Back to Journals » Journal of Asthma and Allergy » Volume 13
Clinical Determinants of Successful Omalizumab Therapy in Severe Allergic Asthma Patients: 4-Year-Long, Real-Life Observation
Authors Kucharczyk A, Więsik-Szewczyk E , Poznańska A , Jahnz-Różyk K
Received 15 September 2020
Accepted for publication 31 October 2020
Published 14 December 2020 Volume 2020:13 Pages 659—668
DOI https://doi.org/10.2147/JAA.S282203
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Amrita Dosanjh
Aleksandra Kucharczyk,1 Ewa Więsik-Szewczyk,1 Anna Poznańska,2 Karina Jahnz-Różyk1
1Department of Internal Medicine, Pulmonology, Allergy and Clinical Immunology, Central Clinical Hospital of the Ministry of National Defence, Military Institute of Medicine, Warsaw, Poland; 2Department of Population Health Monitoring and Analysis, National Institute of Public Health – National Institute of Hygiene, Warsaw, Poland
Correspondence: Ewa Więsik-Szewczyk
Department of Internal Medicine, Pulmonology, Allergy and Clinical Immunology, Central Clinical Hospital of the Ministry of National Defence, Military Institute of Medicine, Szaserów 128, Warsaw 01-141, Poland
Tel/ Fax +48 261 816 581
Email [email protected]
Introduction: Omalizumab is a high-cost therapy recommended for the treatment of severe allergic asthma.
Objective: To find clinical parameters that are related to the sustained response to omalizumab.
Patients and Methods: This retrospective, real-life, 4-year follow-up was provided in Poland between March 2013 and May 2019. The success of omalizumab was assessed based on composed subjective and objective criteria. Simple/multiple regression analyses were performed to search for predictors of the response to omalizumab.
Results: A total of 989 severe allergic asthma patients were referred for omalizumab therapy, of whom 854 patients were considered eligible for treatment. At weeks 16 and 52, omalizumab was successful in 84% and 91% of patients, respectively. Treatment effectiveness was maintained up to the 4-year follow-up. Four predictors of the response to omalizumab were found at week 16 and two at week 52. The results at week 16 may be used as predictors of success at week 52 based on the model including baseline FEV1% and change in ACQ-7 and miniAQLQ score at week 16: the area under the ROC curve equals 0.746 [95% CI: 0.672– 0.820].
Conclusion: Omalizumab therapy is very effective, with this efficacy sustained after 4 years of treatment. Success of the therapy can be predicted from the baseline FEV1% and clinical improvement (based on ACQ-7 and miniAQLQ scores) at week 16.
Keywords: allergic asthma, anti-IgE, biologics, personalized therapy
Introduction
Asthma is a serious health problem worldwide, affecting as many as 300 million people. Approximately 3.7% of this population suffers from severe asthma.1 There are 2 million asthma patients registered in the databases of the National Health Fund in Poland, and approximately 5% suffer from severe asthma.2 According to available epidemiological data in 2014, asthma exacerbations were the cause of 28 870 hospitalizations in adults, 17 508 hospitalizations in children, 1% of in-hospital deaths, and 1% of 30-days post-discharge mortality.3
In severe asthma patients, control is not achieved with Step 4 or Step 5 treatment according to Global Initiative For Asthma (GINA) [eg, medium or high dose of inhaled corticosteroids (ICS)4 with a second controller; maintenance oral corticosteroids (OCS)], despite simultaneous and adequate management of contributory factors or worsening asthma when step down therapy is initiated.1
Biological therapy is currently the treatment of choice for severe asthma, whereas the administration of OCS as the maintenance treatment should be avoided. Biological treatment targets two Th-2 high asthma phenotypes: allergic asthma and eosinophilic asthma. Allergic asthma appears to be the most common asthma phenotype.5 It accounts for approximately 40% of cases in adults and over 70% of cases in children.6 It is characterized by the presence of specific IgE and increased total IgE (tIgE) levels, which play a crucial role in the pathogenesis of the disease.7 For this reason, IgE-mediated immunologic pathways represent an attractive target for therapeutic agents. To date, the only drug known to reduce IgE levels developed for the treatment of allergic asthma is omalizumab, a recombinant humanized IgG1 monoclonal antibody that binds to the Fc part of free IgE, preventing binding to high-affinity IgE receptor (FcϵRI) and downregulating receptor expression. Omalizumab was first registered in 2003 in the United States and in 2005 in Europe. However, long-term data from the real-life experience of large groups of patients are still not satisfactory.4,8 The longest follow-up study lasted 9 years, but it only included a small number of patients.9 In turn, the largest retrospective study to date involved 872 patients, but the follow-up was only 12 months.10 Moreover, it was recently shown that the severe-asthma population in Europe is highly heterogeneous and differs in terms of both clinical characteristics and treatment prior to biological therapy.11,12 This emphasizes the importance of specific population evaluations. To date, little is known about the characteristics and treatments of patients with severe asthma in Poland.3,13
The question of the practical aspects of predictive factors for response to therapy also continues to arise. This follows, on the one hand, the high cost of this therapy and, on the other hand, the possibility of choosing other medications targeting Th2 inflammation, especially in patients with high eosinophilia (anti-IL-5/IL-5R).1,14 Certainly, biological treatment should first be initiated in those patients who are expected to be responders, so there is a need for a tool that allows the prediction of the response to biological treatment in everyday practice. The aim of the present study was to characterize the large group of patients treated with omalizumab and to search for potential predictors of response to therapy.
