings.3 Studies showed that these might differ widely in a product group despite comparable chemical precursors.42,43 For instance, gelling of alginates depends on the ratio of homopolymeric M-regions (consisting of d-mannuronic acid) and G-blocks (formed by l-guluronic acid) in the polysaccharide.44,45 Although the tests for water vapor permeability, fluid affinity, and water uptake are a meaningful way of characterizing the different dressings, they are mainly based on the structure rather than the performance of the dressings. As a result, they are limited in their ability to predict the performance of a dressing in vivo.3 Recently, first efforts have been undertaken to visualize the fluid distribution in dressings with and without compression.46 For the “maceration test”, dressings were applied to an artificial wound in a tissue substitute comprised of 10% (w/v) gelatin and 10% (w/v) milk powder (Figure 2A). Evaluation of fluid uptake and distribution in the dressings was done by video recording. In addition, loss of shape of the dressings, maximal fluid uptake, and time to maceration could be determined. In this study, the alginate dressing showed less fluid uptake when compared with carboxymethyl cellulose and cellulose/cellulose-ethylsulfonate dressings (Figure 2B). Moreover, it was found that these dressings shrank during fluid uptake, while no loss of surface coverage was observed for the alginate (Figure 2C). The results are still limited to a small number of hydroactive dressings, but they may present a valuable tool for evaluation of dressing performance, fluid handling, and maceration risk under conditions mimicking the clinical situation more closely.
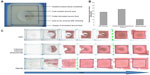  | Figure 2 Maceration model for determination of fluid management by hydroactive dressings (carboxymethyl cellulose [Aquacel Extra], cellulose/cellulose-ethylsulfonate [Suprasorb Liquacel], alginate [Suprasorb A tamponade] over time employing a tissue substitute model (A) and video documentation (VF0700, Creative Labs, Milpitas, CA, USA). Different fluid absorption capacities at the maceration breakpoint were observed for the dressings investigated (B). The spread of the colored, simulated wound fluid solution allows measurement of the breakpoint of maceration (green dotted line) at which the dressings stop taking up fluid and start to leak (C). Image courtesy of Cornelia Wiegand.46 |
Antimicrobial treatment
Infected wounds are commonly painful, display hypersensitivity, and produce odor, resulting in increased discomfort and inconvenience for the patient.47 Further, infected wounds are regularly associated with high exudate levels, increasing the number of dressing changes required, the amount of nursing time involved, and consequently the overall cost to the health care provider. In this regard, chronic wounds, which most readily feature a bioburden consisting of polymicrobial populations of bacteria and fungi, require vigilant monitoring for any signs of microbial progression and infection. If any of those signs are present, timely use of dressings with antimicrobial properties may be required, as well as effective exudate management. Dressings that contain and release antimicrobial agents at the wound surface have now entered the marketplace.7 These dressings usually provide continuous or sustained release of an antiseptic agent (silver, polyhexanide, iodine) at the wound surface to provide long-lasting antimicrobial action in combination with maintenance of a physiologically moist environment for healing. Superabsorbent dressings containing polyacrylates have been found to be beneficial in lowering the bacterial burden by efficiently trapping bacteria in their core structure and reducing the number of viable organisms with each dressing change.39,48–50 Hydrofiber or alginate dressings may allow similar (but probably considerably weaker) entrapment of bacteria and subsequent reduction of microbial progeny.51,52 These dressings are most commonly equipped with ionic or nanocrystalline silver as an antimicrobial agent, which considerably increases the antimicrobial activity of alginate dressings.51 Moreover, increased clinical efficacy was demonstrated for silver-containing alginate7,53,54 and silver-supplemented hydrofiber dressings.55,56 Similarly, foam dressings have been successfully fitted with silver.57
Correction of biochemical imbalance
Newer and more advanced dressings focus on more than just managing moisture levels in the wound environment; they aim to address specific biochemical imbalances commonly found in chronic, non-healing wounds. It is now widely acknowledged that chronic wounds contain high amounts of MMPs14,58 and elevated levels of PMN elastase.13,15,59 Unchecked activity of these proteases leads to substantially decreased amounts of growth factors60 and proteinase inhibitors, like tissue inhibitors of MMPs,61 shifting the balance of matrix degradation and synthesis toward tissue destruction. Hence, current concepts of wound management focus on reduction of these proteolytic enzymes. Active, protease-modulating wound dressings designed to address the biochemical imbalance of the chronic wound are commonly composed of collagen, collagen with oxidized regenerated cellulose, or, recently, a nano-oligosaccharide factor. Studies demonstrated that bovine collagen type I is able to bind considerable amounts of proteases in vitro and ex vivo.62 Further, it aids in establishing a more physiologic wound milieu, supporting leg ulcer healing in a clinical situation.16
