Back to Journals » Breast Cancer: Targets and Therapy » Volume 8
Clinical effects of prior trastuzumab on combination eribulin mesylate plus trastuzumab as first-line treatment for human epidermal growth factor receptor 2 positive locally recurrent or metastatic breast cancer: results from a Phase II, single-arm, multicenter study
Authors Puhalla S, Wilks S, Brufsky AM , O'Shaughnessy J, Schwartzberg LS , Berrak E, Song J, Vahdat L
Received 20 October 2015
Accepted for publication 22 June 2016
Published 7 December 2016 Volume 2016:8 Pages 231—239
DOI https://doi.org/10.2147/BCTT.S98696
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Shannon Puhalla,1 Sharon Wilks,2 Adam M Brufsky,1 Joyce O’Shaughnessy,3 Lee S Schwartzberg,4 Erhan Berrak,5 James Song,5 Linda Vahdat6
1Department of Hematology and Oncology, University of Pittsburgh Medical Center, Pittsburgh, PA, 2Department of Hematology Oncology, US Oncology-Cancer Care Centers of South Texas, San Antonio, TX, 3Department of Medical Oncology, Texas Oncology-Baylor Charles A. Sammons Cancer Center US Oncology, Dallas, TX, 4Department of Hematology/Oncology, West Cancer Center, University of Tennessee Health Science Center, Memphis, TN, 5Department of Medical Affairs, Formerly of Eisai Inc., Woodcliff Lake, NJ, 6Department of Medicine, Weill Cornell Medical College, New York, NY, USA
Abstract: Eribulin mesylate, a novel nontaxane microtubule dynamics inhibitor in the halichondrin class of antineoplastic drugs, is indicated for the treatment of patients with metastatic breast cancer who previously received ≥2 chemotherapy regimens in the metastatic setting. Primary data from a Phase II trial for the first-line combination of eribulin plus trastuzumab in human epidermal growth factor receptor 2 positive patients showed a 71% objective response rate and tolerability consistent with the known profile of these agents. Here, we present prespecified analyses of efficacy of this combination based on prior trastuzumab use. Patients received eribulin mesylate 1.4 mg/m2 (equivalent to 1.23 mg/m2 eribulin [expressed as free base]) intravenously on days 1 and 8 plus trastuzumab (8 mg/kg intravenously/cycle 1, then 6 mg/kg) on day 1 of each 21-day cycle. Objective response rates, progression-free survival, and tolerability were assessed in patients who had and had not received prior adjuvant or neoadjuvant (neo/adjuvant) trastuzumab treatment. Fifty-two patients (median age: 59.5 years) received eribulin/trastuzumab for a median treatment duration of ~31 weeks; 40.4% (n=21) had been previously treated with neo/adjuvant trastuzumab prior to treatment with eribulin plus trastuzumab for metastatic disease (median time between neo/adjuvant and study treatment: 23 months). In trastuzumab-naïve patients (n=31) compared with those who had received prior trastuzumab, objective response rate was 77.4% versus 61.9%, respectively; duration of response was 11.8 versus 9.5 months, respectively; clinical benefit rate was 87.1% versus 81.0%, respectively; and median progression-free survival was 12.2 versus 11.5 months, respectively. The most common grade 3/4 treatment-emergent adverse events (occuring in ≥5% of patients) in patients who received prior trastuzumab versus trastuzumab naïve patients, respectively, were neutropenia (47.6% vs 32.3%), peripheral neuropathy (14.3% vs 25.8%), febrile neutropenia (14.3% vs 3.2%), fatigue (9.5% vs 6.5%), nausea (9.5% vs 0%), vomiting (9.5% vs 3.2%), and leukopenia (9.5% vs 3.2%). In patients with human epidermal growth factor receptor 2 positive metastatic breast cancer, first-line eribulin/trastuzumab treatment demonstrated substantial antitumor activity and was well tolerated, regardless of prior neo/adjuvant trastuzumab treatment.
