Back to Journals » Infection and Drug Resistance » Volume 16
Clinical Characteristics of Six Patients with Chlamydia psittaci Infection Diagnosed by Metagenomic Next-Generation Sequencing: A Case Series
Authors Zhu Z, Wang X, Zhao J, Xie Z, Yang C, Li L, Liu Y
Received 3 November 2022
Accepted for publication 7 January 2023
Published 14 February 2023 Volume 2023:16 Pages 869—878
DOI https://doi.org/10.2147/IDR.S393195
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Zhenghua Zhu,1,* Xiang Wang,1,* Jinhong Zhao,1 Zuozhou Xie,1 Chen Yang,1 Lingyi Li,2 Yi Liu1
1Department of Respiration, The Second People’s Hospital of Kunming, Kunming, People’s Republic of China; 2Department of Medical, Hangzhou Matridx Biotechnology Co., Ltd, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lingyi Li, Department of Medical, Hangzhou Matridx Biotechnology Co., Ltd, No. 2073 Jinchang Road, Yuhang District, Hangzhou, Zhejiang Province, 311100, People’s Republic of China, Tel +8613888173009, Email [email protected] Yi Liu, Department of Respiration, the Second People’s Hospital of Kunming, No. 871 Longquan Road, Panlong District, Kunming City, Yunnan Province, 650051, People’s Republic of China, Tel +8613648898773, Email [email protected]
Abstract: The incidence of psittacosis infection has gradually increased in recent years. Metagenomic next-generation sequencing (mNGS) can be used to comprehensively identify the total DNA and RNA content of the microbiome, as well as identify both known and unexpected pathogens within 24 hours. We diagnosed and treated six patients with psittacosis infection using mNGS, two of whom developed severe disease and most of whom presented with pulmonary symptoms. One of the young female patients also presented with irregular vaginal bleeding and myocarditis. Patients with underlying gastric disorders first showed gastrointestinal symptoms, which is a rare manifestation in patients with psittacosis. Older patients with underlying disease usually showed more severe symptoms. However, rare complications can also occur in immunocompetent young people and develop into severe disease. All patients showed significant congestion at bronchial lumen lesions, which may be associated with a severe inflammatory response to mucosal Chlamydia psittaci (C. psittaci) infection. Overall, mNGS is a rapid and effective tool for the clinical diagnosis of psittacosis caused by C. psittaci, and early diagnosis and treatment can prevent psittacosis from developing into a serious illness.
Keywords: Chlamydia psittaci, diagnosis, evolutionary tree, metagenomic next-generation sequencing, serotype
Introduction
Human psittacosis infection, also known as ornithosis or Parrot Disease, is a relatively rare zoonosis caused by Chlamydia psittaci. Zoonotic transmission of C. psittaci has been documented through contact with infected excreta and secretions, as well as through inhalation.1 A recent study showed that C. psittaci has the potential to evolve, leading to human-to-human transmission via various routes, in Shandong, China.2 Severe psittacosis infection is associated with a wide range of clinical manifestations, including mild influenza-like illnesses and severe atypical pneumonia with symptoms ranging from fever, cough, dyspnea, and fatigue to multiple-organ failure and rapid death. However, not all patients report a history of contact with birds or animals.3 In the last few years, metagenomic next-generation sequencing (mNGS) has been used for precise detection of diseases in medicine, as it demonstrates much greater sensitivity than traditional culture methods. Genetic analysis of C. psittaci based on the gene sequence of outer membrane protein A (ompA) has provided a comprehensive understanding of the possible sources of human infection to facilitate better prevention.4 Here, we describe the clinical characteristics of psittacosis pneumonia diagnosed by mNGS, as well as demonstrating mNGS as an efficient diagnostic method. The identification of psittacosis infection serotypes via molecular methods may improve the study of psittacosis in terms of phylogeny, epidemiology, pathology, clinical diagnosis, and treatment.
