Back to Archived Journals » Biosimilars » Volume 4
Clinical efficacy and safety of Zarzio® (EP2006), a biosimilar recombinant human granulocyte colony-stimulating factor
Authors Tharmarajah S, Mohammed A, Bagalagel A, MacDonald K , Abraham I
Received 12 December 2013
Accepted for publication 1 February 2014
Published 12 March 2014 Volume 2014:4 Pages 1—9
DOI https://doi.org/10.2147/BS.S28710
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Soba Tharmarajah,1,2 Abdulaziz Mohammed,3,4 Alaa Bagalagel,3,4 Karen MacDonald,2 Ivo Abraham2,3,5
1College of Pharmacy, University of Arizona, Tucson, AZ, USA; 2Matrix45, Tucson, AZ, USA; 3Center for Health Outcomes and PharmacoEconomic Research, College of Pharmacy, University of Arizona, Tucson, AZ, USA; 4College of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia; 5Department of Pharmacy Practice and Science, College of Pharmacy, University of Arizona, Tucson, AZ, USA
Abstract: This second review of biosimilar granulocyte colony-stimulating factors approved by the European Medicines Agency evaluates the evidence on the clinical efficacy and safety of prophylaxis of (febrile) neutropenia with Zarzio® in chemotherapy-treated cancer patients relative to the originator product filgrastim (Neupogen®). Source documents include: publicly available documents of the European Medicines Agency; a published article reviewing the (preapproval) clinical development of EP2006 (Zarzio®); and published (postapproval) single-center experience reports on prophylaxis with Zarzio®, including two reports in the cancer setting and one in the setting of autologous peripheral blood stem cell mobilization. Also included is: a pooled analysis of these and other postapproval studies in the cancer setting that includes (interim) data from the two single cancer center reports; one additional single-center experience study; one completed study; and one ongoing multicenter postapproval study. Based on the available therapeutic equivalence and safety data, the clinical and safety outcomes of Zarzio® are likely to be similar to those of Neupogen®. Thus, Zarzio® and Neupogen® may be assumed interchangeable.
Keywords: biosimilars, biosimilar pharmaceuticals, efficacy, safety, granulocyte colony stimulating factor, recombinant proteins
Introduction
The use of chemotherapy agents often leads to myelosuppression and severe neutropenia.1 Grade 4 neutropenia is generally defined as an absolute neutrophil count (ANC) of <500/mm3 or <0.5×109/L. Febrile neutropenia is inferred when patients with grade 4 neutropenia present with an axillary temperature ≥38.5°C or two or more febrile episodes at ≥38°C within a 12-hour period.2 Prophylaxis with granulocyte colony-stimulating factors (GCSF) are recommended when risk of developing febrile neutropenia, according to cancer type and chemotherapy regimen, exceeds 20% or in patients with an intermediate risk (10–20%) of neutropenia who present with risk factors.1 GCSFs are biological growth factors that simulate the production of white blood cells. The European Medicines Agency (EMA) defines a similar biological or biosimilar medicine as a biological medicine that is similar to another biological medicine that has already been authorized for use.3 A similar biological, or biosimilar product is a biologic agent that is similar in terms of quality, safety and efficacy to another biological medicine that has already been authorized for use.
This second review for this journal of the clinical efficacy and safety of biosimilar GCSFs approved by the EMA is focused on Zarzio® (EP2006; Sandoz International GmbH, Holzkirchen, Germany). This review follows a prior similar review of the clinical efficacy and safety of TevaGrastim® (XM02; Teva Pharmaceutical Industries, Ltd, Petach Tikva, Israel).4
Both are biosimilar versions of filgrastim (Neupogen®; Amgen, Thousand Oaks, CA, USA), which was first approved in Europe in February 1991 for the following indications: reduction in the duration and incidence of febrile neutropenia in chemotherapy-treated patients; reduction in the duration of neutropenia in patients undergoing myeloablative therapy followed by bone marrow transplantation; mobilization of peripheral blood progenitor cells; to increase neutrophil counts and reduce the incidence and duration of infection-related events in patients with severe congenital, cyclic, or idiopathic neutropenia with an ANC of ≤0.5×109/L and a history of severe or recurrent infections with long-term administration; and treatment of persistent neutropenia (ANC ≤1.0×109/L) in patients with advanced human immunodeficiency virus (HIV) infection.5 By focusing on clinical efficacy while also updating clinical safety data, this article extends a recent review of the clinical safety of biosimilar GCSFs.6
In the cancer setting, Zarzio® is indicated to reduce the incidence and duration of neutropenia and the incidence of febrile neutropenia in patients undergoing cytotoxic chemotherapy. It is also approved by extrapolation to reduce the duration of neutropenia in high-risk patients undergoing myeloablative therapy followed by bone marrow transplantation and in the mobilization of peripheral blood progenitor cells (Table 1).7 Also by extrapolation, Zarzio® is approved to: treat persistent neutropenia in patients with advanced HIV; reduce the risk of bacterial infections when other treatments are not appropriate; increase neutrophil levels; and reduce infection risk in patients with neutropenia who have a history of severe, repeated infections.