This is the first long-term, real-life, retrospective, observational study of severe allergic asthma in Poland. To our knowledge, this is also the first study with such a large group of patients treated for 4 years.
Patients and Methods
This is a real-life retrospective analysis based on data collected in an electronic database of the National Health Fund (national payer) from 50 centres (Supplementary table 1S) experienced in the treatment of severe asthma patients.
A total of 989 patients with severe allergic asthma were subsequently entered into the database and checked for eligibility for omalizumab treatment according to the inclusion and exclusion criteria of the drug programme (National Treatment Regime) from March 2013 until May 2019.15,16 The inclusion and exclusion criteria of the drug programme are presented in Table 2S. A total of 854 patients started treatment and took at least one dose of omalizumab. The efficacy assessments were performed at week 16, at week 52 (since the baseline), and then every 52 weeks, and follow-up was carried out until the 4th year of treatment. The patients’ flow chart is shown in Figure 1. The effectiveness was assessed based on the following criteria: number of exacerbations in comparison to the period before the treatment, OCS dose, quality of life (mini Asthma Quality of Life Questionnaire – miniAQLQ), asthma control (Asthma Control Questionnaire – ACQ-7), and physician’s overall evaluation according to the Global Evaluation of Treatment Effectiveness (GETE) scale. Patients were treated with omalizumab 150–600 mg every 2 to 4 weeks (with the dose based on the dosing table; if the dose was outside the dosing table, the patient was excluded).
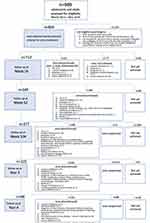 |
Figure 1 Flow chart of patients registered for treatment with omalizumab (oma). |
The treatment was assessed as effective (according to the National Treatment Regime) and could only be continued if the patient met the following criteria:
- GETE scale: very good or good response to treatment
- A decrease in the annual exacerbation rate (any reduction)
- At least two of the following:
- increase in miniAQLQ by > 0.5 points,
- decrease in ACQ-7 by > 0.5 points
- any reduction in the OCS dose.
A severe exacerbation was defined as a significant worsening of asthma requiring systemic corticosteroids for at least 3 days or, for patients on chronic OCS treatment, an increase in the OCS dose regimen for at least 3 days.
The Bioethics Committee of the Military Institute of Medicine in Warsaw approved the protocol of the study (approval no. 14/WIM/2020). Separated patient consent to review their records collected in the database was not required by the IRB due to the retrospective nature of the study. All patient data were confidential and the study procedures complied with the Declaration of Helsinki.
Statistical Analysis
General characteristics of the study population were analysed using methods of descriptive statistics. For the continuous variables, the mean value, standard deviation, and median are presented – most of these characteristics, according to the Shapiro–Wilk test, deviated from a Gaussian distribution.
The distributions of the miniAQLQ, ACQ-7, OCS dose, and their changes in relation to the baseline were compared between checkpoints using the Kruskal–Wallis test, and significantly different pairs were identified by the post hoc Dunn test.