Case Series
Case 1
The first case was a 77-year-old male patient who experienced right-sided chest tightness for 3 days. After becoming disorientated and experiencing blurred vision over the following days, the patient sought medical attention. On admission, the clinical examination revealed a temperature of 39.7°C. Computed tomography (CT) on admission revealed inflammatory lesions in bilateral lungs with ground-glass opacities (GGOs) and consolidation with a surrounding halo sign (Figure 1A, left). We collected the following laboratory test results when the patient was admitted (Table 1). Cefoperazone sodium/sulbactam sodium and arbidol tablets are empirically used to treat respiratory tract infections. The diagnosis of diabetes mellitus was confirmed after the patient underwent two blood glucose tests. The patient achieved good glycemic control on pre-mixed insulin (Mixtard) at a low dose of 5 IU/h. The patient quickly developed severe pneumonia and was transferred to the intensive care unit (ICU). Bronchoalveolar lavage fluid (BALF) was extracted for culture and drug sensitivity testing. Unbiased mNGS of BALF identified 745 specific sequence reads corresponding to C. psittaci and 200 sequence reads corresponding to Candida glabrata on the sixth day of hospitalization. We first administered cefoperazone sodium/sulbactam sodium and meropenem, followed by doxycycline plus azithromycin to treat psittacosis. Itraconazole was used to treat infection with C. glabrata, while traditional Chinese medicine Xuebijing injection was used to reduce systemic inflammation. Obvious lesion absorption in bilateral lungs was observed 25 days after onset (Figure 1A, right). The patient had started housing a Chinese starling 1 month before symptom onset.
 |
Table 1 Clinical Characteristics and Laboratory Inspections Parameters of the Six C. psittaci Pneumonia Cases |
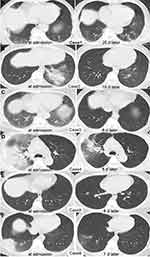 |
Figure 1 Chlamydia psittaci appearance on chest CT in Cases 1–6 (A–F) on the day of admission (left) and after treatment (right). |
Case 2
A 23-year-old female patient experienced a fever and chills after exposure to a cold environment and had a body temperature of 40°C. She exhibited several other symptoms, including dizziness, sweating, headache, chest pain, sore throat, nasal congestion, bloody sputum, general fatigue, and muscle soreness. The patient had finished her menstrual period 10 days prior, but after admission, she developed irregular vaginal bleeding that lasted for 4 days; however, there were no abnormalities on gynecological examination. CT images obtained from the patient in Case 2 observed halo signs, as well as GGOs of bronchial inflation, in the left lower lobe of the lung (Figure 1B, left). Urine antigen testing for Legionella pneumophila was negative. At the beginning of treatment, the patient was treated with arbidol tablets, budesonide, and other symptomatic treatments. The patient experienced fever, chest pain, non-specific T-wave abnormalities, and vaginal bleeding on the third day of hospitalization, and she was strongly suspected of having myocarditis. Abnormal laboratory test results were obtained, as follows: creatine kinase (CK), 287.33 U/L (normal range 24–170 U/L); lactate dehydrogenase (LDH), 264.52 U/L (normal range 100–240 U/L); and hydroxybutyrate dehydrogenase, 199 U/L (normal range 74–199 U/L). Cardiac troponin I (1.114 ng/mL) was also above the reference range (0–0.04 ng/mL). The patient was transferred to the ICU. The Weil-Felix test was significantly positive, with the following results: O antigen, 1:320; H antigen, 1:160; paratyphoid B, 1:80; paratyphoid C, 1:160; O antigen-X19, 1:80; and O antigen-X2, 1:80. The patient was then treated with cefoperazone sodium/sulbactam sodium plus moxifloxacin. Four days after admission, the patient underwent bronchoalveolar lavage. The mNGS of BALF reported 15 sequence reads of C. psittaci (Table 1) and 3 sequence reads of Streptococcus pneumoniae. Moxifloxacin was continued for 10 days. A re-examination by lung CT 15 days after admission showed that the left lower lobe lesions had improved and absorbed (Figure 1B, right). The patient was a researcher, and she collected wild pig feces samples in the 2 weeks prior to symptom onset. Infected wild birds or the environment was the most likely source of C. psittaci.