  | Table 1 Therapeutic indications for Zarzio® as approved by the EMA |
The European Public Assessment Report (EPAR)7 includes one Phase III trial (EP06-301), which constituted the evidence base on which Zarzio® was approved by the EMA. The source documents for this review include: publicly available EMA documents and, in particular, the EPAR;7 a peer-reviewed article reviewing the (preapproval) clinical development of Zarzio®;8 and three peer-reviewed, published (postapproval) single-center experience reports on prophylaxis with Zarzio®, including two reports in the cancer setting9,10 and one report in the setting of autologous peripheral blood stem cell mobilization.11 Each study included here is reviewed in terms of: methods; patients; therapeutic equivalence and other efficacy or effectiveness data; and safety, in particular immunogenicity, bone pain, splenomegaly, allergic reactions, acute respiratory distress syndrome (ARDS), and mortality. For comprehensive safety data, we refer to Abraham et al.6
There is also a published overview12 of: postapproval studies in the cancer setting that includes (interim) data from the two single cancer center reports mentioned earlier in this section;9,10 one additional unpublished single-center experience study; one completed unpublished study; and one ongoing unpublished multicenter postapproval study. This publication is included as a supplemental note as some of its findings have not been published independently as peer-reviewed publications.
Clinical studies
Overview
The late-stage development program of EP2006 included one open single-arm study involving 170 breast cancer patients who were treated with docetaxel/doxorubicin chemotherapy8 with comparison of results to published data from Neupogen® trials.13,14 Following the marketing authorization, two single-center experience studies in the cancer setting were conducted in Italy (SC-Cancer-Italy) and in Germany (SC-Cancer-Germany), as well as one single-center study on autologous peripheral blood stem cell mobilization (SC-BSCM). In addition, a pooled analysis of (interim) data from several studies, including some of the above, was published. A note on this paper is included in this review.
Study EP06-301: breast cancer
Methods
The primary objective of study EP06-3017,8 was to evaluate the therapeutic equivalence and safety of EP2006 relative to Neupogen®. The study was designed as an open, single-arm, multicenter Phase III study in breast cancer patients undergoing chemotherapy. Results associated with Zarzio® prophylaxis were compared to published data from Neupogen® trials.13,14 On day 1 of each chemotherapy cycle, the patients received an IV bolus infusion of 60 mg/m2 doxorubicin, followed by an IV infusion of 75 mg/m2 docetaxel 1 hour later.
Zarzio® was administered from day 2 for up to 14 days or through ANC nadir and ANC reaching 10×109/L in up to four consecutive chemotherapy cycles. Dosing was weight-based with women who weighed less than 60 kg being administered 30 MIU versus 48 MIU for women who weighed more than 60 kg.
The primary endpoints that were compared to published trials12,13 were the incidence and duration (in consecutive days of ANC <0.5×109/L) of grade 4 neutropenia in cycles 1 through 4. Also recorded were the number of days to recovery to ANC ≥1.0×109/L, but no comparative trial data were available.