The analysis of predictive factors was performed in two steps. In the first simple logistic regression, the baseline characteristics significantly related to the success of omalizumab therapy (binary dependent variable) were identified by determining odds ratios (OR), their 95% confidence intervals (95% CI), and statistical significance (p-value). These factors were used as the independent variables in the second step of the analysis. To eliminate their interactions, a final set of predictors was established, and the strength of the association between them and the success of omalizumab therapy were assessed. The multiple logistic regression method was applied. The analyses were performed separately for weeks 16 and 52. The results of treatment after 16 weeks were used as additional predictors for week 52.
Receiver operating characteristics17 and the area under the curve (AUC) were used to assess the quality of the prediction model.
The significance level for all the tests was assumed to equal 0.05. The analysis was performed using SPSS 12.0 PL and R ver. 3.6.1.
Results
At the end of the analysed period (MAY 2019), the database contained data from 989 patients, adults and adolescents ≥12 years of age, reported as being checked for eligibility for omalizumab treatment. As only 4 adolescents (12–17 years of age) participated in the study, no separate analysis was performed for this group. Patient characteristics are presented in Table 1.
 |
Table 1 Characteristics of Severe Allergic Asthma Patients Checked for Eligibility for Omalizumab Treatment |
All the patients had a history of uncontrolled severe allergic asthma as reflected by the number of severe asthma events in the previous year (severe exacerbations: mean value± standard error (SE) 4.7±0.3 and hospitalizations due to asthma in the previous year: 73.8%), a near-fatal asthma (NFA) episode in the past (31.5%), significantly lowered quality of life (miniAQLQ: mean±SE 2.98±0.03) and lack of asthma control (ACQ-7: mean±SE 3.52±0.03). At baseline, 43% of patients were on maintenance OCS treatment (average daily dose 10 mg), while the remaining patients had to receive at least one systemic corticosteroid burst in the 6 months prior to treatment.
The mean serum tIgE level was 339±9 IU/mL. Data on eosinophilia were not collected.
A total of 854 of these patients met the eligibility criteria and received at least one dose of omalizumab.
Long-Term Response to Omalizumab Treatment
Omalizumab treatment improved the quality of life. In the 4-year follow-up period, a systematic increase in miniAQLQ was noted. Although the greatest improvement (1.3 points on average) was observed at week 16, the rate increased throughout the study period by 2.1 points after 4 years (from baseline miniAQLQ = 2.9 to miniAQLQ = 5.0 at the 4th year).
Significant improvement was observed in asthma control. The greatest improvement in ACQ – scores was seen at week 16 (mean 1.2 points). However, from week 52 on, this value remained practically constant (baseline ACQ-7 = 3.8 and year 4 ACQ-7 = 2.1; change by 1.7 points).
The mean OCS dose decreased from 10.8±0.3 mg (mean±SE) observed at baseline to 2.7±0.6 mg after 4 years of treatment. A significant change was observed at week 16 (dose reduction by 6.6±0.3 mg; p=0.001). At the week 16 visit, more than half of the patients (56%) had not used OCS at all.
Distributions of the miniAQLQ, ACQ-7 scores, OCS dose at all follow-up points are shown in the box plots charts (Figure 2).
 |
Figure 2 Distributions of the miniAQLQ (A), ACQ-7 scores (B), OCS dose (C) at all follow-up points (shown in the box plots charts). |
Predictors of Response to Omalizumab Treatment in Logistic Regression Analysis
In the simple logistic regression analysis the success of omalizumab treatment at week 16 was positively associated with higher tIgE (OR=1.015 95% CI: [1.005–1.025]), FEV1% (OR=1.010 95% CI: [1.000–1.019)] and allergy to cat (OR=1.595 95% CI [1.038–2.451]). Negatively correlated with advanced age (OR=0.997 95% CI: [0.962–0.992]), prolonged asthma (OR=0.972 95% CI: [0.948–0.996]), hospitalizations in the preceding year (OR=0.643 95% CI: [0.424–0.975]), NFA episodes (OR=0.561 95% CI: [0.369–0.852]), cardiovascular diseases (OR=0.568 95% CI: [0.374–0.865]), and diabetes (OR=0.418 [0.225–0.777]) (Table 2).