Many studies have been published on other protease-modulating dressings consisting of collagen and oxidized regenerated cellulose, and lately also containing silver. Smeets at el demonstrated a significant reduction in protease activity after only 5 days of treatment with a collagen/oxidized regenerated cellulose matrix compared with a control group that received a hydrocolloid dressing.63 In addition, Gottrup et al observed a significantly increased healing rate using collagen/oxidized regenerated cellulose/silver dressings versus a standard of care control group, which they attributed to the ability of the dressing to rebalance the inflammatory milieu by reducing the elevated protease activity detrimental to wound healing.64 Others have used lipocolloid dressings incorporating nano-oligosaccharide factor, an oligosaccharide that decreases the activity of MMPs,65 and reported outcomes that were clinically superior to alternative treatments.65,66 It has been suggested that these active dressings (directly interacting with wound binding to and inactivating proteases) be reserved for individual cases due to their high costs,1 but other dressings that target exudates and remove proteases through sequestration might also effectively reduce the concentration and activity of proteolytic enzymes and aid healing. Polyacrylate-containing dressings were also shown to sequester and remove MMP and elastase activity in vitro.37,41,49,67,68 Eming et al further reported an indirect effect of SAP particles on MMP activity by binding of essential Ca2+ and Zn2+ ions.37 Protease sequestration capabilities could also been shown for alginates.51 Moreover, alginates and hydrofibers exhibit significant antioxidative capacities.51,69 Such a property is of significance to the wound healing process, because despite the beneficial role that reactive oxygen species play in killing invading microbial pathogens, excessive production of reactive oxygen species can be detrimental to host tissues.69
Negative pressure wound therapy
NPWT, also called controlled negative pressure, topical negative pressure, or vacuum-assisted closure therapy, has been advocated for virtually all acute and chronic wounds. The treatment is based on evenly distributed local negative pressure applied to the wound surface,70 which effectively removes excess exudate. Besides providing a moist wound environment, NPWT may also promote healing by increasing blood flow,71 reducing edema72 and wound area,73 as well as stimulation of formation of granulation tissue,74,75 cell proliferation,76 and angiogenesis.74–76 In addition, it was been proposed that NPWT influences the microenvironment of the wound by eradication of inflammatory proteases77 and decreasing bacterial burden.74 For application of NPWT, a wound dressing, mostly foam but also gauze, which could more correctly be designated as “wound filler”, is placed on the wound. In most systems, the wound filler is “black” foam (large-pored polyurethane foam), although polyvinyl alcohol (“white”) foam, saline-soaked gauze, or antimicrobial-impregnated gauze is also used. Several studies showed that differences in the quality of granulation tissue formed by gauze or foam exist, eg, wound bed tissue grows into the foam during NPWT and often more force is required to remove the foam from the wound when compared with gauze.78–80 In contrast, tissue damage caused by removal of gauze-based NPWT was reported to be less than 2% in a non-comparative series of 152 patients.81 On the other hand, gauze dressings produce lower levels of tissue microdeformation and an uneven distribution of pressure in the wound bed compared with open-cell polyurethane foams.82 Hence, they would lead to a smaller amount of granulation tissue due to smaller micromechanical forces.78 However, it is thought that these physical effects initiate signaling cascades that encourage cell proliferation.83
Conclusion
The customary practice of using the same wound dressing during the entire healing period is no longer reasonable. Instead, multiple types of dressing may be needed for a single wound over its healing trajectory.5 This concept requires selection of the most appropriate dressing with regard to patient needs, wound etiology and localization, economic considerations, and last but not least, wound status.1,5 The latter in particular should drive the choice of local wound product considering wound phase, depth, signs of infection, and the level of exudate. For example, a venous stasis ulcer producing a high amount of exudate will require a highly absorptive dressing. Patient preference will also actively influence the choice of dressing. It is the caregiver’s task to listen to a patient’s opinion and concerns as well as provide the patient with information on the best treatment to ensure compliance.