Keywords: oncology, breast neoplasms, advanced breast cancer, chemotherapy, eribulin mesylate, trastuzumab, HER2
Introduction
In the United States, ~5% of all women have metastatic breast cancer (MBC) at the time of initial diagnosis.1 Recurrent or MBC is incurable and has a 5-year survival rate of ~26.3%.1 For MBC or locally recurrent disease, selection of therapy is guided by several factors, including biology, clinical presentation, hormone receptor (estrogen receptor and progesterone receptor) expression and sensitivity, location and burden of metastases, prior treatment history, patient age and comorbid conditions, patients’ lifestyle choices, menopausal status, and human epidermal growth factor receptor 2 (HER2) expression.2 Surveys have indicated that ~15% of patients with breast cancer have tumors that are positive for the transmembrane tyrosine kinase receptor HER2,3,4 and the frequency of HER2 positivity is increased among patients with metastatic disease.4
Trastuzumab, a humanized monoclonal antibody directed against the extracellular domain of HER2,5 is recommended as part of first-line therapy for patients with HER2+ tumors and metastatic disease.2 Results from multiple clinical trials have demonstrated that the combination of trastuzumab with any of several conventional chemotherapeutic agents, including carboplatin, docetaxel, vinorelbine, or capecitabine, is effective for the treatment of HER2-overexpressing MBC.6–10
Although HER2 overexpression identifies patients who are likely to respond to therapy with trastuzumab, not all patients benefit from treatment and some patients develop disease recurrence in spite of adjuvant or neoadjuvant (neo/adjuvant) trastuzumab treatment, indicating the presence of de novo and/or acquired resistance.11,12 Recently, the treatment of HER2+ MBC has been focused on developing therapeutic agents/combination therapy to either potentiate trastuzumab’s effect or target cells that have become trastuzumab-resistant.11 There remains an unmet need for patients who experience disease recurrence following neo/adjuvant treatment with trastuzumab.
Eribulin mesylate is a nontaxane microtubule inhibitor that is a structurally modified synthetic analog of halichondrin B in the halichondrin class of antineoplastic drugs.13–16 Its novel mode of action is distinct from other tubulin-targeting agents in that eribulin binds only to the growing plus ends, which inhibits the microtubule growth phase without affecting the shortening phase and causes tubulin sequestration into nonproductive aggregates.13–16 Eribulin has demonstrated a survival benefit and antitumor activity in patients with MBC who previously received at least two chemotherapeutic regimens (including an anthracycline and a taxane)17–20 and is approved by the United States Food and Drug Administration for this indication.21 The approval was based on the results from the Phase III EMBRACE study, which demonstrated that single-agent eribulin significantly improved overall survival in patients with MBC compared with treatment of the physician’s choice.20
Due to its antitumor activity in the challenging setting of late-line treatment, we assessed eribulin in the first-line setting for the treatment of MBC. Primary data from a Phase II, multicenter, single-arm study of eribulin mesylate plus trastuzumab as first-line therapy for locally recurrent or metastatic HER2+ breast cancer demonstrated an objective response rate (ORR) of 71%, median time to response of 1.3 months, duration of response (DOR) of 11.1 months, median progression-free survival (PFS) of 11.6 months, and tolerability consistent with the known profile of these agents.22 In this analysis, we present prespecified efficacy and safety/tolerability analyses in the same patient population according to prior neo/adjuvant trastuzumab treatment.
Methods
Study design
This multicenter, single-arm, Phase II trial (ClinicalTrials.gov identifier: NCT01269346) assessed the ORR of eribulin in combination with trastuzumab in patients with locally recurrent or metastatic HER2+ breast cancer. There were three phases in the study: screening and baseline, six 21-day cycles of eribulin + trastuzumab, and an extension phase during which patients who completed the initial six cycles continued to receive study treatment until the development of progressive disease or until another withdrawal criterion was met.