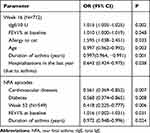 |
Table 2 Patient Characteristics Significantly Related to Response to Therapy at Weeks 16 and 52 (Results of Simple Logistic Regression) |
However, multiple regression analysis showed that four factors significantly influenced the effect of therapy at week 16 – one positively: tIgE level (OR=1.015 95% CI: [1.005–1.025]) and three negatively: asthma duration (OR=0.979 [0.965–0.994]), hospitalizations in the last year due to asthma (OR=0.605 95% CI [0.395–0.928]), and NFA episodes (OR= 0.636 95% CI [0.414–0.977]) (Table 3).
It means that if other parameters are the same, success odds for a patient with NFA episode in the past is lower by 36.4% than for a patient without NFA episode. Any hospitalization (one or more) during the preceding year decreases the success by 39.5%. Each additional 10 years of asthma duration reduces success odds by 19.1%. In contrast, each 100 tIgE units at baseline increases the chance of successful treatment by 16.1% (Figure 3).
Two factors significantly influenced the effect of therapy at week 52. The higher FEV1% was positive factor (OR=1.016 95% CI: [1.002–1.031]). The duration of severe asthma was negative factor (OR=0.972 95% CI [0.948–0.996]). (Table 2).
As predictors of success at week 52, the results at week 16 could also be used. Finally, three factors were the significant success predictors at week 52: improvement in ACQ-7 and miniAQLQ scores at week 16 (OR=2.081 95% CI [1.357–3.192]) and (OR=1.630 95% CI [1.042–2.550]), and FEV1% at baseline (OR=1.016 95% CI [1.001–1.032]). Each additional 0.1 point of improvement in ACQ-7 and miniAQLQ scores observed in week 16 increased success odds by 7.5% and 5.0%, respectively. Each percentage point of FEV1 at baseline increased the chance for success of omalizumab therapy by 1.6%. The strength of association between these factors and therapy success at week 52 is presented in Figure 4.
The three factors, improvement in ACQ-7 and miniAQLQ scores at week 16 and FEV1% at baseline are sufficient to build a fair model of therapy success at week 52. The area under the ROC curve equals 0.746 [95% CI: 0.672–0.820], which implies 80% sensitivity against 56% specificity (Figure 5).
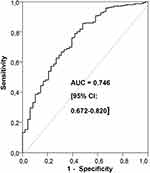 |
Figure 5 ROC curve for the multivariate model of success of omalizumab treatment at week 52. |
Discussion
We present the clinical characteristics of severe allergic asthma patients registered for omalizumab treatment in Poland from 50 severe asthma treatment centers. To our knowledge, this is one of the longest (4 years long) real-life observations that included many patients (n=733 at entry).
The main goal of the analysis was to find predictors of the response to omalizumab therapy. The results have shown that there are four factors that significantly predict treatment results at week 16: the higher the concentration of tIgE, the higher the likelihood of a beneficial response to omalizumab, whereas longer asthma duration, hospitalization within the previous year and an NFA episode in the past increase the risk of treatment failure. We have shown that less impaired lung function (higher FEV1%) favors a good result of omalizumab treatment for week 52. The duration of asthma remains a poor prognostic factor. The most interesting is that the results at week 16 may be used as predictors of success at week 52 as well as in the following years, according to the persistent efficacy confirmed in our study. We found that long-term efficacy could be predicted from a model including three easy-to-use factors: lung function (FEV1%) at baseline and improvement in the ACQ-7 and miniAQLQ scores at week 16 of omalizumab treatment. We confirmed that an improvement in the ACQ-7 score and the miniAQLQ score by one point at week 16 significantly increased the chance for long-term treatment effectiveness.
Comparison of the results of real-life studies is doubtful. On the one hand, the criteria for reimbursement for omalizumab treatment depend on local regulations1,3,10,18,19 and on the other hand, there are no uniform criteria for assessing the effectiveness of therapy.10,18,20,21 In our study, the patient population was determined by the criteria of the National Treatment Regime. Our patient characteristics were similar to those of other severe asthma populations in terms of mean age (46.8 years), gender (62% female), number of exacerbations within the year before omalizumab treatment,4,7 baseline ACQ-7, and miniAQLQ. A similar percentage (44%) of patients at the beginning of omalizumab treatment were taking OCS as the maintenance treatment.22
The efficacy of long-term omalizumab treatment was assessed at strictly defined time points: after 16 and 52 weeks since the beginning of omalizumab therapy and every 52 weeks thereafter. The last monitoring visit took place after the 4th year of treatment.
