Back to Journals » Cancer Management and Research » Volume 11
Clinical efficacy of cisplatin, dexamethasone, gemcitabine and pegaspargase (DDGP) in the initial treatment of advanced stage (stage III–IV) extranodal NK/T-cell lymphoma, and its correlation with Epstein-Barr virus
Authors Zhao Q , Fan S, Chang Y, Liu X, Li W, Ma Q, Li Y, Wang Y, Zhang L, Zhang M
Received 24 October 2018
Accepted for publication 28 February 2019
Published 24 April 2019 Volume 2019:11 Pages 3555—3564
DOI https://doi.org/10.2147/CMAR.S191929
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Beicheng Sun
Qian Zhao,1,2 Shanshan Fan,1,2 Yu Chang,1,2 Xiyang Liu,1,2 Wencai Li,2,3 Qianwen Ma,1,2 Yang Li,1,2 Yan Wang,1,2 Lei Zhang,1,2,* Mingzhi Zhang1,2,*
1Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450000, People’s Republic of China; 2Lymphoma Diagnosis and Treatment Center of Henan Province, Zhengzhou, Henan 450000, People’s Republic of China; 3Department of Pathology, The First Affiliated Hospital of Zhengzhou University, Lymphoma Diagnosis and Treatment Center of Henan Province, Zhengzhou, Henan, People’s Republic of China
*These authors contributed equally to this work
Objective: To evaluate the clinical efficacy and safety of the DDGP regimen in treating extranodal NK/T-cell lymphoma and investigate the correlation between Epstein-Barr virus (EBV)-DNA variation after treatment and the clinical efficacy of NK/T-cell lymphoma.
Methods: Sixty-four patients with extranodal NK/T-cell lymphoma received DDGP regimen-based chemotherapy. Short-term and long-term clinical efficacy and adverse reactions were observed. The relationship between EBV-DNA changes before and after therapy and clinical efficacy was investigated.
Results: After the DDGP regimen was used as the initial treatment, the short-term clinical efficacy included 39 complete remission (CR) (60.94%), 12 partial remission (PR) (18.75%), 2 stable disease (SD) (3.13%) and 11 progressive disease (PD) (17.18%). Objective response rate (ORR) was 79.69% and 82.82% for disease control rate (DCR). 3-year progression-free survival (PFS) was 62.00% and 3-year overall survive (OS) was 74.90%. Hemocytopenia was the predominant adverse effect. Between EBV-DNA positive group and its negative counterpart, a significant difference was noted in OS (P=0.046), but no difference in ORR, DCR or PFS was observed. In the EBV-DNA positive group, ORR, DCR, PFS and OS were higher for patients whose EBV-DNA copy number decreased within a normal range than patients remained positive (93.33% versus 61.53%, P=0.041 for ORR; 93.33% versus 61.53%, P=0.041 for DCR, P=0.003 for PFS, P=0.017 for OS). The main adverse reactions included bone marrow suppression, gastrointestinal reaction and coagulation dysfunction, which were mitigated and treated after expectant or dose-decrement treatment.
Conclusion: DDGP regimen can significantly improve the clinical prognosis of NK/T-cell lymphoma patients with tolerable adverse reactions. The variation in EBV-DNA is correlated with clinical efficacy and prognosis, which provides a theoretical basis for NK/T-cell lymphoma therapy.
Clinical trial: In November 2011, this clinical trial was registered on the website: www.ClinicalTrials.gov (No. NCT01501149).