Patients
Eligible patients were female adult chemotherapy-naïve patients with documented locally advanced or advanced breast cancer or high-risk stage II breast cancer and treated with doxorubicin and docetaxel; had an estimated life expectancy of at least 6 months; had an ECOL (Eastern Cooperative Oncology Group) performance status ≤2; had an ANC ≥1.5×109/L; had a platelet count ≥100×103/L; had aspartate aminotransferase (AST)and alanine aminotransferase (ALT) levels <3× upper limit of normal (provided the alkaline phosphatase level was <5× upper limit of normal); and adequate cardiac, hepatic, and renal function. In total, 170 breast cancer patients were enrolled. Of that number, 153 patients completed all four treatment cycles. Demographic data are summarized in Table 2.
  | Table 2 Patient characteristics in study EP06-301 |
Efficacy
Table 3 presents the efficacy endpoints. At each cycle, the proportion of patients with grade 4 neutropenia in the Zarzio®-treated group was significantly lower than the proportions in the reference trials reported by Holmes et al13 and Green et al14 (all P<0.0001). In cycle 1, the difference in the mean number of consecutive days with ANC <0.5×109/L observed in the Zarzio®-treated patients was not statistically significant from those in the two reference trials (both P not significant [ns]).13,14 In cycles 2 and 4, the mean number of consecutive days of grade 4 neutropenia for Zarzio®-treated patients was significantly higher in the patients in the two reference trials (all P<0.05). In cycle 3, the mean number of consecutive days of grade 4 neutropenia for Zarzio®-treated patients was significantly higher, compared to those in the Green et al14 study, but not in the Holmes et al13 study. The mean (± standard deviation [SD]) number of days required for recovery to ANC ≥1.0×109/L among Zarzio®-treated patients ranged from 1.4±0.6 in cycle 3 to 2.2±0.9 in cycle 1.
  | Table 3 Efficacy endpoints in study EP06-301 |
Safety
No patients developed binding and/or neutralizing antibodies during the study. In addition, 35 patients reported adverse events, classified as bone pain. No patients experienced splenomegaly, allergic reactions, acute respiratory distress syndrome (ARDS), or died during the active treatment phases. Three patients died subsequent to the study, including two due to disease progression from stage IV breast cancer and one in an automobile accident. Hence, these deaths are unrelated to Zarzio® treatment. Comprehensive safety data have been reviewed elsewhere.6
Study SC-Italy: single-center experience with biosimilar GCSF
Methods
The primary objective of study SC-Italy9 was to evaluate the therapeutic equivalence and safety of Zarzio® for the primary and secondary prophylaxis of chemotherapy-induced febrile neutropenia. The study was designed as an observational, single-center study. Patients included in this study were scheduled to receive Zarzio® 30 MIU subcutaneously per day after the end of chemotherapy for a minimum of 4 days and a maximum of 14 days. Zarzio® injections were suspended if the ANC after the expected nadir was ≥10×109/L.
The primary endpoints of interest to this review were the incidence and the duration of febrile neutropenia. Relevant secondary endpoints were the safety of Zarzio® after the end of each cycle up to 180 days after the Zarzio® administration.
Patients
The sample consisted of male and female chemotherapy-naïve adult patients with: an ECOG score ≤2; ANC ≥1.5×109/L; platelet count ≥100×109/L; and adequate cardiac, liver, and renal function. 48 patients were enrolled to receive Zarzio® following chemotherapy treatment. Of these, 37 received Zarzio® as primary prophylaxis and eleven as secondary prophylaxis. Characteristics of patients enrolled in this study are summarized in Table 4, including median duration of Zarzio® administration stratified by primary versus secondary prophylaxis and tumor type.
  | Table 4 Characteristics of patients in study SC-Italy |
Efficacy
The median duration of prophylaxis with Zarzio® was 7 days. Three cases of febrile neutropenia were reported in patients receiving Zarzio® as primary prophylaxis. Two of these cases concerned patients with prostate cancer following the fourth chemotherapy cycle, both of whom had a history of secondary bone involvement and had received palliative radiotherapy. The third case concerned a patient with squamous cell lung cancer and multiple secondary skeletal lesions after the third cycle. These patients were treated with antibiotics and did not require hospitalization. Six cases of grade 4 nonfebrile neutropenia were reported in patients receiving primary prophylaxis with Zarzio®. Outcomes data were not presented in the article, but we computed crude incidence rates of 6.25% for febrile neutropenia and 12.5% for grade 4 nonfebrile neutropenia. This made for a total crude rate of 18.75% across all cycles.
Safety
No safety data were reported except that Zarzio® “was well-tolerated with no unexpected adverse effects being reported”.9
Study SC-Germany: single-center retrospective chart review of outcomes before and after center switch from originator to biosimilar GCSF
Methods
The primary objective of this study10 was to evaluate the therapeutic equivalence and safety of Zarzio® compared to Neupogen®. The study was designed as an observational, retrospective review of patient charts for a 2.5-year period in a single community oncology center in Germany. During this period, the center gradually switched from originator (Neupogen®) to biosimilar filgrastim (Zarzio®). Hence, a panel of Neupogen®-treated patients and a panel of Zarzio®-treated patients were compared. There was no direct comparison of both agents in the same patient.