There is no universal definition of a responder to biological therapies for asthma. In our study, the efficacy criteria were detailed by the National Treatment Regime. The patients had to meet subjective (GETE scale, improvement in ACQ-7 and miniAQLQ score) and objective criteria (reduction in the number of exacerbations and reduction in the OCS dose, regardless of what extent). For this reason, the assessment seems to be reliable and consistent with current recommendations.
Treatment with omalizumab was successful in most patients (84% at week 16), which is in agreement with other studies.22 Significant improvements in quality of life and asthma control were achieved, the greatest at week 16, but gradually increasing and lasting up to the 4th year of treatment. Similarly, the mean dose of OCS decreased significantly. At the end of the study, dose reduction achieved 75%, and most patients did not require any systemic corticoids. Significant improvements in GETE, lung function and exacerbation rates were also demonstrated.
In the subgroup of patients, we observed that the expected effectiveness of treatment is achieved after more than 16 weeks (at least 6 months), which is consistent with other studies.19,23 In our analysis at the first checkpoint (week 16), failure was observed in 111 patients (16%). Nevertheless, only 28 patients (3.9%) were excluded from treatment. At week 52, ineffectiveness of therapy was observed in only 9% of patients. This confirms that there are late responders and that the decision to discontinue omalizumab treatment should be made later than on week 16.
Although the effectiveness of omalizumab was assessed in real-life studies, either the treatment period was short (16–52 weeks to a maximum of 2 years) or the group of patients was small.20–27
In the first published study of the response to omalizumab treatment, episodes of NFA in the previous year, a high beclomethasone dipropionate dose and poor lung function (FEV1≤65%) were good clinical predictors.28 These results are inconsistent with our study. The outcome of our analysis may have been influenced by the inclusion of patients with FEV1>80%, who were usually excluded in other studies, or by the fact that the precise inclusion criteria for treatment were not given.23
We confirmed that tIgE level can be a predictor of successful omalizumab therapy at week 16. The relationship between the tIgE concentration and treatment results was not found in two other studies. This discrepancy may be due to differences in the mean baseline tIgE concentration: a large group of patients with tIgE between 0 and 100 IU/mL were included in one study, and in the other, the mean concentration of tIgE was 198 IU/mL; by contrast, in our group, the mean value was much higher (339 IU/mL|).29,30
One of the limitations of our study is that we did not have access to data on eosinophilia. The results of previous studies on the impact of eosinophilia on the number of exacerbations and efficacy of omalizumab treatment are divergent or no such relationship was observed.10 It seems that the results of controlled studies pointing to eosinophils as predictors of the efficacy of omalizumab therapy may be stressed with a methodological bias.3,7,31,32
The other limitation of our study was its retrospective design, but even though RCTs are still the most reliable form of scientific evidence, real-life studies allow for the assessment of a more diverse group of patients who would not be included in a clinical trial, for instance, because of FEV1% values that are too high or too low.3,8 Unfortunately, we did not have access for the results of global pulmonary function tests which were not collected in the database. However we made analysis based on the model including baseline FEV1%.
Regardless of these limitations, our study is of interest because it covers the entire population of Polish patients treated for severe allergic asthma in real-life setting. Our patient characteristics were similar to those of other severe asthma populations. Despite its retrospective nature, we selected checkpoints (monitoring visits) with the assessment of strictly defined criteria of therapy effectiveness. Finally, each dose of omalizumab was administered in the center (every 2 to 4 weeks), which resulted in very high compliance. It means that the results can be compared with those obtained in other studies.
Conclusion
In conclusion, data from our 4-year study and a large patient sample confirmed the effectiveness of omalizumab treatment in real-life setting. Biological therapies are now the treatment of choice in patients with severe asthma.1 With an increasing number of available target therapies, predefined criteria for selection of the most suitable treatment for individual patients are needed. In our opinion, it should be based on simple parameters that are easily available for physicians. A predictive model of the long-term response to omalizumab treatment, might include baseline FEV1%, improvement in ACQ-7 and miniAQLQ scores at treatment week 16.