Approval was obtained from independent ethics committees and regulatory authorities (US Oncology, Inc. Institutional Review Board; Western Institutional Review Board; Medical University of South Carolina, Office of Research Integrity, Institutional Review Board for Human Research; Committee on Research Involving Human Subjects; Biomedical Research Alliance of New York, LLC; Weill Cornell Medical College Institutional Review Board; Mercy Institutional Review Board, Cancer Resource Center; Northridge Hospital Medical Center Institutional Review Board). All patients provided written informed consent before undergoing any study-related procedures. The study was conducted in accordance with the Declaration of Helsinki (2008).
Patients
Inclusion criteria were females ≥18 years of age with histologically or cytologically proven recurrent or metastatic adenocarcinoma of the breast with ≥1 measurable lesion according to Response Evaluation Criteria in Solid Tumors, version 1.1; a HER2+ tumor as determined by a score of 3+ on immunohistochemistry staining or gene amplification by fluorescence in situ hybridization; life expectancy of ≥24 weeks; Eastern Cooperative Oncology Group performance status of 0, 1, or 2; ≥12 months since prior neo/adjuvant chemotherapy (no washout period for prior adjuvant trastuzumab); ≥2 weeks since prior radiotherapy or endocrine therapy, or trastuzumab, or lapatinib, with complete recovery from the effects of these interventions; and adequate renal, bone marrow, liver, and cardiac function. Exclusion criteria were prior chemotherapy, biologic therapy, or investigational therapy for locally recurrent or metastatic HER2+ breast cancer; prior exposure of >360 mg/m2 of doxorubicin or >720 mg/m2 of epirubicin; or preexisting grade 3 or 4 neuropathy.
Treatment
Patients received six cycles of eribulin mesylate 1.4 mg/m2 (equivalent to 1.23 mg/m2 eribulin [expressed as free base]) administered via intravenous infusion over 2 to 5 minutes on days 1 and 8 of each 21-day cycle and received trastuzumab 8 mg/kg administered intravenously over 90 minutes on day 1 of cycle 1. Thereafter, trastuzumab 6 mg/kg was infused over 30 minutes on day 1 of each subsequent 21-day cycle. Dose reductions were permitted for eribulin, but not trastuzumab; eribulin or trastuzumab could be continued as monotherapy if the other drug was discontinued.
Concomitant medications
Medications that were considered necessary for patient welfare and were not expected to interfere with study treatment evaluations could be administered at the discretion of the investigator. These treatments included granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and erythropoietin, administered according to American Society of Clinical Oncology guidelines and standard practice; stable bisphosphonate doses; and palliative radiotherapy (<10% of bone marrow). Other antitumor therapies were not permitted.
Endpoints
Baseline tumor assessments (computed tomography or magnetic resonance imaging scans) of the chest, abdomen, pelvis, and other areas of known disease were performed >28 days before the first infusion of study treatment, every 6 weeks during the treatment phase, and every 12 weeks in the extension phase. The primary endpoint of the study was ORR defined as the proportion of subjects who achieved a complete response (CR) plus those who achieved a partial response (PR) based on Response Evaluation Criteria in Solid Tumors version 1.1 criteria. PR and CR were assessed by changes in tumor measurements confirmed by repeat evaluations carried out ≥4 weeks after the response criteria were first met. Secondary endpoints were PFS, stable disease (SD), durable SD (SD >6 months), progressive disease, and DOR for patients whose best overall response was CR or PR, and clinical benefit rate (CBR) calculated using CR + PR + durable SD. Exploratory endpoints included efficacy and safety of first-line eribulin plus trastuzumab therapy in trastuzumab-naïve versus trastuzumab-pretreated patients.