Several main types of dressings, including hydrogels, hydrocolloids, alginates, hydrofibers, foams, and superabsorbent dressings, have been summarized here and evaluated with regard to their efficacy for highly exuding wounds. Their ability to manage exudate increases from hydrogels to hydrocolloids, alginates, hydrofibers, and foams, to superabsorbent dressings containing polyacrylates (Figure 1). Hence, the latter seems most favorable for highly exuding wounds. While large randomized trials confirming the superiority of one dressing over another are still lacking, management of exudate remains a key point in chronic wound therapy, and one that has to be addressed daily by caregivers.
Acknowledgment
The authors thank Astrid Hoppe for fruitful discussions concerning the handling of wound dressings.
Disclosure
The authors have no conflicts of interest in this work.
References
Karl T. Hydroaktives stadienadaptiertes Wundmanagement [Hydroactive, stage-oriented wound management]. Unfallchirurg. 2012;115:783–791. German. | |
Skórkowska-Telichowska K, Czemplik M, Kulma A, Szopa J. The local treatment and available dressings designed for chronic wounds. J Am Acad Dermatol. 2013;68:e117–e126. | |
Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97:2892–2923. | |
Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 2008;58:185–206. | |
Baranoski S, Ayello EA. Wound dressings: an evolving art and science. Adv Skin Wound Care. 2012;25:81–92. | |
Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systemic approach to wound management. Wound Repair Regen. 2000;8:347–352. | |
Ovington LG. Advances in wound dressings. Clin Dermatol. 2007;25:33–38. | |
Winter GD. Formation of the scab and the rate of epithelisation of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293–294. | |
Bolton LL, Johnson CL, Van Rijswijk L. Occlusive dressings: therapeutic agents and effects on drug delivery. Clin Dermatol. 1991;9:573–583. | |
Bolton L. Operational definition of moist wound healing. J Wound Ostomy Continence Nurs. 2007;34:23–29. | |
Zoellner P, Kapp H, Smola H. A prospective, open-label study to assess the clinical performance of a foam dressing in the management of chronic wounds. Ostomy Wound Manag. 2006;52:2. | |
Diehm C, Lawall H. Evaluation of Tielle hydropolymer dressings in the management of chronic exuding wounds in primary care. Int Wound J. 2005;2:26–35. | |
Barrick B, Campell EJ, Owen CA. Leukocyte proteinases in wound healing: roles in physiologic and pathologic processes. Wound Repair Regen. 1999;7:410–422. | |
Trengrove NJ, Stacey MC, MacAuley S, et al. Analysis of acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7:442–452. | |
Yager DR, Nwomeh BC. The proteolytic environment of chronic wounds. Wound Repair Regen. 1999;7:433–441. | |
Wiegand C, Schönfelder U, Abel M, Ruth P, Kaatz M, Hipler UC. Protease and pro-inflammatory cytokine concentrations are elevated in chronic compared to acute wounds and can be modulated by collagen type I in vitro. Arch Dermatol Res. 2010;302:419–428. | |
Yager DR, Chen SM, Ward SI, Olutoye OO, Diegelman RF, Cohen IK. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen. 1997;5:23–32. | |
Whitney JD. Overview: acute and chronic wounds. Nurs Clin North Am. 2005;40:191–205. | |
Moor NA, Vachon DJ, Gould LJ. Proteolytic activity in wound fluids and tissue derived from chronic venous leg ulcers. Wound Repair Regen. 2009;17:832–839. | |
Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol. 2009;158:951–961. | |
Liu Y, Min D, Bolton T, et al. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabet Care. 2009;32:117–119. | |
Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen. 2002;10:26–37. | |
Grinnel F, Zhu M. Identification of neutrophil elastase as the protease in burn wound fluid responsible for degradation of fibronectin. J Invest Dermatol. 1994;103:155–161. | |