Keywords: gemcitabine, pegaspargase, extranodal NK/T-cell lymphoma, Epstein-Barr virus-DNA, prognosis
Introduction
Extranodal natural killer/T-cell lymphoma (nasal type, ENKTL) is a relatively rare type of malignant lymphoma that is highly malignant.1 Nasal cavity-type ENKTL originates from the nasal cavity with a predilection for the nasal septum and inferior nasal concha. Common nasal diseases include nasal tumors, nasal obstruction and nosebleeds. Along with the progression of diseases, patients present with nasal space-occupying lesions, extensive involvement with adjacent soft tissues, ulcers and bone destruction.2–4 Extranasal-type ENKTL affects the skin, gastrointestinal tract and reproductive organs with diverse manifestations according to the different organs involved. A clinical trial is recommended for the treatment of NK/T-cell lymphoma. The treatment for these patients mainly includes chemotherapy, radiotherapy or hematopoietic stem-cell transplantation. Better efficacy for radiotherapy was observed in early stage but poor efficacy in the advanced stage. In recent years, checkpoint inhibitors have been explored as a novel therapeutic approach by enhancing antitumor immunity by blocking immune check points with antibodies, a pathway that has been shown to be increasingly important in ENKTL. PD-1/PD-L1 are responsible for this negative immune regulation and are the targets of the antitumor effect.5,6 However, effective first-line treatment of NK/T-cell lymphoma remains to be explored. In the earliest, anthracycline-containing chemotherapy regimens, such as CHOP, were the mainstay of advanced ENKTL treatment. However, as P-glycoprotein in lymphoma cells, which leads to drug efflux and resistance to treatment, these regimens yield poor outcomes.7,8 To explore more effective chemotherapy regimens for ENKTL, a series of studies on ENKTL therapy have been carried out since 2009 in the Lymphoma Diagnosis and Treatment Center, Henan, China. The IC50 values of 30 types of chemotherapy agents (such as anthracycline, vincristine, breviscapine, dacarbazine, cisplatin, methotrexate, gemcitabine, colaspase and pegaspargase) on the NK/T-cell lymphoma cell line SNK-6 were detected in vitro to identify the medications with low IC50 values, high sensitivity, different action sites and no overlapping side effects.9 The DDGP chemotherapy regimen alone (cisplatin, dexamethasone, gemcitabine and pegaspargase) was applied to treat advanced NK/T-cell lymphoma. The clinical efficacy and safety of the DDGP regimen were evaluated. From August 2010 to May 2012, our center enrolled 12 newly-diagnosed stage II–IV ENKTL patients treated with DDGP regimen, and 100% RR was achieved (ten patients (10/12, 83.3%) achieved CR and two (2/12, 16.7%) achieved PR).10 In addition, Zhang et al investigated the efficacy and safety of 25 newly diagnosed, advanced-stage ENKTL patients treated with DDGP regimen between March 2011 to September 2013, the PR and the CR rate were 16.67% and 70.83%, respectively. The 1- and 2-year PFS was 75.00% and 61.8%, respectively, and the 1- and 2-year OS was 79.20% and 68.50%, respectively. 11 In order to further verify the clinical efficacy and safety of DDGP regimen, we are recruiting more patients to further evaluate the efficacy of DDGP regimen. Previous studies have demonstrated that Epstein-Barr virus (EBV) infection is intimately correlated with the incidence and progression of ENKTL, but the precise etiology remains undetermined.13–16 EBV-DNA detection can serve as a pivotal parameter for the assessment of the clinical prognosis of NK/T-cell lymphoma patients. Nevertheless, the relationship between the dynamic variation in copy number of EBV-DNA and clinical prognosis during the treatment of ENKTL has been rarely investigated. In this study, EBV-DNA variation before and after the DDGP regimen was studied to investigate the relationship between EBV and the clinical efficacy and prognosis of NK/T-cell lymphoma.
Materials and methods
General data
Sixty-four patients with advanced stage NK/T-cell lymphoma admitted to the First Affiliated Hospital of Zhengzhou University between March 2011 and April 2018 were recruited in this clinical trial. The diagnosis of ENKTL was validated by more than two experienced professors from the Department of Pathology.
Patients with no medical history of chemotherapy or radiotherapy received 4–6 cycles of the DDGP regimen as the initial treatment. The clinical characteristics of all patients are illustrated in Table 1.
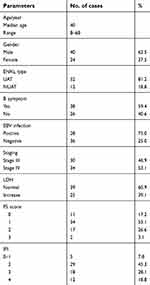 | Table 1 Baseline data of all patients (n=64) |
Clinical treatment
All 64 patients received the DDGP regimen consisting of 800 mg/m2 gemcitabine via intravenous drip at day 1 and 8, 20 mg/m2 cisplatin via intravenous drip from day 1 to 4, 15 mg/m2 dexamethasone via intravenous drip from day 1 to 5 and intramuscular administration of 2,500 IU/m2 pegaspargase at day 1. Six cycles of the DDGP regimen were delivered, 21 days for each cycle. Clinical efficacy was evaluated every two cycles. Routine examinations were performed prior to chemotherapy. Symptomatic treatment was implemented before and after chemotherapy. The dose of chemotherapy agent was lowered by 20% upon the appearance of severe grade IV adverse reactions.
