Back to Journals » Journal of Hepatocellular Carcinoma » Volume 8
Clinical Characteristics, Treatment Patterns, and Healthcare Costs and Utilization for Hepatocellular Carcinoma (HCC) Patients Treated at a Large Referral Center in Washington State 2007–2018
Authors Shankaran V, Chennupati S, Sanchez H, Sun Q, Li L, Fedorenko C, Aly A, Healey M, Seal B
Received 22 July 2021
Accepted for publication 25 November 2021
Published 14 December 2021 Volume 2021:8 Pages 1597—1606
DOI https://doi.org/10.2147/JHC.S328274
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Tim Meyer
Veena Shankaran,1,2 Shasank Chennupati,1 Hayley Sanchez,1 Qin Sun,1 Li Li,1 Catherine Fedorenko,1 Abdalla Aly,3 Marcus Healey,3 Brian Seal3
1Hutchinson Institute for Cancer Outcomes Research, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; 2Division of Medical Oncology, University of Washington, Seattle, WA, USA; 3AstraZeneca, Gaithersburg, MD, USA
Correspondence: Veena Shankaran
Clinical Research Division, Fred Hutchinson Cancer Research Center, 825 Eastlake Ave. E, MS G4-830, Seattle, WA, 98109, USA
Tel +1 206 667-7844
Fax +1 206 606-2042
Email [email protected]
Introduction: Though the treatment landscape for hepatocellular carcinoma (HCC) has evolved significantly with the refinement of liver-directed therapy techniques and the introduction of new drugs, few studies have investigated the impact of the changing treatment landscape on lifetime treatment costs, particularly in Barcelona Clinic Liver Cancer (BCLC) stage C disease. We sought to investigate real-world clinical characteristics, treatment patterns, and healthcare costs in a cohort of HCC patients treated at a single high-volume institution in Washington (WA) state.
Methods: We conducted a retrospective cohort study of patients diagnosed with HCC between 2007 and 2018 using abstracted electronic medical record (EMR) data linked to cancer registry data and health claims from commercial plans, Medicare, and Medicaid. We described clinical and treatment characteristics, including BCLC stage and Child Pugh score. We investigated median survival and mean lifetime treatment costs by BCLC stage using Kaplan–Meier cost estimator methods. A multivariate Cox proportional hazards model was used to investigate factors associated with overall survival.
Results: The final cohort included 215 patients, the majority of whom were white (71%), male (68%), and with underlying hepatitis C (61%). Mean per patient lifetime costs were highest in BCLC A and BCLC C patients. Mean lifetime costs in BCLC A patients ($292,134) was driven by surgery, hospital, pharmacy, imaging, and outpatient costs. Chemotherapy costs were highest in BCLC C patients, though not the predominant area of spending. Median survival was highest in patients with BCLC 0 and A disease; BCLC stage C and higher area deprivation index (ADI) were associated with poorer survival.
Conclusion: In a cohort of WA state HCC patients, mean lifetime costs were highest in patients with BCLC A disease, attributable to surgery and hospital costs. As increased utilization of newer and less toxic therapies improves survival in BCLC C patients, mean lifetime costs in this group may also rise.
Keywords: hepatocellular carcinoma, health economics, health services research, cost of care
Introduction
Hepatocellular carcinoma (HCC) is the fifth leading cause of cancer-related death in men and the eighth leading cause of cancer-related death in women in the United States (US).1 In 2020, an estimated 30,160 individuals are expected to die from this disease.1 HCC typically develops in the context of chronic liver disease, most commonly from hepatitis B or C virus infection and chronic excessive alcohol consumption. In the US, the obesity epidemic has also contributed to increasing rates of HCC due to a rise in type 2 diabetes and non-alcoholic fatty liver disease (NAFLD).