Grinnel F, Zhu M. Fibronectin degradation in chronic wounds depends on the relative levels of elastase, alpha1-proteinase inhibitor, and alpha2-macroglobulin. J Invest Dermatol. 1996;106:335–341. | |
Horn T. Lokale Wundauflagen: Übersicht und Klassifikation [Wound Dressings: Overview and Classification]. Chir Gastroenterol. 2006;22:147–154. German. | |
DGfW. S3-Leitlinie: Lokaltherapie chronischer Wunden in Patienten mit den Risiken periphere arterielle Verschlusskrankheit, Diabetis mellitus, chronische venöse Insuffizienz [S3 guideline: local therapy of chronic wounds in patients with arterial obstructive disease, diabetis mellitus, chronic venous insufficiency]. 2012. German. | |
Abdelrahman T, Newton H. Wound dressings: principles and practice. Surgery. 2011;29:491–495. | |
Chaby G, Senet P, Vaneau M, et al. Dressings for acute and chronic wounds – a systematic review. Arch Dermatol. 2007;143:1297–1304. | |
Braumann C, Guenther N, Menenakos C, et al. Clinical experiences derived from implementation of an easy to use concept for treatment of wound healing by secondary intention and guidance in selection of appropriate dressings. Int Wound J. 2011;8:253–260. | |
Hoekstra MJ, Hermans MH, Richters CD, Dutrieux RP. A histological comparison of acute inflammatory responses with a hydrofiber or tulle gauze dressing. J Wound Care. 2002;11:113–117. | |
White RJ. New developments in the use of dressings on surgical wounds. Br J Nurs. 2001;10:S70. | |
Gloeckner Powers J, Morton LM, Phillips TJ. Dressings for chronic wounds. Dermatol Ther. 2013;26:197–206. | |
Renner R, Simon JC, Treudler R. Contact sensitization to modern wound dressings in 70 patients with chronic leg ulcers. Dermatitis. 2013;24:60–63. | |
Sasseville D, Tennstedt D, Lachapelle JM. Allergic contact dermatitis from hydrocolloid dressings. Am J Contact Dermat. 1997;8:236–238. | |
Stojadinovic A, Carlson JW, Schultz GS, Davis TA, Elster EA. Topical advances in wound care. Gynecol Oncol. 2008;111(2 Suppl):S70–S80. | |
Barnea Y, Weiss J, Gur E. A review of the applications of the hydrofiber dressing with silver (Aquacel Ag) in wound care. Ther Clin Risk Manag. 2010;6:21–27. | |
Eming S, Smola H, Hartmann B, et al. The inhibition of matrix metalloproteinase activity in chronic wounds by a polyacrylate superabsorber. Biomaterials. 2008;29:2932–2940. | |
Paustian C, Stegman MR. Preparing the wound for healing: the effect of activated polyacrylate dressing on debridement. Ostomy Wound Manag. 2003;49:9. | |
Mahr R. The mode of action of a superabsorbent polymer wound dressing (TenderWet®). Ostomy Wound Manag. 2003;49:2A. | |
Zohuriaan-Mehr MJ, Kabiri K. Superabsorbent polymer materials: a review. Iran Polym J. 2008;17:451–477. | |
Wiegand C, Hipler UC. In vitro studies on the beneficial effect of a hydrokinetic fiber dressing on wound healing by reduction of protease activity. J Wound Care. 2013;22:592–598. | |
Waring MJ, Parsons D. Physico-chemical characterization of carboxymethylated spun cellulose fibres. Biomaterials. 2001;22:903–912. | |
Ferrari F, Bertoni M, Caramella C, Waring MJ. Comparative evaluation of hydrocolloid dressings by means of water uptake and swelling force measurements. Int J Pharm. 1994;112:29–36. | |
Thomas S. Alginate dressings in surgery and wound management – part 1. J Wound Care. 2000;9:56–60. | |
Thomas A, Harding KG, Moore K. Alginates from wound dressings activate human macrophages to secrete tumour necrosis factor-α. Biomaterials. 2000;21:1797–1802. | |
Wiegand C, Reddersen K, Springer S, Abel M, Ruth P, Hipler UC. In-vitro-Analyse des Flüssigkeitsmanagements durch hydroaktive Wundverbände mit Hilfe eines Mazerationsmodells [In-vitro-Analysis of the fluid management by hydroactive wound dressings using a maceration model]. German. Presented at the conference of the DGfW in 2014. | |