Before and after the institutional switch, the GCSF agent (respectively, Neupogen® or Zarzio®) was administered subcutaneously daily at 300 μg for 5 days. Treatment with GCSF was continued beyond 5 days if neutropenia was present. GCSF was administered to patients in accordance with the clinical guidelines of the European Organisation for Research and Treatment of Cancer.2 Patients with a febrile neutropenia risk of ≥20% also received prophylactic oral antibiotics.
The primary endpoints of interest to this review were the proportions of patients with a febrile neutropenia risk of ≥20% and those with a risk <10% treated with GCSF in the originator and biosimilar groups, as well as the proportions receiving GCSF as primary versus secondary prophylaxis. Relevant secondary endpoints were the proportions of patients in the originator and biosimilar groups who developed febrile neutropenia, had chemotherapy-dose reductions, and dose discontinuations because of neutropenia.
Patients
The sample consisted of male and female adults aged ≥18 years with a solid tumor or hematological malignancy and who were treated with GCSF during at least one chemotherapy cycle. Charts were reviewed for a total of 77 Zarzio®-treated patients compared to 25 Neupogen®-treated patients. As detailed in Table 5, both treatment groups were similar in terms of sex, age, cancer, and tumor type.
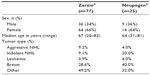  | Table 5 Characteristics of patients in study SC-Germany |
Efficacy
Proportions of GCSF-treated patients receiving chemotherapy with febrile neutropenia risk ≥20% or <10%, as well as proportions receiving GCSF as primary prophylaxis, and all this stratified by Zarzio® versus Neupogen®, are summarized in Table 6. In terms of treatment patterns, the proportions were more favorable for the Zarzio® group relative to the Neupogen® group (all P<0.0001). Proportions for febrile neutropenia and chemotherapy changes were statistically similar between both groups (P=ns).
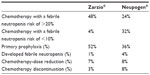  | Table 6 Efficacy endpoints in study SC-Germany |
Safety
No safety data were reported except that in the Zarzio® patients “no unexpected safety findings were observed”.10 No safety information is provided for the Neupogen® patients.
Study SC-BSCM: single-center study of autologous peripheral blood stem cell mobilization with biosimilar GCSF
Methods
The main objective of this study11 was to evaluate the therapeutic equivalence and safety of Zarzio® and Neupogen® for the mobilization and the collection of autologous peripheral blood stem cells (PBSC) in patients with hematological disease.
The study was designed as an open, single-center study of prospectively included patients scheduled to receive Zarzio® following chemotherapy for treatment and autologous PBSC mobilization. These patients were matched in terms of age, diagnosis, chemotherapy received, and mobilization to a historical control group who had received Neupogen® at the same center. Both groups were treated according to the same clinical protocol.
The primary endpoints of interest were: the median duration of GCSF administration; median preleukapheresis peripheral blood white cell count and CD34+; and the median number of blood masses processed by leukapheresis (initiated when the CD34+ cell concentration reached 10/μL). Relevant secondary endpoints were: median 106 CD34+/kg collected for first leukapheresis; median number of leukapheresis to collect 3 ×106 CD34+ cells/kg; and the number of PBSC mobilization failures.
Patients
The sample consisted of male and female adults with lymphoma or myeloma. Patients with a previous history of PBSC mobilization were excluded. Also, 40 patients enrolled to receive Zarzio® following chemotherapy treatment were historically matched to 41 patients who had received Neupogen®. As detailed in Table 7, both arms were similar in terms of age, diagnosis, previous chemotherapy, and PBSC mobilization regimen.
Efficacy
As Table 8 shows, there were no statistically significant differences between: the Zarzio®-treated patients and their matched Neupogen®-treated patients in the median duration of GCSF administration; the median preleukapheresis peripheral blood white cell count and CD34+; the median number of blood masses processed by leukapheresis (initiated when the CD34+ cell concentration reached 10/μL); the median 106 CD34+/kg collected for first leukapheresis; the median number of leukapheresis to collect 3 ×106 CD34+ cells/kg; and the number of PBSC mobilization failures (all P=ns) Also, there was no significant difference in terms of duration of recovery to ANC ≥0.5 ×109/L or platelet count ≥20 ×109/L between both treatment groups (Table 5).