Safety and tolerability
Adverse events (AEs) and serious AEs were assessed. AEs were graded on a 5-point scale according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Other safety parameters included: hematology and clinical chemistry; physical examinations; periodic measurement of vital signs and electrocardiograms; and evaluation of left ventricular ejection fraction by multigated acquisition scans or echocardiograms. Standardized MedDRA queries analyses were conducted for peripheral neuropathy that included the following search terms: neuropathy peripheral, neuropathy, peripheral motor neuropathy, polyneuropathy, peripheral sensory neuropathy, peripheral sensorimotor neuropathy, demyelinating polyneuropathy, and paresthesia.22
Statistical analysis
Efficacy analyses were based on the full analysis set by prior trastuzumab treatment and included all patients who received ≥1 dose(s) of study treatment. Baseline demographic and clinical characteristics were summarized by prior trastuzumab use. The ORR was determined along with corresponding two-sided, exact binomial 95% confidence intervals (CIs). DOR and PFS were analyzed using Kaplan–Meier product-limit estimates. CBR and 95% CIs were also determined.
Results
Patient characteristics
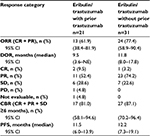
Fifty-two patients (median age: 59.5 years) received combination eribulin plus trastuzumab for first-line MBC therapy for a median treatment duration of 31 weeks; 40.4% (n=21) had been previously treated with trastuzumab as neo/adjuvant therapy (Table 1). The median time from stopping neo/adjuvant trastuzumab until beginning eribulin plus trastuzumab in the study was 23 months. Both trastuzumab-pretreated and trastuzumab-naïve patients received a median of eleven cycles of eribulin. The median duration of eribulin treatment was 31.1 weeks among patients who had received prior trastuzumab, and 30.3 weeks among patients who were trastuzumab-naïve.
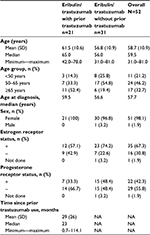  | Table 1 Demographic and clinical characteristics of study patients Abbreviations: NA, not assessed; SD, standard deviation; min, minimum; max, maximum. |
Efficacy
Overall, 52 patients in the full analysis set were evaluable for ORR (Table 2). Efficacy results were similar in patients who had received prior trastuzumab (n=21) compared with patients who had not received prior trastuzumab (n=31). The ORR was 61.9% in patients who had received prior trastuzumab treatment compared with 77.4% among those who had not, with a median DOR of 9.5 and 11.8 months, respectively; CBRs were 81.0% and 87.1%, respectively.
A summary of percentage changes in the total sum of target lesion diameters is shown in Figure 1. The median percentage change from baseline was −62.4% overall,22 −53.5% among trastuzumab-pretreated patients, and −64.0% among trastuzumab-naïve patients. Differences between treatment groups for these endpoints were not significant. Median PFS was 12.2 months (95% CI 7.3–19.1) in patients who had not received prior trastuzumab versus 11.5 (95% CI 6.0–13.9) months in those who had (Figure 2).
  | Figure 2 Kaplan–Meier plot of progression-free survival. Abbreviation: T, trastuzumab. |
Safety
All patients experienced at least one treatment-emergent AE (TEAE; Table 3). Grade ≥3 TEAEs were reported by 76.2% of patients who had received prior trastuzumab and 67.7% of those who had not. TEAEs that led to dose modification occurred in 66.7% of patients who had received prior trastuzumab and 54.8% of patients who had not. The most frequent TEAEs that led to dose modification, with a difference in incidence (≥10%) between patients who had received prior trastuzumab versus trastuzumab-naïve patients, were febrile neutropenia (14.3% vs 3.2%, respectively), neutropenia (42.9% vs 16.1%, respectively), fatigue (14.3% vs 0%, respectively), peripheral neuropathy (9.5% vs 22.6%, respectively), and peripheral sensory neuropathy (0% vs 9.7%, respectively). TEAEs that led to drug discontinuation occurred in 23.8% of patients who had received prior trastuzumab and 19.4% of those who had not. At least one serious AE was experienced by 33.3% of patients who had received prior trastuzumab and 25.8% of those who were trastuzumab-naïve. The most frequent serious AEs in patients who received prior trastuzumab treatment compared with those who did not (all grade 3/4) were febrile neutropenia (14.3% vs 3.2%, respectively) and peripheral neuropathy (0% vs 9.7%, respectively). An analysis for peripheral neuropathy that included multiple preferred terms found grade 1–3 peripheral neuropathy in 66.7% of patients who had received prior trastuzumab and in 71.0% of patients who had not. There were no grade 4/5 neuropathy events.