Over the last several years, the treatment landscape for HCC has changed significantly. In addition to the increased utilization of catheter-based therapies such as radioembolization and chemoembolization, several newly Food and Drug Administration (FDA)-approved systemic therapeutic options such as cabozantinib, nivolumab, lenvatinib, and combination therapy with atezolizumab + bevacizumab have expanded the portfolio of treatment options in advanced and metastatic HCC. However, despite emerging therapies, HCC can be particularly challenging to manage given the frequent coexistence of cirrhosis and other comorbidities. Previous studies have reported significant economic and healthcare utilization impacts of HCC in the United States due to drug therapies, hospitalizations, and imaging studies.2–4 However, these studies have not been updated to reflect the likely increased economic impact related to newer therapeutics and diagnostics.
In the context of recent changes in HCC treatment, we sought to investigate real-world clinical characteristics, treatment patterns, healthcare utilization, and costs in patients with HCC treated at a single high-volume institution. Focusing on a single institution HCC population diagnosed in 2007–2018, we used a combination of medical record and health claims data to describe the clinical characteristics of newly diagnosed HCC patients and provide an estimate of the healthcare resource utilization and direct medical costs associated with diagnosis and treatment.
Methods
Study Design and Data Sources
We conducted a retrospective cohort study of patients diagnosed with HCC between 2007 and 2018 at a single clinical cancer center (Seattle Cancer Care Alliance (SCCA)/University of Washington (UW)). We linked data from the electronic medical record (EMR) data warehouse at SCCA/UW to the Hutchinson Institute for Cancer Outcomes Research (HICOR) data repository, which links cancer registry records from the Western Washington Surveillance Epidemiology and End Results (SEER) to insurance enrollment and claims data from regional commercial payers (Premera Blue Cross and Regence Blue Shield), Medicare, and Medicaid. This study received approval from the Fred Hutchinson Cancer Consortium Institutional Review Board (IRB). All data acquisition, linkage, analysis, and reporting procedures adhered to the relevant data use agreements and privacy regulations.
Study Cohort
All patients from the SCCA/UW EMR data warehouse as having HCC or “liver cancer” as a primary diagnosis during the period of interest (2007–2018) and matched with a record in the HICOR data repository (resided in one of the 13 Western Washington CSS counties at the time of diagnosis and had evidence of enrollment in Premera Blue Cross, Regence Blue Shield, Medicare, or Medicaid) were identified. Of these, only patients who were age 18 years or greater, had continuous insurance enrollment for at least one month following diagnosis, had at least one claim identifying receipt of care at SCCA/UW by Clinic Tax Identification Numbers (TIN) codes, and had available records in the EMR for abstraction (and confirmed HCC on chart abstraction) were included in the analysis. Patients were excluded if their diagnosis year was missing, if their diagnosis and death date were the same, and if the diagnosis was made by autopsy or death certificate. These exclusions were applied to ensure that all patients in the final cohort were indeed seen or treated at UW/SCCA and had sufficient observation time and claims data available to describe healthcare utilization and estimate costs. Given that this is a patterns of care study at a single treatment center rather than a population-based analysis of all HCC patients diagnosed in the region, these exclusion criteria were felt to be necessary to accomplish the stated study objectives.
Demographic and Clinical Data
Demographic and clinical data were obtained from both the regional cancer registry and the EMR. Age at diagnosis, race/ethnicity, insurance type, HCC diagnosis year, and American Joint Committee on Cancer (AJCC) diagnosis stage were all obtained from the cancer registry. International Classification of Diseases (ICD-9 and ICD-10) diagnosis codes from insurance claims were used to assign each patient a Charlson Comorbidity Index (CCI) score, a predictor of mortality in patients with multiple specific comorbidities.5 Residential zip codes obtained from the tumor registry were used to assign each individual an area deprivation index (ADI) score. ADI is a measure of socioeconomic disadvantage at the census block level, ranked 1–10, with 10 being the most disadvantaged decile.6 This index is a composite of variables that assess factors including educational attainment, income and housing at an area level and correlates with mortality disparities. Data abstractors reviewed the EMR for additional demographic and clinical characteristics at diagnosis including primary language, marital status, smoking status, alcohol use, BCLC stage, Child Pugh scores, extent of disease (number of metastatic sites), and the presence of other cancer comorbidities. For patients in whom Child Pugh and BCLC score were not identified in the EMR, these scores were imputed using laboratory (albumin, INR, total bilirubin) and other clinical data (presence of ascites, hepatic encephalopathy, HCC nodule number, size, and location) available in the EMR, when possible.