Ethics Approval and Informed Consent
All patients provided written inform consent before omalizumab treatment according to local low. The authors obtained written consent for using database for scientific research purposes, in accordance with regulations of local law. The Bioethics Committee of the Military Institute of Medicine in Warsaw approved the protocol of the study (approval no. 14/WIM/2020). Since this is a retrospective study, separated patient consent to review their records collected in the database was not required. All patient data were confidential and the study procedures complied with the Declaration of Helsinki.
Disclosure
AK received lecture fees from Astra Zeneca and Novartis, ad board fees from Novartis, Teva, and GSK, and reports statistical analysis, advisory board, and lectures for Novartis, during the conduct of the study. EWS has nothing to declare. AP reports personal fees from Novartis, outside the submitted work and is an author of Epidemiological Statistical Report concerning biologically treated patients for Novartis (2019) that did not influence the findings presented in this article.KJR received lecture and ad board fees from Astra Zeneca, Novartis, GSK, and Sanofi Aventis, outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
1. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2020. Available from: www.ginasthma.org.
2. Mapa Potrzeb Zdrowotnych MPZ ((Health Need Maps). Avaiable from mz.www.gov.pl.
3. Jahnz-Różyk K, Lis J, Warchoł M, Kucharczyk A. Clinical and economic impact of a one-year treatment with omalizumab in patients with severe allergic asthma within a drug programme in Poland. BMC Pulm Med. 2018;18(1):48. doi:10.1186/s12890-018-0610-z
4. Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
5. Schatz M, Rosenwasser L. The allergic asthma phenotype. J Allergy Clin Immunol Pract. 2014;2(6):645–648. (). doi:10.1016/j.jaip.2014.09.004
6. Akar-Ghibril N, Casale T, Custovic A, Phipatanakul W. Allergic endotypes and phenotypes of asthma [published correction appears in J Allergy Clin Immunol Pract. 2020 May;8(5):1779]. J Allergy Clin Immunol Pract. 2020;8(2):429–440. doi:10.1016/j.jaip.2019.11.008
7. Corry DB, Kheradmand F. Induction and regulation of the IgE response. Nature. 1999;402(6760 Suppl):B18–23. doi:10.1038/35037014
8. Alhossan A, Lee CS, MacDonald K, Abraham I. “Real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: meta-analysis. J Allergy Clin Immunol Pract. 2017;5(5):1362–1370.e2. doi:10.1016/j.jaip.2017.02.002
9. Mansur AH, Srivastava S, Mitchell V, Sullivan J, Kasujee I. Longterm clinical outcomes of omalizumab therapy in severe allergic asthma: study of efficacy and safety. Respir Med. 2017;124:36–43. doi:10.1016/j.rmed.2017.01.008
10. Humbert M, Taille C, Mala L, et al. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: the STELLAIR study. Eur Respir J. 2018;51(5):1702523. doi:10.1183/13993003.02523-2017
11. van Bragt J, Adcock IM, Bel EHD, et al. Characteristics and treatment regimens across ERS SHARP severe asthma registries. Eur Respir J. 2020;55:1. doi:10.1183/13993003.01163-2019
12. Cabrejos S, Moreira A, Ramirez A, et al. FENOMA study: achieving full control in patients with severe allergic asthma. J Asthma Allergy. 2020;13:159–166. doi:10.2147/JAA.S246902
13. Kupryś-Lipińska I, Majak P, Molinska J, Kuna P. Effectiveness of the Polish program for the treatment of severe allergic asthma with omalizumab: a single-center experience. BMC Pulm Med. 2016;16(1):61. doi:10.1186/s12890-016-0224-2
14. Uddin M, Watz H, Malmgren A, Pedersen F. NETopathic Inflammation in Chronic Obstructive Pulmonary Disease and Severe Asthma. Front Immunol. 2019;10:47. doi:10.3389/fimmu.2019.00047
15. Available from https://www.gov.pl/web/zdrowie/programy-lekowe.
16. NOTICE OF THE MINISTER OF HEALTH of 26 October 2012 on the list of reimbursed drugs, foodstuffs intended for particular nutritional uses and medical devices at the date of 1 November 2012. Available on http://www.mz.gov.pl/leki/refundacja/lista-lekow-refundowanych-obwieszczenia-ministra-zdrowia/obwieszczenie-ministra-zdrowia-z-dnia-26-pazdziernika-2012-r.