  | Table 3 Treatment-emergent adverse events Notes: aIn >25% of patients. bIn >5% of patients. Abbreviation: TEAE, treatment-emergent adverse event. |
Discussion
In this Phase II, single-arm trial in patients with HER2+ MBC, eribulin plus trastuzumab demonstrated substantial antitumor activity and was well tolerated as first-line treatment, irrespective of prior neo/adjuvant trastuzumab treatment. Efficacy, assessed by ORR, CBR, PFS, and DOR, was very similar in patients who had received prior trastuzumab treatment compared with patients who had not received prior trastuzumab.
Although trastuzumab substantially improves outcomes in both early-stage and MBC, not all patients respond to trastuzumab (de novo HER2 resistance), and many progress after realizing an initial response (acquired HER2 resistance).23 In early-stage breast cancer, the addition of trastuzumab to neo/adjuvant chemotherapy is associated with a pathologic CR of the breast and lymph nodes in 38% to 55% of patients, suggesting a de novo resistance rate of 45% to 62%.23 In patients with MBC treated with trastuzumab and chemotherapy, the median duration of PR or CR is 9.1 months, suggesting that within 1 year, patients acquire resistance.23–25 In previous trials of patients with MBC treated with trastuzumab in combination with other targeted agents, DORs have also been less than 1 year.26–28 Several resistance mechanisms have been proposed, including, 1) altered receptor–antibody interaction, 2) activation of the downstream pathways by increased signaling from either other members of the HER family or other receptors, or 3) constitutive activation of downstream elements.11 Results from the present trial and data from several additional trials have demonstrated the benefit of continued trastuzumab plus chemotherapy following disease progression on a trastuzumab-containing regimen.2,29–31 Moreover, National Comprehensive Cancer Network treatment guidelines recommend continuation of HER2 blockade for patients with HER2+ MBC who progress on first-line trastuzumab-containing regimens.2 This also applies to patients who are diagnosed with HER2+ metastatic disease following prior exposure to trastuzumab in the adjuvant setting.2
Irrespective of prior trastuzumab treatment, eribulin plus trastuzumab demonstrated activity as first-line treatment for patients with HER2+ locally advanced or MBC comparable to that reported for other chemotherapy combinations evaluated in a similar setting. Docetaxel, vinorelbine, capecitabine, and paclitaxel with or without carboplatin are chemotherapeutic agents that are currently recommended in combination with trastuzumab as first-line treatment for patients with HER2+ MBC.2 Results from multiple trials for trastuzumab plus docetaxel have indicated time to progression (TTP) ranging from 8.3 to 12.4 months and ORR ranging from 45% to 61%.32–35 A study comparing vinorelbine plus trastuzumab and docetaxel plus trastuzumab demonstrated a TTP of 15.3 and 12.4 months, respectively, and an ORR of 59.3% in both arms.34 In trials that combined trastuzumab and capecitabine, TTP results ranged from 7.8 to 9.1 months and ORR ranged from 38% to 65% in patients with HER2+ MBC.36,37 Results for the combination of trastuzumab and paclitaxel indicated TTP ranging from 8.6 to 12.1 months and ORR of 36% to 75%.38–40 A PFS of 10.7 months and an ORR of 52% have been reported for the combination of trastuzumab plus paclitaxel and carboplatin.40 Only one of the aforementioned studies32 reported having a substantial percentage of patients who had received prior trastuzumab (27% of patients treated with docetaxel plus trastuzumab).32
The combination of eribulin plus trastuzumab showed an acceptable safety profile similar to other single agents that are commonly combined with trastuzumab as first-line therapy. The rate of febrile neutropenia and neutropenia (all grades) in patients who received prior trastuzumab was numerically higher than those observed in the pivotal Phase III EMBRACE study20 (14% vs 5% febrile neutropenia in this study compared to EMBRACE, respectively; 62% vs 52% neutropenia in this study compared to EMBRACE, respectively). A numerical increase in the incidence of neutropenia with the combination of chemotherapy and trastuzumab compared with chemotherapy alone has also been observed in previous trials.10
Of note, while in the current era dual HER2 blockade may be considered the treatment of choice for HER2+ MBC in the first-line setting, in the CLEOPATRA trial41 there were differences in efficacy whether or not patients had received prior trastuzumab therapy. In those patients who did receive prior trastuzumab, the median PFS was 10.4 months in the docetaxel–trastuzumab arm and was 16.9 months in the docetaxel–trastuzumab–pertuzumab arm; in patients who had not received prior trastuzumab, the median PFS was 12.6 and 21.6 months in these arms, respectively.41 Overall, in the current study, eribulin’s activity combined with trastuzumab was comparable with that of other combinations currently recommended for HER2+ MBC.