Treatment and Healthcare Utilization
All treatment received from diagnosis until end of follow-up (either disenrollment from their insurance plan or end of available claims in the HICOR data repository) was determined from healthcare claims and was categorized as systemic therapy, catheter-based therapy, ablation, and surgery. In addition, utilization of endoscopy, advanced imaging, and emergency department (ED) and hospital admissions were determined from administrative claims. Utilization of systemic chemotherapeutics following diagnosis was abstracted from the EMR. Patients who enrolled in at least one clinical trial were identified. In addition, all chemotherapy administration from diagnosis until end of follow-up (disenrollment of end of available claims in Dec 2019) or death was identified from claims using J codes for specific drugs (including in the outpatient prescription files) and chemotherapy administration codes.
Healthcare Costs
Mean lifetime costs for all patients was determined by tabulating all paid claims from diagnosis to death or end of follow-up. Costs were grouped by the type of associated claim: chemotherapy, surgery, ablation, catheter-based therapy, endoscopic procedures, radiation, imaging, and pharmacy. Inpatient, ED, and outpatient costs attributable to specific procedures were included in the total costs of the corresponding procedure; other inpatient, ED, and outpatient costs not attributable to the specific procedures are grouped separately.
Statistical Methods
Summary statistics were used to describe the demographic and clinical characteristics of the study population. Median survival for the entire cohort and for BCLC stage groups was determined using Kaplan-Meier methods, adjusting for censoring. A Cox proportional hazards model estimated the association between BCLC stage, Child Pugh score, and survival, adjusting for relevant clinical and demographic factors such as age, race, CCI, ADI, and insurance type, respecting the minimum ratio of 10 events per predictor.7,8 For these analyses, BCLC D and unknown patients were excluded due to very small numbers. In addition, BCLC 0 and A patients were combined to improve statistical power and given that BCLC 0 and A patients follow a generally similar trajectory in terms of treatments and prognosis. The proportional hazards assumption was checked using Kaplan-Meier curves, ensuring that the curves were parallel and thus assumption was satisfied. The proportion of patients receiving specific therapies or procedures were reported. The mean number of imaging studies and endoscopic procedures per patient was also reported. The Kaplan-Meier sample average (KMSA) cost estimator method was used to calculate total and mean per patient lifetime costs. This method sums the Kaplan-Meier probability of surviving to the beginning of each month multiplied by the mean costs for patients alive at the beginning of each month. This approach avoids bias associated with limiting cost estimation only to patients who have died during follow-up and accounts for censoring of individuals who disenroll.9–11 SAS® analytic software (version 9.4) was used data cleaning and analysis; R software (version 3.5.1) was used for calculating KMSA cost estimates.
Reporting Requirements
In accordance with the SEER data use requirements, cell sizes of 11 or less were not reported in any of the tables and, where appropriate, categories were combined such that results could be reported while abiding by these guidelines.
Results
A total of 1912 potential records associated with a diagnosis of HCC or “liver cancer” were identified from the EMR data warehouse at UW/SCCA of which 1395 records matched to the HICOR database (cancer diagnosed in one of the 13 Western Washington SEER counties and had evidence of enrollment with either Premera, Regence, Medicare, or Medicaid). After applying the exclusion criteria discussed above and eliminating duplicate records, the final study cohort included 215 individual patients with available EMR data, cancer registry records, and health claims available for analysis (Figure 1).