The study was carried out with Good Clinical Practice Guidelines and the Helsinki Declaration. This work was approved by the Local Ethics Committee of Zhengzhou University and the Scientific Council of Faculty of Medicine. All patients were fully informed about the nature and possible toxicities of the treatment protocol and submitted written informed consent. For patients under 18 years of age, written informed consent was provided by parents or legal guardians.
Evaluation criteria
Imaging methods to evaluate the efficacy of all patients included CT or PET-CT, if necessary we usually combined with color doppler ultrasound and MRI. The clinical efficacy was assessed according to the WHO evaluation criteria as the complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).13 The objective response rate (ORR) was calculated according to the percentage of CR + PR patients among all patients. The disease control rate (DCR) was calculated from the percentage of CR + PR + SD patients among all individuals. According to the WHO criteria for acute and subacute toxic reactions to anticancer drugs, the severity of chemotherapy-induced toxicity was classified as grade I-IV. The long-term clinical efficacy was assessed with progression-free survival (PFS) and overall survival (OS). PFS is defined as the time interval starting from the day of chemotherapy to disease progression or death. OS refers to the time interval starting from the day of chemotherapy to death or final follow-up.
Statistical analysis
SPSS 21.0 statistical software was utilized for data analysis. Kaplan-Meier survival analysis was performed. Fisher’s exact test was used for statistical processing. The ORR and DCR among different EBV-DNA copy number groups were statistically compared by the χ2 test. A value of P<0.05 was considered statistically significant.
Quantification of EBV- DNA
A 5 mL patient peripheral blood was obtained, sent to the PCR laboratory (The First Affiliated Hospital of Zhengzhou University), then separate the plasma sample. Amplification was performed according to the instructions of EBV nucleic acid amplification fluorescence quantitative detection kit (Da An Gene Co., Ltd. of Sun Yat-sen University, Guangzhou, China). The amplification result with less than 5.00E + 02 copies/mL is defined as negative for EBV-DNA. More than 5.00E + 02 copies/mL is defined as positive for EBV-DNA.
Results
Baseline characteristics of patients
Sixty- four patients were enrolled from March 2011 to April 2018. Their baseline characteristics are listed in Table 1. Among them, there are 30 patients in stage III and 34 in stage IV. The median age was 40 years old (range 10– 68 years old) and the female: male ratio was 1:1.667. Fifty- two patients (81.2%) were diagnosed with upper aerodigestive tract ENKTL. B symptoms were observed in 38 patients (59.5%), and elevated lactate dehydrogenase (LDH) was observed in 25 patients (39.1%).
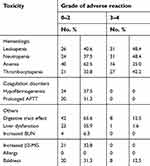 | Table 2 DDGP regime-induced adverse reactions |
Short- term clinical efficacy
The short-term clinical efficacy in 64 ENKTL patients was assessed, including 39 cases of CR (60.94%), 12 PR (18.75%), 2 SD (3.13%) and 11 PD (17.18%). The ORR was calculated as 79.69% and 82.82% for DCR. During subsequent follow-up, 28 among 64 patients were positive and 36 were negative in EBV-DNA levels. The infection rate was 43.75% (28/64). In the EBV-DNA-positive group, the ORR was 78.57% (22/28), and in the EBV-DNA-negative group, it was 80.56% (29/36). No significant difference was observed between the two groups (P=0.845). The DCR was 78.57% (22/28) in the EBV-DNA-positive group and 86.11% (31/36) in the EBV-DNA-negative group, with no significant difference (P=0.428). In the EBV-DNA-positive group, the copy number of EBV-DNA decreased within normal range included 15 cases after the DDGP regimen. The negative conversion ratio was 53.57% (15/28). The ORR of the negative group was 93.33% (14/15), which significantly differed from 61.53% (8/13) for the positive group (P=0.041), and the DCR was the same.