Conclusion
Irrespective of prior trastuzumab use, in this Phase II single-arm trial in patients with HER2+ MBC, the combination of eribulin plus trastuzumab as first-line therapy resulted in high ORRs and CBRs, as well as a prolonged median PFS with an acceptable safety profile similar to other single agents that are commonly combined with trastuzumab as first-line therapy. Moreover, ORRs and PFS, as well as tolerability, were comparable between patients who had received prior trastuzumab treatment and those who were trastuzumab-naïve. The most common grade 3/4 TEAEs in trastuzumab-naïve patients versus those who received prior trastuzumab were neutropenia, peripheral neuropathy, febrile neutropenia, fatigue, nausea, vomiting, and leukopenia.
Acknowledgments
The authors thank Maura Dickler, Erica Mayer, Antoinette Tan, Kevin Kalinsky, and Weichung Joseph Shih of the Data Safety Monitoring Board for all their substantial contributions to this study, and Leonard Lionnet PhD and Sherri D Jones PhD of MedVal Scientific Information Services, LLC, for providing medical writing and editorial assistance. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: The GPP2 Guidelines.” Funding to support this study and the preparation of this manuscript was provided by Eisai Inc.
Data from this paper were previously presented:
- Wilks S, Puhalla S, O’Shaughnessy J, Schwartzberg L, Berrak E, Song J, Cox D, Vahdat L. Phase 2, multicenter, single-arm study of eribulin mesylate + trastuzumab as first-line therapy for locally recurrent or metastatic HER2 positive breast cancer. Poster presented at the San Antonio Breast Cancer Symposium. December 10–14, 2013. Poster P4-12-12.
- Puhalla S, Wilks S, Brufsky A, et al. Clinical effects of prior trastuzumab on combination eribulin mesylate plus trastuzumab as first-line treatment for HER2+ locally recurrent or metastatic breast cancer (MBC): Results from a phase II, single-arm, multicenter study [abstract]. J Clin Oncol. 2014;32(15 suppl):abstr 635.
- O’Shaughnessy J, Huggins-Puhalla SL, Wilks S, Brufsky A, Schwartzberg LS, Berrak E, Song JX, Cox D, Vahdat LT. Clinical effects of prior trastuzumab on combination eribulin mesylate plus trastuzumab as first-line treatment for HER2+ locally recurrent or metastatic breast cancer (MBC): Results from a phase 2, single-arm, multicenter study [abstract]. J Clin Oncol. 2014;32(suppl 26):abstr 139.
- O’Shaughnessy J, Puhalla S, Wilks S, Brufsky A, Schwartzberg L, Berrak E, Song J, Cox D, Vahdat L. Clinical effects of prior trastuzumab on combination eribulin mesylate + trastuzumab as first-line treatment for HER2+ locally recurrent or metastatic breast cancer: results from a phase 2, single-arm, multicenter study. Poster presented at the VIII Franco Brazilian Congress of Oncology. October 9–11, 2014. Poster E56.