Case 3
A 59-year-old female patient experienced recurrent high fever (40°C) with chills after feeding her chickens. She complained of muscular stiffness, dry throat, dizziness, and headache, and she visited the local clinic. The patient was treated with penicillin and cephalosporin for 2 days, but no improvement in symptoms was noted. The laboratory results at admission are shown in Table 1. Cardiac enzymes showed elevated CK (932.62 U/L), LDH (284.85 U/L), and myoglobin (106.83 ng/mL) (normal range 14–65.8 ng/mL) concentrations. Chest CT showed peripheral GGOs with vascular enlargement representing halo signs in the posterior segment of the right lower lobe, as well as small bilateral pleural effusion (Figure 1C, left). After admission, moxifloxacin treatment was administered for anti-infection due to the patient’s history of avian exposure. Sodium chloride was injected for adequate rehydration, and potassium chloride was administrated to maintain rehydration and electrolyte balance. mNGS of BALF detected 803 specific C. psittaci sequences (Figure 2A). The patient continued treatment with a full course of doxycycline plus moxifloxacin. To trace the source of C. psittaci, swabs were collected from the beaks and feathers of the patient’s chickens and sent for mNGS. A variety of poultry pathogens were detected, including Avibacterium paragallinarum, Blastocystis hominis, Plasmodium gallinaceum, Eimeria necatrix, and Avian coronavirus. However, the investigation and its results were limited because we did not swab all of the chickens. Poultry or environmental contamination was considered to be the most probable source of C. psittaci. After 8 days of treatment, the patient’s symptoms gradually subsided. The last chest CT examination showed significant lesion absorption in both lungs (Figure 1C, right).
Case 4
A 66-year-old male patient experienced fever, chills, generalized aches and pains, cough, and sputum for 7 days, with a peak temperature of 39.5°C. Chest CT showed GGOs (halo sign) in the periphery of the right upper lobe and air bronchogram in the right subpleural area (Figure 1D, left). The patient had a history of hypertension. The patient was admitted with community-acquired pneumonia, which was treated with moxifloxacin and azithromycin. The patient underwent a bronchoscopic examination on the second day. The sample testing with mNGS detected C. psittaci (Table 1) with 90 specific sequence reads (Figure 2B). Then, we changed his treatment to doxycycline to target C. psittaci. The patient was treated with symptomatic and supportive treatment for 2 weeks, and the clinical symptoms of cough, sputum, and chest tightness improved significantly. CT images showed a significantly reduced lesion on the upper right lung (Figure 1D, right). The patient had visited the parrot park 2 weeks prior to symptom onset.
Case 5
A 23-year-old male patient experienced a dry cough for 3 days, which was aggravated at night, following a cold. He developed generalized muscle aches and fever up to 38.5°C. The laboratory results at admission are shown in Table 1. Chest CT showed right upper lobe consolidation and nodular consolidation with adjacent GGOs on admission (Figure 1E, left). The patient was treated with moxifloxacin. The mNGS results confirmed that the patient was infected with C. psittaci with 2 specific sequence reads (Figure 2C) and Porphyromonas gingivalis with 3634 specific sequence reads (Table 1). The patient showed no signs of Porphyromonas gingivalis infection, probably oral colonization brought in from the upper respiratory tract during sampling. Doxycycline was initiated, and the patient’s body temperature returned to normal the next day. The patient was discharged soon after in a stable condition. Chest CT showed no inflammatory exudate, and the lesions were absorbed (Figure 1E, right). The patient was unable to recall any definite living animal exposure.
Case 6
A 55-year-old female patient suffered from generalized body aches and pains. Fever developed, with the patient’s body temperature suddenly reaching 42°C, which was accompanied by chills and rigor. The patient had black, loose, watery stools. She also complained of nausea, decreased appetite, occasional palpitations, chest tightness, chest pain, paroxysmal cough, and sputum after being treated with ibuprofen (100 mg/5 mL oral suspension). The patient had a medical history of chronic atrophic gastritis and hypothyroidism. The laboratory results at admission are shown in Table 1. The patient was treated with eprazole, monopeptide, cefpiramide, and compound amino acids to inhibit gastric acid secretion, ensure mucosal protection, provide nutritional support, and enhance immunity, respectively. Chest CT images obtained on the second day of hospitalization showed an air bronchogram in the right subpleural area with GGOs and halo signs (Figure 1F, left). The patient worked as a nanny, and her employer started housing a parrot 4 weeks before the patient’s admission. The patient received anti-infective treatment with doxycycline plus cefpiramide for her symptoms because an atypical pathogen infection was considered. Bronchoscopy was performed 4 days after admission. BALF was collected and sent for mNGS, which revealed the detection of sequence reads mapped to C. psittaci (Table 1). After 6 days of doxycycline treatment, the patient’s right upper lobe lesions improved and were totally absorbed (Figure 1F, right).