Long- term clinical efficacy
The final date of follow- up was in April 2018. The median follow- up was 40 months. Among the 64 ENKTL patients, 15 died. Nine patients died of disease progression, 2 patients died of cerebral hemorrhage, 2 patients died of gastrointestinal hemorrhage, 1 patient died of multiple organ failure, and 1 patient died of unknown causes. Six patients presenting with disease progression and 3 released patients were switched to an alternative regimen and were subsequently excluded from this clinical trial. Among them 3 patients were positive for EBV and 6 patients were negative for EBV. Finally, the OS of 55 patients was assessed. The 1- , 2- and 3-year PFS was 77.00%, 67.80% and 62.00%, respectively, and the 1-, 2- and 3-year OS was 81.50% 74.9% and 74.90%, respectively, as illustrated in Figure 1A and B. In the EBV-DNA-positive group, the 1-, 2- and 3-year PFS was 70.30%, 67.50% and 67.50%, respectively. In the EBV-DNA-negative group, the 1-, 2- and 3-year PFS was 79.90%, 68.80% and 58.6%, respectively. No significant difference was noted in PFS between the two groups (P=0.812). In the EBV-DNA-positive group, the 1-, 2- and 3-year OS was 73.75%, 62.6% and 62.60%, respectively. In the EBV-DNA-negative group, the 1-, 2- and 3-year OS was 89.90%, 85.4% and 85.40%, respectively. A significant difference was noted in OS between the two groups (P=0.046), as illustrated in Figure 2A and B. In the EBV-DNA-positive group, the 1-year PFS of the negative group was 90.90%, which was significantly higher than that (43.1%) in the positive group (P=0.003). Moreover, the 1-year OS was 93.30% and 44.90% in the negative and positive groups, respectively, which were significantly different (P=0.017), as illustrated in Figure 3A and B.
Adverse reactions
Adverse reactions mainly included bone marrow suppression, digestive tract reactions, coagulation dysfunction, etc. Among the 64 patients, 48.4% presented with grade III–IV leukopenia; 62.5%, with grade I–II anemia; 42.2%, with grade III–IV thrombocytopenia; 65.6%, with digestive tract reactions; and 37.5%, with slight coagulation dysfunction, as illustrated in Table 2.
Discussion
The prevalence of ENKTL is relatively low, and ENKTL is rarely seen in clinical practice. Few case-control studies have been conducted. At present, no standard treatment or principles have been established. Common therapies mainly include radiotherapy, chemotherapy and hematopoietic stem cell transplantation. The ENKTL lesions are predominantly located in the nasal cavity and surrounding tissues.17 For patients with stage III–IV or refractory recurrent ENKTL, synchronized chemo-radio therapy or combined chemotherapy has been recommended in clinical trials by NCCN guidelines.18 Although few studies have been performed, chemotherapy plays a pivotal role in the treatment of middle- and advanced-stage or refractory recurrent ENKTL. Since ENKTL rarely affects the bone marrow, hematopoietic stem cell transplantation has been more frequently applied in clinical practice in recent years. Even though this technique is in the initial stages, it can serve as a remedy and consolidate therapy after chemotherapy for patients with advanced-stage ENKTL.19
Gemcitabine is a novel type of cytidine derivative. It is a deoxycytidine analog and a new difluorine nucleotide anti-metabolism anticancer drug. Gemcitabine is a cell cycle-specific agent.20 Gemcitabine functions by eliminating the cells at the S phase and blocking the cell proliferation from the G1 phase to the S phase. Active metabolites of diphosphate and triphosphate difluorine deoxycytidine insert into the DNA of tumor cells, leading to the interruption of DNA synthesis and cellular apoptosis. This agent exerts a broad-spectrum antitumor effect and plays a synergistic role in combination with platinum, vindesine and hormone medications.21 In vitro experiments also revealed that gemcitabine possesses favorable membrane penetrability and strong affinity for deoxycytidine kinase and is retained within the cells for a long time, suggesting that it has relatively high antitumor activity. Relevant investigations have been carried out in our center.9,22 in which the IC50 values of different chemotherapy agents upon the NK/T-cell lymphoma SNK-6 cell line are studied. The results demonstrate that gemcitabine (0.002 μg/mL) has the lowest IC50 value and acts in a time- and dose-dependent manner.23–25
Currently, a pegaspargase-based chemotherapy regimen is recommended as the first-line treatment for patients with middle- and advanced-stage and refractory recurrent ENKTL according to the NCCN guidelines. Asparagine is an essential amino acid for protein synthesis. Normal cells in the human body can not only uptake asparagine from the blood circulation but also synthesize asparagine via asparagine synthetase. However, tumor cells can only uptake exogenous asparagine due to the lack of asparagine synthetase.26 Pegaspargase is able to hydrolyze asparagine in the blood into aspartic acid and ammonia. Thus, tumor cells without asparagine synthetase lack an essential amino acid, which inhibits the synthesis of DNA, RNA and protein, blocks the exogenous source of asparagine for tumor cells and suppresses the synthesis of protein. Consequently, pegaspargase exerts an antitumor effect.27,28 Nagafuji et al29 first reported that patients for whom ENKTL recurred after autologous peripheral blood stem cell transplantation obtained partial response after the administration of L-asparagine (L-ASP) and were still surviving during an 18-month follow-up. Due to relatively severe allergic reactions, L-ASP is rarely utilized in clinical practice.30 L-ASP functions via a distinct mechanism with explicit clinical efficacy. However, due to the relatively short half-life of L-ASP, it should be administered multiple times to maintain the required drug concentration. Along with the increasing frequency of medicine administration, the incidence of allergic reactions is elevated accordingly. Hence, the use of L-ASP is strictly restricted in clinical settings.