- Wilks S, Puhalla S, O’Shaughnessy J, Schwartzberg L, Berrak E, Song J, Cox D, Vahdat L. Final results from a phase 2, multicenter, single-arm study of eribulin mesylate + trastuzumab as first-line therapy for locally recurrent or metastatic HER2+ breast cancer. Poster presented at the 31st Annual Miami Breast Cancer Conference. March 6–9, 2014.
Disclosure
SP: consultant for Celldex, Medimmune, and Pfizer; SW: no conflicts; AMB: no conflicts; JO: consultant for Eisai Inc.; LSS: consultant for and research support provided for Eisai Inc.; EB: Eisai employee; JS: Eisai employee; and LV: consultant for and served on the speaker’s bureau for Eisai Inc.
References
SEER Cancer Statistics Factsheets: Female Breast Cancer. National Cancer Institute. Bethesda, MD. Available from: http://seer.cancer.gov/statfacts/html/breast.html. Accessed May 13, 2016. | ||
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast Cancer. Version 2. 2016. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed May 13, 2016. | ||
Spitale A, Mazzola P, Soldini D, Mazzucchelli L, Bordoni A. Breast cancer classification according to immunohistochemical markers: clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann Oncol. 2009;20(4):628–635. | ||
Lund MJ, Butler EN, Hair BY, et al. Age/race differences in HER2 testing and in incidence rates for breast cancer triple subtypes: a population-based study and first report. Cancer. 2010;116(11):2549–2559. | ||
Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18(6):977–984. | ||
Heinemann V, Di Gioia D, Vehling-Kaiser U, et al. A prospective multicenter phase II study of oral and i.v. vinorelbine plus trastuzumab as first-line therapy in HER2-overexpressing metastatic breast cancer. Ann Oncol. 2011;22(3):603–608. | ||
Redana S, Donadio M, Nolè F, et al. Trastuzumab with either docetaxel or vinorelbine as first-line treatment for patients with HER2-positive advanced breast cancer: a retrospective comparison. BMC Cancer. 2010;10:28. | ||
Ishida T, Kiba T, Takeda M, et al. Phase II study of capecitabine and trastuzumab combination chemotherapy in patients with HER2 overexpressing metastatic breast cancers resistant to both anthracyclines and taxanes. Cancer Chemother Pharmacol. 2009;64(2):361–369. | ||
Sato N, Sano M, Tabei T, et al. Combination docetaxel and trastuzumab treatment for patients with HER-2-overexpressing metastatic breast cancer: a multicenter, phase-II study. Breast Cancer. 2006;13(2):166–171. | ||
Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. | ||
Kümler I, Tuxen MK, Nielsen DL. A systematic review of dual targeting in HER2-positive breast cancer. Cancer Treat Rev. 2014;40(2):259–270. | ||
Chung A, Cui X, Audeh W, Giuliano A. Current status of anti-human epidermal growth factor receptor 2 therapies: predicting and overcoming herceptin resistance. Clin Breast Cancer. 2013;13(4):223–232. | ||
Jordan MA, Kamath K, Manna T, et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005;4(7):1086–1095. | ||
Okouneva T, Azarenko O, Wilson L, Littlefield BA, Jordan MA. Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Mol Cancer Ther. 2008;7(7):2003–2011. | ||
Smith JA, Wilson L, Azarenko O, Zhu X, Lewis BM, Littlefield BA, Jordan MA. Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry. 2010;49(6):1331–1337. | ||
Jordan MA, Kamath K. How do microtubule-targeted drugs work? An overview. Curr Cancer Drug Targets. 2007;7(8):730–742. | ||
Vahdat LT, Pruitt B, Fabian CJ, et al. Phase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2009;27(18):2954–2961. | ||
Cortes J, Vahdat L, Blum JL, et al. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2010;28(25):3922–3928. | ||
Aogi K, Iwata H, Masuda N, et al. A phase II study of eribulin in Japanese patients with heavily pretreated metastatic breast cancer. Ann Oncol. 2012;23(6):1441–1448. | ||