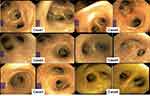
Of the patients reported, three were female and three were male, with an age range of 23–77 years (median age, 51 years). The underlying diseases differed between patients, but included diabetes mellitus (Case 1), hypertension (Case 4), and hypothyroidism and chronic atrophic gastritis (Case 6). Five of six patients had a history of recent avian or pig exposure. The patients’ detailed information is summarized in Table 1. In addition, we detected C. psittaci ompA DNA fragments in the remaining samples from patients 3, 4, and 5, which confirmed the positive mNGS result. All three samples were greater than 1000 bp in size (Figure 2D). The polymerase chain reaction (PCR) band of Case 5 was weak, which indicated a very low C. psittaci ompA DNA fragment concentration in the sample. On the basis of ompA sequences, a phylogenetic analysis of C. psittaci was performed. All C. psittaci-positive samples shared the highest ompA similarity with C. psittaci strain 41A12, which belongs to the B genotype(Figure 2E). Detailed methods for all sample collection (Sample and Information Collection), mNGS(mNGS assay), PCR and phylogenetic tree construction (PCR and Evolutionary tree construction) are given in the Supplementary. Significant congestion in the bronchial lumen of the lung was observed in all patients in fiberoptic bronchoscopy (Figure 3). This feature made psittacosis clearly distinguishable from L. pneumophila infection (Legionnaire’s disease), despite their similar symptoms.
Discussion
Atypical pneumonia in humans can be caused by Legionella, Mycoplasma, Coxiella, and influenza.5 When atypical pneumonia presents due to a history of exposure to birds, the diagnosis of psittacosis should definitely be considered. In our study, 83% of patients (5/6) had a history of exposure to live animal. All patients had a high fever (temperature of >38.5°C) and presented with flu-like symptoms, such as muscle aches and weakness. All patients had a rapid onset of illness. Initial blood investigations showed a weakly elevated white blood cell count of 8.3 × 109/L (normal range 3.5–9.5 × 109/L). Eighty-three percent of patients (5/6) showed an increased neutrophil percentage above the normal range. All patients had a low lymphocyte ratio. C-reactive protein was elevated in all patients with a mean value of 115.21 mg/L (normal range 0.01–6 mg/L) (Table 1). Aspartate aminotransferase and alanine transaminase concentrations were relatively normal at the time of admission and increased with psittacosis progression, but they returned to normal when the patient was discharged. Patients with psittacosis with liver involvement often present with marked hepatosplenomegaly, markedly elevated transaminases, and in some cases, intestinal discomfort. In severe cases, patients may also experience black stools. The patient in Case 6 had chronic atrophic gastritis and underwent gastric polypectomy 6 months prior to psittacosis onset. After infection with C. psittaci, this patient’s symptoms first manifested in the digestive system.
C. psittaci infection is on the rise as animal migration and the housing of birds as pets increase. Test results identified C. psittaci as the most prevalent chlamydial species in community-acquired pneumonia in Germany.6 People infected with C. psittaci experience symptoms such as fever, chills, headache, malaise, myalgia, and non-productive coughing.7,8 The clinical presentation of psittacosis can vary widely in severity, ranging from asymptomatic or respiratory involvement to acute respiratory distress syndrome and death or gastrointestinal symptoms.9 The patient in Case 2 had an elevated cardiac troponin I concentration that was 10-fold greater than the normal reference range (1.107 ng/mL; normal range 0–0.04 ng/mL) on admission. The main features of psittacosis pneumonia on chest radiography were Inflammatory infiltrated in the upper lung field, and GGOs were present in the bilateral lower lung fields. Nodular or consolidated shadows were commonly found in pathological areas. Lesions present on chest X-ray or CT depended on disease severity, but were not specific. All six patients had similar imaging features to those shown in previous studies.8,10,11 In an animal study, in type 1 alveolar epithelial cells of the lung, C. psittaci replicated, which was followed by an influx of neutrophils, vascular leakage, and fibrinous exudation, after which the area of consolidation increased.12 Air bronchograms were observed in bilateral lungs. With psittacosis progression, multiple lesions are common. The lesions can be patchy, nodular, or lumpy. They commonly have an uneven density and GGOs, interlobular thickening, and consolidation. The clinical presentation of atypical pathogenic pneumonia is similar.9 In the present study, the patient in Case 2 was suspected of having community pneumonia, either caused by the Legionella genus or the Salmonella genus, before mNGS. However, pathogens were not detected in the blood or sputum or in BALF cultures, but mNGS eventually detected C. psittaci in BALF. The patient in Case 2 also had irregular vaginal bleeding, which although not heavy, has not been reported in previous studies. The gynecological consultation did not exclude that the patient’s mood swings led to hormonal fluctuations causing withdrawal bleeding. C. psittaci can infect mucous membrane and invade epithelial columnar cells.3 In addition to the obvious congestion of the bronchial tract, vaginal bleeding may have occurred due to vaginal mucosal involvement caused by C. psittaci.