Carville P, Cuddigan J, Fletcher J, et al. Wound infection in clinical practice. An international consensus. Int Wound J. 2008;5:S3–S11. | |
Wiegand C, Abel M, Muldoon J, Ruth P, Hipler UC. SAP-containing dressings exhibit sustained antimicrobial effects over 7 days in vitro. J Wound Care. 2013;22:120–127. | |
Wiegand C, Abel M, Ruth P, Hipler UC. Superabsorbent polymer-containing wound dressings have a beneficial effect on wound healing by reducing PMN elastase concentration and inhibiting microbial growth. J Mater Sci Mater Med. 2011;22:2583–2590. | |
Bruggisser R. Bacterial and fungal absorption properties of a hydrogel dressing with a superabsorbent polymer core. J Wound Care. 2005;14:438–442. | |
Wiegand C, Heinze T, Hipler UC. Comparative in vitro study on cytotoxicity, antimicrobial activity, and binding capacity for pathophysiological factors in chronic wounds of alginate and silver-containing alginate. Wound Repair Regen. 2009;17:511–521. | |
Walker M, Hobot JA, Newman GR, Bowler PG. Scanning electron microscopic examination of bacterial immobilisation in a carboxymethyl cellulose (Aquacel®) and alginate dressings. Biomaterials. 2003;24:883–890. | |
Beele H, Meuleneire F, Nahuys M, Percival SL. A prospective randomised open label study to evaluate the potential of a new silver alginate/carboxymethyl-cellulose antimicrobial wound dressing to promote wound healing. Int Wound J. 2010;7:262–270. | |
Trial C, Darbas H, Lavigne JP, et al. Assessment of the antimicrobial effectiveness of a new silver alginate wound dressing: a RCT. J Wound Care. 2010;19:20–26. | |
Harding K, Gottrup F, Jawien A, et al. A prospective, multi-centre, randomized, open label, parallel, comparative study to evaluate effects of Aquacel Ag and Urgotul silver dressing on healing of chronic venous leg ulcers. Int Wound J. 2012;9:285–294. | |
Coutts P, Sibbald RG. The effect of a silver-containing hydrofiber dressing on superficial wound bed and bacterial balance of chronic wounds. Int Wound J. 2005;2:348–356. | |
Jørgensen B, Price P, Andersen KE, et al. The silver-releasing foam dressing, Contreet foam, promotes faster healing of critically colonised venous leg ulcers: a randomised, controlled trial. Int Wound J. 2005; 2:64–73. | |
Wysocki AB, Staiano-Coico L, Grinnel F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993;101:64–68. | |
Herrick S, Ashcroft G, Ireland G, Horan M, McCollum C, Ferguson M. Up-regulation of elastase in acute wounds of healthy aged humans and chronic venous leg ulcers are associated with matrix degradation. Lab Invest. 1997;77:281–288. | |
He C, Hughes MA, Cherry GW, Arnold F. Effects of chronic wound fluid on the bioactivity of platelet-derived growth factor in serum-free medium and its direct effect on fibroblast growth. Wound Repair Regen. 1999;7:97–105. | |
Chen SM, Ward SI, Olutoye OO, Diegelmann RF, Cohen IK. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen. 1997;5:23–32. | |
Schönfelder U, Abel M, Wiegand C, Klemm D, Elsner P, Hipler UC. Influence of selected wound dressings on PMN elastase in chronic wound fluid and their antioxidative potential in vitro. Biomaterials. 2005;26:6664–6673. | |
Smeets R, Ulrich D, Unglaub F, Wültje M, Pallua N. Effect of oxidised regenerated cellulose/collagen matrix on proteases in wound exudate of patients with chronic venous ulceration. Int Wound J. 2008;5:195–203. | |
Gottrup F, Cullen BM, Karlsmark T, Bischoff-Mikkelsen M, Nisbet L, Gibson MC. Randomized controlled trial on collagen/oxidized regenerated cellulose/silver treatment. Wound Repair Regen. 2013;21:216–225. | |
Schmutz J-L, Meaume S, Fays S, et al. Evaluation of the nano-oligosaccharide factor lipido-colloid matrix in the local management of venous leg ulcers: results of a randomised, controlled trial. Int Wound J. 2008;5:172–182. | |