Safety
In addition, 14 patients in the Zarzio® group reported bone pain and/or headache. Safety events in the Neupogen® group could not be ascertained retrospectively. No other safety data are reported.
Note on Gascón et al summary of postapproval studies of Zarzio®
A recent 2013 paper12 brings together selected findings from the peer-reviewed Salesi et al9 and Verpoort et al10 single-center studies summarized. Also included are an additional Italian single-center study, a German multicenter study, and a European multicenter study published only in abstract form (and thus not meeting the inclusion criteria for this review).
Some pooled calculations were performed using these studies: 36% of patients were treated with chemotherapy regimens with a febrile neutropenia risk ≥20%; 40% with a risk between 10%–20%; 12% with a risk <10% (12% unknown); and mainly for primary (57%) compared to secondary (27%) prophylaxis (16% unknown). As to clinical events, 2.2% of patients experienced an episode of febrile neutropenia and 8.5% experienced grade 4 neutropenia. Patients receiving secondary prophylaxis were twice as likely to experience these neutropenic complications. Though limited to data from two studies, chemotherapy-dose reductions or chemotherapy discontinuation occurred in 9% of patients.
The article also summarizes safety data and/or provides additional evidence. The occurrence of bone pain in 8% of patients is stated to be consistent with the reported incidence for Neupogen® after adjusting for the fact that bone pain is now recognized as a common side effect associated with GCSF therapy. There have been no reports of neutralizing antibodies in the postapproval pharmacovigilance period.
Comments
The regulatory and peer-reviewed published evidence of the clinical efficacy and the safety of Zarzio® accumulated to date includes one multicenter preapproval study in breast cancer patients, two postapproval single-center experience studies in oncology clinics, and one single-center experience study in peripheral blood stem cell mobilization.7,8–11 Two completed postapproval studies (one single-center and one multicenter) have not yet been published, and one multicenter, multicountry study recently completed data collection and is entering the data management and analysis phase.12
The Phase III EP06-301 study was the scientific base for Zarzio® included in the EPAR document, and the EMA accepted this study as sufficient for marketing authorization.7 The findings from this single-arm study on breast cancer patients treated with Zarzio® were compared to those from published trials for Neupogen®.13,14 The incidence rates of patients with severe neutropenia despite GCSF prophylaxis were significantly higher in the Neupogen® reference trials across all four cycles of chemotherapy. This may be due to all the patients in the EP06-301 study being chemotherapy-naïve, compared to 20% of patients in the reference trials who had previously undergone chemotherapy treatment.
On the other hand, with the exception of cycle 1, where the mean consecutive days with ANC <0.5×109/L were similar for Zarzio® versus Neupogen®-treated patients, patients in the Neupogen® reference trials had fewer mean consecutive days of ANC ≥1.0×109/L (severe neutropenia) by 0.2–0.7 days. It may be that the (fewer) patients with grade 4 neutropenia in the EP06-301 study experienced more serious neutropenia and therefore required longer recovery times. In contrast, the larger proportions of patients with grade 4 neutropenia in the reference trials may have included a significant number of patients with borderline grade 4 neutropenia who required relatively less recovery time.
The single-center studies SC-Italy and SC-Germany reported crude incidence rates of febrile neutropenia of 6% and 1%, respectively. These are encouraging results, but the limited sample sizes in these reports call for caution: selection bias may have been present; patients may have presented with fewer risk factors; and the severity of illness may have been lower. While this may have influenced the results, it also draws attention to: the importance of carefully evaluating patients; assessing the neutropenia risk associated with chemotherapy regimens (in particular, risk ≥20%); evaluating risk factors (especially for regimens with 10%–20% febrile neutropenia risk); and using GCSF in the first instance as primary prophylaxis. This must be examined in large multicenter studies. Study SC-Germany also provides some data on chemotherapy-dose reduction and chemotherapy discontinuation. These are outcomes to be evaluated in large multicenter studies as well.
It may be important to consider time (and, relatedly, clinical experience with GCSF) when comparing the results from studies EP06-301, SC-Italy, and SC-Germany with controlled and noncontrolled studies of Neupogen®. The Zarzio® studies were conducted after significant clinical experience with GCSF had accumulated, and GCSF prophylaxis had become the virtual standard of care. In addition, the two single-center studies were conducted concurrent with or after the dissemination of the 2010 European Organisation for Research and Treatment of Cancer guidelines,2 which is likely to have provided clinicians at these centers with significantly more guidance than may have been available before.