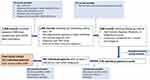 |
Figure 1 Study Cohort. |
Most patients in the final cohort were male (68.4%), white (70.7%), and married (63.3%). There were a substantial proportion (20%) of Asian patients, the largest fraction of which were Vietnamese-speaking, reflective of the large Asian immigrant population in the Puget Sound region in Western WA and the higher risk of chronic hepatitis B infection and HCC in Asians (Table 1). Approximately 61% of the cohort had hepatitis C virus, and over 75% had documented cirrhosis. Alcohol abuse was common (46%) in this population. Most patients had Child Pugh A (76%) disease and either BCLC 0 or A stage (50.6%). A very small proportion of patients had missing Child Pugh scores (<2%) or unknown BCLC stage (6%).
 |  | 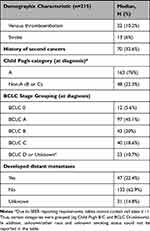 |
Table 1 Demographic and Clinical Characteristics |
Treatment Characteristics
Because of the predominance of earlier stage patients, surgery (including transplantation) or ablative therapy was the initial treatment for 42.7% of patients (Table 2). Another 48.8% received embolization (either TACE or radioembolization) as the initial form of therapy. Only 3% of patients received chemotherapy or radiation as their initial treatment. Of the 23% of patients who were identified in the EMR as having received chemotherapy at some point after diagnosis, 32% were participants in clinical trials. A total of 115 (53.5%) patients were identified from claims data as having received chemotherapy, predominantly sorafenib. There was little evidence of receipt of newer agents such as afatinib, pembrolizumab, lenvatinib, or cabozantinib in the claims data, though review of EMR suggested use of some of these agents in clinical trials.
 |
Table 2 Treatment Characteristics |
Survival
Median survival was highest in patients with BCLC 0/A disease (7.49 years) and lower in BCLC B (2.85 years) and BCLC C (2.13 years) disease (Table 3, Figure 2). In a Cox proportional hazards model, higher ADI and BCLC C stage was associated with poorer survival (Table 4).
 |
Table 3 Median Survival by BCLC Categorya |
 |
Table 4 Factors Associated with Survival in HCC |
 |
Figure 2 Median Survival by BCLC Stage Group. |
Healthcare Utilization and Lifetime Treatment Costs
Mean number of imaging studies (CT, PET, MRI) per patient from diagnosis to death or end of follow-up was 14.4. Mean number of ED visits and inpatient hospital stays per patient were 4.5 and 1.6, respectively. The top five categories of spending for the overall patient cohort included outpatient clinic visits, other services (eg laboratory, skilled nursing facility costs, hospice, dialysis), ED and hospital visits, pharmacy, and surgery (Table 5). However, the types and amount of spending by category differed by BCLC stage grouping (Table 6). In general, mean lifetime costs were highest in early BCLC stage patients and lowest in BCLC D patients, likely due to the limited therapeutic interventions that can be offered to this cohort. In general, costs associated with surgery and ablation were highest in BCLC 0 patients and declined consistently with increasing stage while costs for chemotherapy and catheter-based therapies increased with stage (Table 6). BCLC A patients had the highest mean lifetime costs ($292,134), driven largely by costs for surgery, hospital-based care, advanced imaging, pharmacy, and outpatient visits. BCLC C patients had the next highest lifetime costs ($255,431) with similarly high spending for imaging, outpatient visits, pharmacy, and hospital care but also higher costs for chemotherapy and interventional radiology. Chemotherapy costs were over twice as high in BCLC C patients compared with BCLC A patients ($10,799 vs $2998) but was not the predominant category of spending even among BCLC C patients.