As a novel type of asparagine enzyme preparation, pegaspargase integrates polyethylene glycol into the non-active site of L-ASP to form a macromolecular vector medication with a t1/2 of 4–6 times higher than that of colaspase. Consequently, it is more convenient to utilize and possesses longer action times in clinical practice compared with colaspas.10,31,32 Due to its low immunogenicity, it is safe to use pegaspargase wieth a low incidence of allergic reactions. It is applicable for patients who are allergic to colaspase. In the present study, to resolve the potential allergic reactions of kidrolase, a large dose of hormone was supplemented to decrease the formation of kidrolase antibody, thereby reducing the incidence of allergic reactions. Moreover, the hormone can also eliminate lymph tumor cells.33,34
The findings in this study demonstrate that the DDGP regimen (cisplatin, dexamethasone, gemcitabine and pegaspargase) is efficacious for ENKTL patients.33,34 In fact, our center had conducted a study to compare the clinical efficacy of DDGP regimen and SMILE regimen in 2016. The result showed that DDGP chemotherapy resulted in significant improvement in PFS, OS and better tolerability compared with SMILE chemotherapy for newly diagnosed advanced NK/T cell lymphoma patients.34 In addition, we collect the reports on the treatment regimen of gemcitabine or/and L-asparaginase for NK/T cell lymphoma in recent years and Table 3 summarizes the latest published results.35–38 Quantitative detection of serum EBV-DNA found that in 53.57% of patients with positive EBV-DNA turn negative for EBV-DNA after DDGP treatment. The ORR and DCR in the EBV-DNA-positive group were 79.69% (22/28) and 79.69% (22/28), and those in the EBV-DNA-negative group were 80.56% (29/36) and 86.11% (31/36), respectively. In the EBV-positive group, the ORR and DCR were both 93.3% (14/15) for patients who turned negative for EBV and 61.53% (8/13) for those who remained positive for EBV. A significant difference was observed in the ORR and DCR between patients positive and negative for EBV. This indicated that the patients with advanced NK/T-cell lymphoma achieved good therapeutic effects after the DDGP regimen. However, a refractory high copy number of EBV is an unfavorable factor affecting the therapeutic effect. In the EBV-positive group, the 1-, 2- and 3-year PFS was 70.30%, 67.50% and 67.50%, respectively. In the EBV-negative group, the 1-, 2- and 3-year PFS was 79.9%, 68.80% and 58.6%, respectively. In the EBV-positive group, the 1-, 2- and 3-year OS was 73.57%, 62.6% and 62.60%, respectively. In the EBV-negative group, the 1-, 2- and 3-year OS was 89.9%, 58.4% and 85.4%, respectively. There was a significant difference in OS (P=0.046), but no significant difference was noted in the PFS (P=0.812) between the two groups, possibly because of the small sample size. However, the PFS values in the EBV-negative group were higher than those in the EBV-positive group. In the EBV-positive group, the 1-year PFS and 1-year OS in patients negative for EBV were 90.9% and 93.3%, respectively, and 43.1% and 44.9%, respectively, in those positive for EBV. Kumai et al also found that the copy number in over half of patients positive for EBV was negative after treatment,39 prompting the possibility of inhibiting the copy number of plasma EBV-DNA through anti-virus therapy or alternative methods or performing prognosis stratification analysis and establishing individualized therapeutic regimens according to the dynamic detection of the EBV-DNA copy number before, during and after the therapy, thereby improving the clinical prognosis of ENKTL patients. In this study, the overall ORR was 79.69%, the overall DCR was 82.82%, the 1-, 2- and 3-year PFS was 77.00%, 67.80% and 58.6%, respectively, and the 1-, 2- and 3-year OS was 81.50%, 74.9% and 74.90%, respectively, after the DDGP regimen. The main adverse events included bone marrow suppression, often manifested as leukopenia, slight anemia and thrombocytopenia. The administration of granulocyte colony stimulating factor, recombinant human thrombopoietin or interleukin-11 was recommended as the expectant treatment. Preventive anti-infection therapy and platelet infusion were also necessary. The dose of chemotherapy agent was reduced by 20% in the next cycle of chemotherapy upon the appearance of grade IV adverse events. In addition, 65.6% of patients presented with digestive tract reaction, 35.9% of patients with liver function injury and 37.5% of patients with mild