Cortes J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377(9769):914–923. | ||
Halaven (eribulin mesylate) injection [prescribing information]. Woodcliff Lake, NJ: Eisai Inc.; 2016. | ||
Wilks S, Puhalla S, O’Shaughnessy J, et al. Phase 2, multicenter, single-arm study of eribulin mesylate with trastuzumab as first-line therapy for locally recurrent or metastatic HER2-positive breast cancer. Clin Breast Cancer. 2014;14(6):405–412. | ||
Mohd Sharial MS, Crown J, Hennessy BT. Overcoming resistance and restoring sensitivity to HER2-targeted therapies in breast cancer. Ann Oncol. 2012;23(12):3007–3016. | ||
Olson E, Mullins D. When standard therapy fails in breast cancer: current and future options for HER2-positive disease. J Clin Trials. 2013;3:1000129. | ||
Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. | ||
Saura C, Garcia-Saenz JA, Xu B, et al. Safety and efficacy of neratinib in combination with capecitabine in patients with metastatic human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2014;32(32):3626–3633. | ||
Zhao M, Pan X, Layman R, et al. A Phase II study of bevacizumab in combination with trastuzumab and docetaxel in HER2 positive metastatic breast cancer. Invest New Drugs. 2014;32(6):1285–1294. | ||
Bachelot T, Garcia-Saenz JA, Verma S, et al. Sunitinib in combination with trastuzumab for the treatment of advanced breast cancer: activity and safety results from a phase II study. BMC Cancer. 2014;14:166. | ||
von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast international group 03-05 study. J Clin Oncol. 2009;27(12):1999–2006. | ||
Von Minckwitz G, Zielinski C, Maarteense E, et al. Capecitabine vs. capecitabine + trastuzumab in patients with HER2-positive metastatic breast cancer progressing during trastuzumab treatment: The TBP phase III study (GBG 26/BIG 3–05) [abstract]. J Clin Oncol. 2008;26(15S):1025. | ||
Bartsch R, Wenzel C, Altorjai G, et al. Capecitabine and trastuzumab in heavily pretreated metastatic breast cancer. J Clin Oncol. 2007;25(25):3853–3858. | ||
Hurvitz SA, Dirix L, Kocsis J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31(9):1157–1163. | ||
Servitja S, Ramos M, Gil M, et al. Multicenter, phase II, nonrandomized study of docetaxel plus trastuzumab every 21 days as the primary therapy in metastatic breast cancer overexpressing HER2. Anticancer Drugs. 2012;23(2):239–246. | ||
Andersson M, Lidbrink E, Bjerre K, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol. 2011;29(3):264–271. | ||
Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23(19):4265–4274. | ||
Yamamoto D, Iwase S, Kitamura K, Odagiri H, Yamamoto C, Nagumo Y. A phase II study of trastuzumab and capecitabine for patients with HER2-overexpressing metastatic breast cancer: Japan Breast Cancer Research Network (JBCRN) 00 Trial. Cancer Chemother Pharmacol. 2008;61(3):509–514. | ||
Michalaki V, Fotiou S, Gennatas S, Gennatas C. Trastuzumab plus capecitabine and docetaxel as first-line therapy for HER2-positive metastatic breast cancer: phase II results. Anticancer Res. 2010;30(7):3051–3054. | ||
Fountzilas G, Tsavdaridis D, Kalogera-Fountzila A, et al. Weekly paclitaxel as first-line chemotherapy and trastuzumab in patients with advanced breast cancer. A Hellenic Cooperative Oncology Group phase II study. Ann Oncol. 2001;12(11):1545–1551. | ||
Gasparini G, Gion M, Mariani L, et al. Randomized phase II trial of weekly paclitaxel alone versus trastuzumab plus weekly paclitaxel as first-line therapy of patients with Her-2 positive advanced breast cancer. Breast Cancer Res Treat. 2007;101(3):355–365. | ||
Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006;24(18):2786–2792. | ||
Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–119. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.