 |
Table 5 Mean Overall per Patient Lifetime Costs |
 |
Table 6 Mean per Patient Lifetime Costs by BCLC Stage Group |
Discussion
In summary, we conducted one of the first analyses of health care utilization, costs, and outcomes using EMR, cancer registry, and claims data in a large cohort of HCC patients. We found that healthcare utilization and costs differed by BCLC stage. Costs associated with advanced imaging, hospital-based care, and outpatient visits were highest in the overall cohort and in each BCLC category. Despite the increase in costs for chemotherapy and catheter-based therapies with increasing BCLC stage, these costs did not comprise the predominant area of spending. This was true even among BCLC C patients for whom chemotherapy utilization might be expected to represent the costliest aspect of care. Our findings contrast with a previous analysis estimating the annual financial burden of HCC in the United States using 1999 SEER-Medicare data.3 In that analysis by Lang et al, total costs were highest in localized disease and significantly lower in more advanced disease with an overall per-patient annual cost of $32,907.3 Our study shows that the overall costs were much higher and similar across BCLC 0-C disease, likely reflecting newer techniques and therapies in advanced disease. As utilization of newer and less toxic systemic therapies increases, chemotherapy costs may comprise a greater proportion of spending in BCLC C patients and potentially push lifetime costs highest in this group. Despite the differences noted above, lifetime treatment costs were in a similar range across BCLC categories 0-C, in contrast to median survival, which varied significantly by BCLC stage and was much lower in BCLC C patients compared with BCLC 0 and A patients. Significant increases in cost without improvements in survival, as is true more generally in oncology, may place undue financial burdens on patients and the healthcare system. Indeed, recent studies have concluded that many newer therapies for advanced HCC are not cost-effective by accepted thresholds given the high price of these agents relative to the modest survival benefits.12–18 Our study provides a baseline cost estimate for future real-world cost analyses as newer therapies are increasingly taken up in clinical practice. If newer therapies for advanced BCLC stage HCC have improved toxicity profiles and result in decreased inpatient and ED utilization and outpatient care, the higher costs of the treatments may be offset by savings in other areas. At the same time, less costly but similarly effective interventions across all BCLC stages should be investigated as a strategy to decrease healthcare spending while maintaining outcomes.
Several limitations should be acknowledged in interpreting our study findings. First, because our data repository only includes patients with specific types of health insurance, we were not able to report on healthcare utilization and costs for patients who lacked health insurance or were insured by a payer outside our linkage. We expect that patients with HCC who lack health insurance have very poor outcomes and limited access to treatment given the high cost of care; the exclusion of these individuals from our analysis therefore limits generalizability. We also limited our analyses to patients with continuous enrollment for at least one month following diagnosis, which is typical of many other studies using administrative claims data to assess patterns of care; this requirement may also have excluded patients who disenrolled shortly after diagnosis and either remained uninsured or enrolled in an alternate health plan not represented in our database. Next, we could not determine BCLC stage and Child Pugh score in a substantial proportion of patients in our sample because of lack of adequate documentation of liver function, ECOG Performance Status, and imaging characteristics in the EMR. This information is also not available in the cancer registry or claims database but is critical to treatment decision making and survival in HCC. Finally, our analysis likely underestimates the proportion of patients who received newer chemotherapy agents, given that many newer agents obtained through compassionate use or clinical trial could not be identified from claims data. Thus, our lifetime cost estimates may underestimate actual costs that would have been incurred had these individuals received treatment billed as standard of care, outside of a clinical trial.
Despite the limitations, our study clearly shows differences in survival, healthcare utilization, and lifetime treatment costs by BCLC stage group in a cohort of patients seen at a large referral center in WA state. Our findings establish a clear reference point for real world lifetime treatment costs in HCC that can be used to frame future analyses, particularly as new treatment strategies emerge.
Funding
Research funding from AstraZeneca.
Disclosure
Dr Veena Shankaran reports grants from AstraZeneca, during the conduct of the study, and has also received grants from BMS and Merck, outside the submitted work. Dr Abdalla Aly and Dr Brian Seal were employees of AstraZeneca during the conduct of the study. Dr Abdalla Aly is now an employee of Novo Nordisk; Dr Brian Seal is now an employee at Organon. Dr Marcus Healey is affiliated with AstraZeneca. The authors report no other conflicts of interest in this work.
References
1. American Cancer Society. Cancer facts and figures 2018; 2018. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html.