The single-center SC-BSCM study provides evidence on the efficacy of Zarzio® in peripheral blood stem cell mobilization. This is important because the indication for “mobilization of peripheral blood progenitor cells” was granted by extrapolation under the EMA biosimilar pathway.15 While further evidence is necessary, the study draws attention to the need for postapproval support for the HIV and infections indications in the EMA-approved label for Zarzio®. This, of course, is not unique to Zarzio®, but to all biosimilar GCSFs, if not biosimilar agents in general.
Postapproval safety data for Zarzio® remain limited. While this may be of lesser concern than, for instance, for biosimilar erythropoietins, continued pharmacovigilance is important. Single-center studies do not have the statistical power to detect low-frequency safety events. It might be better for reports on small sample studies to cite observed safety events rather than state that no unexpected adverse effects were observed.
Multicenter multicountry studies evaluating the clinical effectiveness and safety of Zarzio®, and the determinants thereof, will be critical to further document the clinical effectiveness and safety of Zarzio® – and biosimilar agents in general. As Aapro points out,16 prescribers ask the same questions about biosimilars as they asked years ago about generics: similarity of the biosimilar to the originator product in terms of activity; safety; and quality. In addition, clinicians will seek assurances that the lower cost of biosimilars will not impair their ability to practice in accordance with international guidelines.
Sandoz, of the Novartis Group, is currently sponsoring the Multi-level Evaluation of Chemotherapy-induced Febrile Neutropenia Prophylaxis, Outcomes, and Determinants With Granulocyte-colony Stimulating Factor (MONITOR-GCSF) in 126 centers in 12 countries in Europe. This is a pharmacoepidemiological study of the determinants, predictors, and clinical outcomes of febrile neutropenia prophylaxis with Zarzio®.17,18
As reported by Gascón et al,12 an interim analysis on enrollment data for 741 patients showed that 409 received primary prophylaxis and 129 received secondary prophylaxis with Zarzio®, while the type of prophylaxis was unknown (as of yet) for 203 patients. This study is likely to provide significant insights into the treatment patterns, outcomes, and associated determinants of neutropenia prophylaxis with Zarzio®, as well as evidence on the impact of neutropenia episodes in chemotherapy regimens, including dose reductions, dose delays, and chemotherapy cancellations.
Based on the studies reviewed here, the clinical and safety outcomes of Zarzio® are likely to be similar to those of Neupogen®. Both GCSFs can be assumed to be interchangeable in the EMA-approved indications. However, as the three EMA-approved biosimilars TevaGrastim® (Teva Pharmaceutical Industries, Ltd), Zarzio® (Sandoz Pharmaceuticals), and Nivestim™ (Hospira Inc., Lake Forest, IL, USA) have not been studied relative to each other, these biosimilars must not be assumed to be interchangeable.
Conclusion
The clinical efficacy and safety of EP2006/Zarzio® was assessed in one Phase III study included in the EMA approval dossier as well as three postapproval studies. While continued assessment of effectiveness and safety is recommended, prophylaxis with Zarzio® should produce similar patient outcomes within similar safety parameters as compared to Neupogen®. Commercial Zarzio® and Neupogen® might be interchangeable products; however, further studies are needed.
Acknowledgments
Soba Tharmarajah is a doctor of pharmacy candidate at the University Of Arizona College of Pharmacy. Alaa Bagalagel and Abdulaziz Mohammed were supported as fellows and Ivo Abraham as director of the Postdoctoral Fellowship Program in Clinical Research in Human Therapeutics at the University of Arizona and funded by King Abdulaziz University, Jeddah, Saudi Arabia. Ivo Abraham was also supported as director of the Arizona Area Health Education Center Interprofessional Fellowship Program in Clinical Outcomes and Comparative Effectiveness Research, funded by the Bureau of Health Professions, US Department of Health and Human Services through the Arizona Area Health Education Centers Program. The services of Karen MacDonald were contributed pro bono by Matrix45.