Methods for the laboratory identification of C. psittaci infection include culture, serological assay, and PCR.13–15 However, at most hospitals, C. psittaci detection methods, such as PCR, complement fixation, and serological and micro-immunofluorescence tests are not routinely available. In patients with CAP, empiric treatment does not always cover atypical pathogens. Early diagnosis and immediate treatment initiation are essential for an effective C. psittaci control program. As a high-throughput sequencing technology, mNGS allows for hypothesis-free and culture-independent pathogen detection directly from samples. The technique can complement current conventional identification methods in the diagnosis of C. psittaci.16 With the widespread use of mNGS in the clinic, its use to diagnose C. psittaci pneumonia has increased.8,10,11,17–20 All patients in the present study were diagnosed with C. psittaci infection solely using mNGS. Among the samples that were positive for C. psittaci detected using mNGS, three ompA fragments (1.4 kb) were successfully amplified and sequenced. Sample from the patient in Case 5 had minimal sequencing reads (only 2 mapping reads to the C. psittaci genome) and showed an ambiguous, faint DNA band. The specific reads of microbial sequences that mapped to C. psittaci reference genomes varied among patients, but the sequence number detected with mNGS was affected by many factors, including human background reads, sample collection, pathogen load, host depletion methods, and PCR-free library construction.21 OmpA is commonly used for C. psittaci strain detection and identification.22 The ompA sequence of C. psittaci obtained in this study was identical to a strain isolated from pigeon (41A12), which was classified as genotype B. Pigeons most frequently carry genotype B, but some parrots are also susceptible to this genotype.23 The patient in Case 3 raised chickens, but mNGS of random samples obtained from the beaks and feathers of these chickens showed that the samples were negative for C. psittaci; thus, the source of infection could not be traced. The patient in Case 4 spent time in the parrot park prior to psittacosis onset and possibly contracted the infection from sick birds or from the environment. Although C. psittaci is an intracellular bacterium, it could survive in vitro long enough to enable transmission through the environment.24
Conclusion
In conclusion, the epidemiology, clinical characteristics, results of mNGS, and imaging features of patients with C. psittaci pneumonia have been summarized, which help us to better understand and fight the disease. MNGS is a comprehensive, rapid, and newly emerging tool that assists in diagnosis of C. psittaci, and minimize the emergence of antibiotic-resistant. MNGS will be critical to improve the disease outcomes and prevent the potential spread of C. psittaci.
Associated Data
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Data Sharing Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/sra, accession number.
Ethics Approval and Informed Consent
The Ethics Committees of the Second People’s Hospital of Kunming approved this study. Informed consent was obtained from the patient and guardians.