coagulation dysfunction. No patients showed allergic reactions. In addition to the regular detection of liver function and coagulation functions, fresh frozen plasma, cryoprecipitate or human fibrinogen should be administered in a timely manner to treat the hypofibrinogenemia induced throughout the therapy. Moreover, corresponding measures should be taken to protect the liver and stomach from vomiting. During subsequent follow-up, no patient died from adverse reactions. Taken together, this prospective study demonstrates that the DDGP regimen (cisplatin, dexamethasone, gemcitabine and pegaspargase) yields relatively high efficacy in the treatment of ENKTL, which can effectively minimize the tumor size, mitigate relevant symptoms and induce few adverse reactions with excellent tolerance. Nevertheless, the sample size is small, and the follow-up duration is short. Consequently, long-term clinical trials with larger sample sizes are urgently required. Currently, we are still recruiting more patients in our center to more accurately evaluate the clinical efficacy and safety of the DDGP regimen. Along with the rapid development of immune inhibitors, chemotherapy in combination with checkpoint inhibitors enhances the clinical efficacy in treating lymphoa.40 Hence, this regimen combined with checkpoint inhibitors is currently a promising treatment direction. More clinical trials are urgently required to validate the results. The good prognosis of the copy number of EBV-DNA declined within normal range in the positive group also reminded anti-EBV therapy may be an effective treatment for NK/T cell lymphoma. Currently, EB-virsus targeted immunotherapy is under development, we look forward to its arrival and bring another breakthrough in the treatment of lymphoma.
 | Table 3 Comparison of pegaspargase- or L-asparaginase- or gemcitabine-based regimens in the treatment of extranodal NK/T-cell lymphoma |
Conclusion
Our study demonstrated that the DDGP regimen can significantly improve the clinical prognosis of NK/T-cell lymphoma patients with tolerable adverse reactions. The EBV-DNA variation is correlated with clinical efficacy and prognosis, which provides a theoretical basis for NK/T-cell lymphoma therapy.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81172118) and Medical Science and Technology Plan Project of Henan Province, China (contract/grant no.: 201302001). The authors would like to thank Ling Li, Xin Li, Xinhua Wang, Xiaorui Fu, Jingjing Wu, Zhenchang Sun, Xudong Zhang, Zhiyuan Zhou, Feifei Nan, Jiaqin Yan, and Zhaoming Li from the Lymphoma Diagnosis and Treatment Centre of Henan Province for providing clinical data.
Data sharing statement
Individual participant data will be available. All of the individual participant data collected during the trial, after deidentification, will be shared. Other study-related documents (study protocol, statistical analysis plan, informed consent form, and analytic code) will be made available. Proposals should be directed to [email protected]. Once approved, the data will be sent through the mailbox. The data will be made available beginning 3 months and ending 36 months following article publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hanakawa H, Orita Y, Sato Y, et al. Novel and simple prognostic index for nasal natural killer/T-cell lymphoma. Head Neck. 2014;36(4):551–556. doi:10.1002/hed.23322
2. Cai Q, Luo X, Zhang G, et al. New prognostic model for extranodal natural killer/T cell lymphoma, nasal type. Ann Hematol. 2014;93(9):1541–1549. doi:10.1007/s00277-014-2089-x
3. Tse E, Kwong YL. How I treat NK/T-cell lymphomas. Blood. 2013;121(25):4997–5005. doi:10.1182/blood-2013-01-453233
4. Lima M. Aggressive mature natural killer cell neoplasms: from epidemiology to diagnosis. Orphanet J Rare Dis. 2013;8:95. doi:10.1186/1750-1172-8-95
5. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106. doi:10.1097/COC.0000000000000239
6. Suzuki R. NK/T cell lymphoma: updates in therapy. Curr Hematol Malig Rep. 2018;13(1):7–12. doi:10.1007/s11899-018-0430-5
7. Suzuki R, Takeuchi K, Ohshima K, Nakamura S. Extranodal NK/T-cell lymphoma: diagnosis and treatment cues. Hematol Oncol. 2008;26(2):66–72. doi:10.1002/hon.847
8. Oshimi K. Progress in understanding and managing natural killer-cell malignancies. Br J Haematol. 2007;139(4):532–544. doi:10.1111/j.1365-2141.2007.06835.x
9. Cao L, Zhang M, Hu Y, Zhang X, Sun S, Wen J. Apoptosis of NK/T lymphoma cell line SNK-6 induced by gemcitabine. Can Res Prev Treat. 2013;40(8):733–736.