Disclosure
Karen MacDonald and Ivo Abraham are principals at Matrix45; Soba Tharmarajah is an intern at Matrix45. The company has received research grants and contracts related to granulocyte colony-stimulating factors from Sandoz/Novartis. By company policy, they cannot hold equity in sponsor organizations, nor can they receive direct personal benefits – financial or other – from sponsor organizations.Matrix45 provides similar services to other biopharmaceutical companies without exclusivity constraints. The present paper was prepared independently. The manufacturer of the biosimilar reviewed here was not contacted for data, publications, or other sources of information, nor did it have any input on the review activities or in the preparation of the manuscript. Abdulaziz Mohammed and Alaa Bagalagel report no conflicts of interest in this work.
References
Bennett CL, Djulbegovic B, Norris LB, Armitage JO. Colony-stimulating factors for febrile neutropenia during cancer therapy. N Engl J Med. 2013;368(12):1131–1139. | |
Aapro MS, Bohlius J, Cameron DA, et al; European Organisation for Research and Treatment of Cancer. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adults patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32. | |
ema.europa.eu [homepage on the Internet]. Biosimilar medicines. European Medicines Agency; 2014; [updated September 28, 2012; cited January 28, 2014]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/document_listing/document_listing_000318.jsp&mid=WC0b01ac0580281bf0. Accessed January 29, 2014. | |
Bagalagel A, Mohammed A, MacDonald K, Abraham I. Clinical efficacy and safety of Tevagrastim® (XM02), a biosimilar recombinant human granulocyte colony-stimulating factor. Biosimilars. 2013;3:55–62. | |
European Medicines Agency. European Public Assessment Report Zarzio®. London: European Medicines Agency; 2009. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000917/WC500046526.pdf. Accessed January 28, 2014. | |
Abraham I, Tharmarajah S, MacDonald K. Clinical safety of biosimilar recombinant human granulocyte colony-stimulating factors. Expert Opin Drug Saf. 2013;12(2):235–246. | |
ema.europa.eu [homepage on the Internet]. Zarzio®. European Medicines Agency; 2013 [updated April 12, 2013; cited January 28, 2014]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000917/human_med_001170.jsp&mid=WC0b01ac058001d124. Accessed December 11, 2013. | |
Gascón P, Fuhr U, Sörgel F, et al. Development of a new G-CSF product based on biosimilarity assessment. Ann Oncol. 2010;21(7):1419–1429. | |
Salesi N, Di Cocco B, Colonna M, Veltri E. Biosimilar medicines in oncology: single-center experience with biosimilar G-CSF. Future Oncol. 2012;8(5):625–630. | |
Verpoort K, Möhler TM. A non-interventional study of biosimilar granulocyte colony-stimulating factor as prophylaxis for chemotherapy-induced neutropenia in a community oncology center. Ther Adv Med Oncol. 2012;4(6):289–293. | |
Lefrère F, Brignier AC, Elie C, et al. First experience of autologous peripheral blood stem cell mobilization with biosimilar granulocyte colony-stimulating factor. Adv Ther. 2011;28(4):304–310. | |
Gascón P, Tesch H, Verpoort K, et al. Clinical experience with Zarzio® in Europe: what have we learned? Support Care Cancer. 2013;21(10):2925–2932. | |
Holmes FA, O’Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002;20(3):727–731. | |
Green MD, Koelbl H, Baselga J, et al. International Pegfilgrastim 749 Study Group. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003;14(1):29–35. | |
ema.europa.eu [homepage on the Internet]. Concept paper on extrapolation of efficacy and safety in medicine development. European Medicines Agency; 2012 [updated March 19, 2013; cited January 28, 2014]. Available from http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/04/WC500142358.pdf. Accessed January 27, 2014. | |
Aapro MS. What do prescribers think of biosimilars? Targ Oncol. 2012;7(Suppl 1):S51–S55. | |
Gascón P, Aapro M, Ludwig H, et al. Background and methodology of MONITOR-GCSF, a pharmaco-epidemiological study of the multi-level determinants, predictors, and clinical outcomes of febrile neutropenia prophylaxis with biosimilar granulocyte-colony stimulating factor filgrastim. Crit Rev Oncol Hematol. 2011;77(3):184–197. | |
Gascón P, Aapro M, Ludwig H, et al. Update on the MONITOR-GCSF study of biosimilar filgrastim to reduce the incidence of chemotherapy-induced febrile neutropenia in cancer patients: Protocol amendments. Crit Rev Oncol Hematol. 2011;77(3):198–200. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