2. Bonafede MM, Korytowsky B, Singh P, et al. Treatment patterns and economic burden by lines of therapy among patients with advanced hepatocellular carcinoma treated with systemic cancer therapy. J Gastrointest Cancer. 2020;51(1):217–226. doi:10.1007/s12029-019-00230-z
3. Lang K, Danchenko N, Gondek K, Shah S, Thompson D. The burden of illness associated with hepatocellular carcinoma in the United States. J Hepatol. 2009;50(1):89–99. doi:10.1016/j.jhep.2008.07.029
4. Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59(5):638–644. doi:10.1136/gut.2009.187286
5. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
6. Durfey SNM, Kind AJH, Buckingham WR, DuGoff EH, Trivedi AN. Neighborhood disadvantage and chronic disease management. Health Serv Res. 2019;54(Suppl 1):206–216. doi:10.1111/1475-6773.13092
7. Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48(12):1503–1510. doi:10.1016/0895-4356(95)00048-8
8. Concato J, Peduzzi P, Holford TR, Feinstein AR. Importance of events per independent variable in proportional hazards analysis. I. Background, goals, and general strategy. J Clin Epidemiol. 1995;48(12):1495–1501. doi:10.1016/0895-4356(95)00510-2
9. Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-medicare data. Med Care. 2002;40(8 Suppl):
10. Lin DY. Proportional means regression for censored medical costs. Biometrics. 2000;56(3):775–778. doi:10.1111/j.0006-341X.2000.00775.x
11. Lin DY, Feuer EJ, Etzioni R, Wax Y. Estimating medical costs from incomplete follow-up data. Biometrics. 1997;53(2):419–434. doi:10.2307/2533947
12. Chiang CL, Chan SK, Lee SF, Wong IO, Choi HC. Cost-effectiveness of pembrolizumab as a second-line therapy for hepatocellular carcinoma. JAMA Netw Open. 2021;4(1):e2033761. doi:10.1001/jamanetworkopen.2020.33761
13. Marqueen KE, Kim E, Ang C, Mazumdar M, Buckstein M, Ferket BS. Cost-effectiveness analysis of selective internal radiotherapy with Yttrium-90 versus Sorafenib in locally advanced hepatocellular carcinoma. JCO Oncol Pract. 2021;17(2):e266–e277. doi:10.1200/OP.20.00443
14. Shlomai A, Leshno M, Goldstein DA. Regorafenib treatment for patients with hepatocellular carcinoma who progressed on sorafenib-A cost-effectiveness analysis. PLoS One. 2018;13(11):e0207132. doi:10.1371/journal.pone.0207132
15. Sieg M, Hartmann M, Settmacher U, Arefian H. Comparative cost-effectiveness of cabozantinib as second-line therapy for patients with advanced hepatocellular carcinoma in Germany and the United States. BMC Gastroenterol. 2020;20(1):120. doi:10.1186/s12876-020-01241-y
16. Soto-perez-de-celis E, Aguiar PN, Cordon ML, Chavarri-Guerra Y, Lopes GL. Cost-effectiveness of cabozantinib in the second-line treatment of advanced hepatocellular carcinoma. J Natl Compr Canc Netw. 2019;17(6):669–675. doi:10.6004/jnccn.2018.7275
17. Wen F, Zheng H, Zhang P, Liao W, Zhou K, Li Q. Atezolizumab and bevacizumab combination compared with sorafenib as the first-line systemic treatment for patients with unresectable hepatocellular carcinoma: a cost-effectiveness analysis in China and the United States. Liver Int. 2021;41(5):1097–1104. doi:10.1111/liv.14795
18. Zheng H, Qin Z, Qiu X, Zhan M, Wen F, Xu T. Cost-effectiveness analysis of ramucirumab treatment for patients with hepatocellular carcinoma who progressed on sorafenib with alpha-fetoprotein concentrations of at least 400 ng/mL. J Med Econ. 2020;23(4):347–352. doi:10.1080/13696998.2019.1707211
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