17. McDonald VM, Hiles SA, Godbout K, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Respirology. 2019;24(1):37–47. doi:10.1111/resp.13389
18. Brusselle G, Michils A, Louis R, et al. “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med. 2009;103(11):1633–1642. doi:10.1016/j.rmed.2009.06.014
19. Casale TB, Pacou M, Mesana L, Farge G, Sun SX, Castro M. Reslizumab Compared with Benralizumab in Patients with Eosinophilic Asthma: A Systematic Literature Review and Network Meta-Analysis. J Allergy Clin Immunol Pract. 2019;7(1):122–130.e1. doi:10.1016/j.jaip.2018.08.036
20. Tzortzaki EG, Georgiou A, Kampas D, et al. Long-term omalizumab treatment in severe allergic asthma: the South-Eastern Mediterranean “real-life” experience. Pulm Pharmacol Ther. 2012;25(1):77–82.
21. Singh H, Peters JI, Kaur Y, Maselli DJ, Diaz JD. Long-term evaluation of response to omalizumab therapy in real life by a novel multimodular approach: the Real-life Effectiveness of Omalizumab Therapy (REALITY) study. Ann Allergy Asthma Immunol. 2019;1(5):476–482. doi:10.1016/j.anai.2019.07.026
22. MacDonald KM, Kavati A, Ortiz B, Alhossan A, Lee CS, Abraham I. Short- and long-term real-world effectiveness of omalizumab in severe allergic asthma: systematic review of 42 studies published 2008-2018. Expert Rev Clin Immunol. 2019;15(5):553–569. doi:10.1080/1744666X.2019.1574571
23. Kallieri M, Papaioannou AI, Papathanasiou E, Ntontsi P, Papiris S, Loukides S. Predictors of response to therapy with omalizumab in patients with severe allergic asthma - a real life study. Postgrad Med. 2017;129(6):598–604. doi:10.1080/00325481.2017.1321945
24. Yorgancıoğlu A, Öner Erkekol F, Mungan D, et al. Long-term omalizumab treatment: a multicenter, real-life, 5-year trial. Int Arch Allergy Immunol. 2018;176(3–4):225–233. doi:10.1159/000488349
25. Nurkić J, Al-Ahmad M, Maher A, Arifhodžić N, Jusufović E. Most common, real life factors affecting effectiveness of omalizumab asthma treatment: a 10-year study. Med Glas (Zenica). 2019;16(1):45–52.
26. Braunstahl GJ, Chlumský J, Peachey G, Chen CW. Reduction in oral corticosteroid use in patients receiving omalizumab for allergic asthma in the real-world setting. Allergy Asthma Clin Immunol. 2013;9(1):47. doi:10.1186/1710-1492-9-47
27. Vennera Mdel C, Pérez De Llano L, Bardagí Set al., Omalizumab therapy in severe asthma: experience from the Spanish registry–some new approaches. J Asthma. 2012;49(4):416–422. doi:10.3109/02770903.2012.668255
28. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125(4):1378–1386. doi:10.1378/chest.125.4.1378
29. Wahn U, Martin C, Freeman P, Blogg M, Jimenez P. Relationship between pretreatment specific IgE and the response to omalizumab therapy. Allergy. 2009;64(12):1780–1787. doi:10.1111/j.1398-9995.2009.02119.x
30. Bousquet J, Rabe K, Humbert M, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med. 2007;101(7):1483–1492. doi:10.1016/j.rmed.2007.01.011
31. Busse WW. Are peripheral blood eosinophil counts a guideline for omalizumab treatment? STELLAIR Says No! Eur Respir J. 2018;51:5.
32. Casale TB, Chipps BE, Rosén K, et al. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy. 2018;73(2):490–497. doi:10.1111/all.13302
33. Henriksen DP, Bodtger U, Sidenius K, et al. Efficacy of omalizumab in children, adolescents, and adults with severe allergic asthma: a systematic review, meta-analysis, and call for new trials using current guidelines for assessment of severe asthma. Allergy Asthma Clin Immunol. 2020;16(1):49. doi:10.1186/s13223-020-00442-0
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