Meaume S, Truchetet F, Cambazard F, et al. A randomized, controlled, double-blind prospective trial with a lipidocolloid technology nano-oligosaccharide factor wound dressing in the local management of venous leg ulcers. Wound Repair Regen. 2012;20:500–511. | |
Wiegand C, White R. Binding and inhibition of protease enzymes, including MMPs, by a superabsorbent dressing in vitro. J Wound Care. 2013;22:221–227. | |
Wiegand C, Hipler UC. A superabsorbent polymer-containing wound dressing efficiently sequesters MMPs and inhibits collagenase activity in vitro. J Mater Sci Mater Med. 2013;24:2473–2478. | |
Moseley R, Walker M, Waddington RJ, Chen WYJ. Comparison of the antioxidant properties of wound dressing materials – carboxymethylcellulose, hyaluronan benzyl ester and hyaluronan, towards polymorphonuclear leukocyte derived reactive oxygen species. Biomaterials. 2003;24:1549–1557. | |
Vikatmaa P, Juutilainen V, Kuukasjärvi P, Malmivaara A. Negative pressure wound therapy: a systematic review on effectiveness and safety. Eur J Vasc Endovasc Surg. 2008;36:438–448. | |
Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC. Effects of varying levels of subatmosphereic pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg. 2001;47:547–551. | |
European Wound Management Association. Understanding topical negative pressure therapy. In: Gustaffson R, Sjögren J, Ingemansson R, editors. EWMA position document: Topical negative pressure in wound management. 2007. Available from: http://ewma.org/fileadmin/user_upload/EWMA/pdf/Position_Documents/2007/EWMA_Eng_07_final.pdf. Accessed April 30, 2015. | |
Isago T, Nozaki M, Kikuchi Y, Honda T, Nakazawa H. Effects of different negative pressures on reduction of wounds in negative pressure wound dressings. J Dermatol. 2003;30:569–601. | |
Zhou M, Yu A, Wu G, Xia C, Hu X, Qi B. Role of different negative pressure values in the proves of infected wounds healing treated by vacuum-assisted closure: an experimental study. Int Wound J. 2012;10:508–515. | |
Jacobs S, Simhaee DA, Marsano A, Fomovsky GM, Niedt G, Wu JK. Efficacy and mechanisms of vacuum-assisted closure (VAC) therapy in promoting wound healing: a rodent model. J Plast Reconstr Surg. 2009;62:1331–1338. | |
Scherer SS, Pietramaggiori G, Mathews JC, Orgill DP. Short periodic applications of the vacuum-assisted closure device cause an extended tissue response in the diabetic mouse model. Plast Reconstr Surg. 2009;124:1458–1465. | |
Moues CM, van Toorenenbergen AW, Heule F, Hop WC, Hovius SER. The role of topical negative pressure in wound repair: expression of biochemical markers in wound fluid during wound healing. Wound Repair Regen. 2008;16:488–494. | |
Borgquist O, Gustafsson L, Ingemansson R, Malmsjö M. Tissue ingrowth into foam but not into gauze during negative pressure wound therapy. Wounds. 2009;21:302–309. | |
Campbell PE, Smith GS, Smith JM. Retrospective clinical evaluation of gauze-based negative pressure wound therapy. Int Wound J. 2008;5:280–286. | |
Apostoli A, Caula C. Dolore e attività funzionali di base durante la V.A.C.® terapia in un gruppo di pazienti ospedalizzati portatori di lesioni cutanee [Pain and basic functional activities in a group of patients with cutaneous wounds under VAC therapy in hospital setting]. Prof Inferm. 2008;61:158–164. Italian. | |
Hurd T, Chadwick P, Cote J, Cockwill J, Mole TR, Smith JM. Impact of gauze-based NPWT on the patient and nursing experience in the treatment of challenging wounds. Int Wound J. 2010;7:448–455. | |
Wilkes R, Zhao Y, Cunningham K, Kieswetter K, Haridas B. 3D strain measurement in soft tissue: demonstration of a novel inverse finite element model algorithm on microCT images of tissue phantom exposed to negative pressure wound therapy. J Mechan Behav Biomed Mater. 2009;2:272–287. | |
Wiegand C, White R. Microdeformation in wound healing. Wound Repair Regen. 2013;21:793–799. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