10. Li L, Zhang C, Zhang L, et al. Efficacy of a pegaspargase-based regimen in the treatment of newly-diagnosed extranodal natural killer/T-cell lymphoma. Neoplasma. 2014;61(2):225–232. doi:10.4149/neo_2014_029
11. Zhang L, Jia S, Ma Y, et al. Efficacy and safety of cisplatin, dexamethasone, gemcitabine and pegaspargase (DDGP) regimen in newly diagnosed, advanced-stage extranodal natural killer/T-cell lymphoma: interim analysis of a phase 4 study NCT01501149. Oncotarget 2016;7(34):55721–55731.
12. Liang R. Advances in the management and monitoring of extranodal NK/T-cell lymphoma, nasal type. Br J Haematol. 2009;147(1):13–21. doi:10.1111/j.1365-2141.2009.07802.x
13. Liu W, Nan F, Jia S, et al. Detecting EB virus to determine curative effect in extranodal natural killer/T-cell lymphoma. Chin J Clin Oncol. 2015;(2)105–108.
14. Jia S, Nan F, Sucai LI, et al. The clinical features and prognosis of EBER negative extranodal natural killer/T-cell lymphoma. China Oncol. 2016;26(6):533–537.
15. Kimura H. EBV in T-/NK-cell tumorigenesis. Adv Exp Med Biol. 2018;1045:459–475. doi:10.1007/978-981-10-7230-7_21
16. Chaudhary RK, Bhatt VR, Vose JM. Management of extranodal natural killer/t-cell lymphoma, nasal type. Clin Lymphoma Myeloma Leuk. 2015;15(5):245–252. doi:10.1016/j.clml.2014.12.014
17. Chauchet A, Michallet AS, Berger F, et al. Complete remission after first-line radio-chemotherapy as predictor of survival in extranodal NK/T cell lymphoma. J Hematol Oncol. 2012;5:27. doi:10.1186/1756-8722-5-27
18. Kim SJ, Park S, Kang ES, et al. Induction treatment with SMILE and consolidation with autologous stem cell transplantation for newly diagnosed stage IV extranodal natural killer/T-cell lymphoma patients. Ann Hematol. 2015;94(1):71–78. doi:10.1007/s00277-014-2171-4
19. Dong M, He XH, Liu P, et al. Gemcitabine-based combination regimen in patients with peripheral T-cell lymphoma. Med Oncol. 2013;30(1):351. doi:10.1007/s12032-012-0351-4
20. Ahn HK, Kim SJ, Hwang DW, et al. Gemcitabine alone and/or containing chemotherapy is efficient in refractory or relapsed NK/T-cell lymphoma. Invest New Drugs. 2013;31(2):469–472.
21. Chen YC, Zhang MZ, Zhang XD, et al. TUBB3 and ERCC1 expression levels are correlated with chemosensitivity to platinum and anti-microtubule reagents in human lymphoma cell lines. J Xian Jiaotong Univ. 2012;33(5):539–543.
22. Park BB, Kim WS, Suh C, et al. Salvage chemotherapy of gemcitabine, dexamethasone, and cisplatin (GDP) for patients with relapsed or refractory peripheral T-cell lymphomas: a consortium for improving survival of lymphoma (CISL) trial. Ann Hematol. 2015;94(11):1845–1851. doi:10.1007/s00277-015-2468-y
23. Ramzi M, Rezvani A, Dehghani M. Correction: GDP versus ESHAP regimen in relapsed and/or refractory hodgkin lymphoma: a comparison study. Int J Hematol Oncol Stem Cell Res. 2015;9(4):218.
24. Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG. J Clin Oncol. 2014;32(31):3490–3496. doi:10.1200/JCO.2013.53.9593
25. Yong W, Zheng W, Zhu J, et al. L-asparaginase in the treatment of refractory and relapsed extranodal NK/T-cell lymphoma, nasal type. Ann Hematol. 2009;88(7):647–652. doi:10.1007/s00277-008-0669-3
26. Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120(15):2973–2980. doi:10.1182/blood-2012-05-431460
27. Chan A, Tang T, Ng T, et al. To SMILE or not: supportive care matters. J Clin Oncol. 2012;30(9):1015–1017. doi:10.1200/JCO.2011.40.7098
28. Nagafuji K, Fujisaki T, Arima F, Ohshima K. L-asparaginase induced durable remission of relapsed nasal NK/T-cell lymphoma after autologous peripheral blood stem cell transplantation. Int J Hematol. 2001;74(4):447–450.
29. Silverman LB, Supko JG, Stevenson KE, et al. Intravenous PEG-asparaginase during remission induction in children and adolescents with newly diagnosed acute lymphoblastic leukemia. Blood. 2010;115(7):1351–1353. doi:10.1182/blood-2009-09-245951
30. Jung KS, Cho SH, Kim SJ, Ko YH, Kang ES, Kim WS. L-asparaginase-based regimens followed by allogeneic hematopoietic stem cell transplantation improve outcomes in aggressive natural killer cell leukemia. J Hematol Oncol. 2016;9:41. doi:10.1186/s13045-016-0271-4
31. Guo HQ, Liu L, Wang XF, et al. Efficacy of gemcitabine combined with oxaliplatin, L-asparaginase and dexamethasone in patients with newly-diagnosed extranodal NK/T-cell lymphoma. Mol Clin Oncol. 2014;2(6):1172–1176. doi:10.3892/mco.2014.368
32. Zhou Z, Li X, Chen C, et al. Effectiveness of gemcitabine, pegaspargase, cisplatin, and dexamethasone (DDGP) combination chemotherapy in the treatment of relapsed/refractory extranodal NK/T cell lymphoma: a retrospective study of 17 patients. Ann Hematol. 2014;93(11):1889–1894. doi:10.1007/s00277-014-2136-7
33. Zhang L, Li S, Jia S, et al. The DDGP (cisplatin, dexamethasone, gemcitabine, and pegaspargase) regimen for treatment of extranodal natural killer (NK)/T-cell lymphoma, nasal type. Oncotarget. 2016;7(36):58396–58404. doi:10.18632/oncotarget.11135
34. Li X, Cui Y, Sun Z, et al. DDGP versus SMILE in newly diagnosed advanced natural killer/T-cell lymphoma: a randomized controlled, multicenter, Open-label Study in China. Clin Cancer Res. 2016;22(21):5223–5228. doi:10.1158/1078-0432.CCR-16-0153
35. Wang JH, Wang H, Wang YJ, et al. Analysis of the efficacy and safety of a combined gemcitabine, oxaliplatin and pegaspargase regimen for NK/T-cell lymphoma. Oncotarget. 2016;7(23):35412–35422. doi:10.18632/oncotarget.8643
36. Yamaguchi M, Suzuki R, Kwong YL, et al. Phase I study of dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) chemotherapy for advanced-stage, relapsed or refractory extranodal natural killer (NK)/T-cell lymphoma and leukemia. Cancer Sci. 2010;99(5):1016–1020. doi:10.1111/j.1349-7006.2008.00768.x
37. Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29(33):4410–4416. doi:10.1200/JCO.2011.35.6287
38. Jaccard A, Gachard N, Marin B, et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117(6):1834–1839. doi:10.1182/blood-2010-09-307454
39. Kumai T, Kobayashi H, Harabuchi Y. Novel targets for natural killer/T-cell lymphoma immunotherapy. Immunotherapy-UK. 2016;8(1):45–55. doi:10.2217/imt.15.103
40. Kumai T, Nagato T, Kobayashi H, et al. CCL17 and CCL22/CCR4 signaling is a strong candidate for novel targeted therapy against nasal natural killer/T-cell lymphoma. Cancer Immunol Immunother. 2015;64(6):697–705. doi:10.1007/s00262-015-1675-7
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