Consent for Publication
All six patients provided written informed consent for their case details and any accompanying images to be published.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by a research grant from Health Scientific research Project of Kunming Health Commission (Grant No. 2020-03-02-117).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Balsamo G, Maxted AM, Midla JW, et al. Compendium of measures to control chlamydia psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis), 2017. J Avian Med Surg. 2017;31(3):262–282. doi:10.1647/217-265
2. Zhang Z, Zhou H, Cao H, et al. Human-to-human transmission of Chlamydia psittaci in China, 2020: an epidemiological and aetiological investigation. Lancet Microbe. 2022;3(7):e512–e20. doi:10.1016/s2666-5247(22)00064-7
3. Beeckman DS, Vanrompay DC. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin Microbiol Infect. 2009;15(1):11–17. doi:10.1111/j.1469-0691.2008.02669.x
4. Heddema ER, van Hannen EJ, Duim B, Vandenbroucke-Grauls CM, Pannekoek Y. Genotyping of Chlamydophila psittaci in human samples. Emerg Infect Dis. 2006;12(12):1989–1990. doi:10.3201/eid1212.051633
5. Mi H, Li H, Yu J. Psittacosis. Radiol Infect Dis. 2015;2:207–212. doi:10.1007/978-94-017-9876-1_20
6. Dumke R, Schnee C, Pletz MW, et al. Mycoplasma pneumoniae and Chlamydia spp. infection in community-acquired pneumonia, Germany, 2011–2012. Emerg Infect Dis. 2015;21(3):426–434. doi:10.3201/eid2103.140927
7. Teng XQ, Gong WC, Qi TT, et al. Clinical analysis of metagenomic next-generation sequencing confirmed chlamydia psittaci pneumonia: a case series and literature review. Infect Drug Resist. 2021;14:1481–1492. doi:10.2147/idr.S305790
8. Gu L, Liu W, Ru M, et al. The application of metagenomic next-generation sequencing in diagnosing Chlamydia psittaci pneumonia: a report of five cases. BMC Pulm Med. 2020;20(1):65. doi:10.1186/s12890-020-1098-x
9. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371(17):1619–1628. doi:10.1056/NEJMra1312885
10. Chen X, Cao K, Wei Y, et al. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection. 2020;48(4):535–542. doi:10.1007/s15010-020-01429-0
11. Wu HH, Feng LF, Fang SY. Application of metagenomic next-generation sequencing in the diagnosis of severe pneumonia caused by Chlamydia psittaci. BMC Pulm Med. 2021;21(1):300. doi:10.1186/s12890-021-01673-6
12. Knittler MR, Berndt A, Böcker S, et al. Chlamydia psittaci: new insights into genomic diversity, clinical pathology, host–pathogen interaction and anti-bacterial immunity. Int J Med Microbiol. 2014;304(7):877–893. doi:10.1016/j.ijmm.2014.06.010
13. Cong W, Huang SY, Zhang XX, et al. Chlamydia psittaci exposure in pet birds. J Med Microbiol. 2014;63(Pt 4):578–581. doi:10.1099/jmm.0.070003-0
14. Madani SA, Peighambari SM. PCR-based diagnosis, molecular characterization and detection of atypical strains of avian Chlamydia psittaci in companion and wild birds. Avian Pathol. 2013;42(1):38–44. doi:10.1080/03079457.2012.757288
15. Thomas R, Davison HC, Wilsmore AJ. Use of the IDEIA ELISA to detect Chlamydia psittaci (ovis) in material from aborted fetal membranes and milk from ewes affected by ovine enzootic abortion. Br Vet J. 1990;146(4):364–367. doi:10.1016/s0007-1935(11)80031-x
16. Davar K, Wilson MR, Miller S, Chiu CY, Vijayan T, Rare Bird A. Diagnosis of psittacosis meningitis by clinical metagenomic next-generation sequencing. Open Forum Infect Dis. 2021;8(12):ofab555. doi:10.1093/ofid/ofab555
17. Xiao Q, Shen W, Zou Y, et al. Sixteen cases of severe pneumonia caused by Chlamydia psittaci in South China investigated via metagenomic next-generation sequencing. J Med Microbiol. 2021;70(11). doi:10.1099/jmm.0.001456
18. Yuan Y, Zhang X, Gui C. Detection of Chlamydia psittaci in both blood and bronchoalveolar lavage fluid using metagenomic next-generation sequencing: a case report. Medicine. 2021;100(27):e26514. doi:10.1097/md.0000000000026514
19. Wen W, Gu L, Zhao LW, et al. Diagnosis and treatment of Chlamydia psittaci pneumonia: experiences of 8 cases. Chin J Tubercul Respir Dis. 2021;44(6):531–536. doi:10.3760/cma.j.cn112147-20210205-00097
20. Kong C-Y, Zhu J, Lu -J-J, Xu Z-H, Lyu P. Clinical characteristics of Chlamydia psittaci pneumonia. Chin Med J. 2021;134(03):353–355. doi:10.1097/CM9.0000000000001313
21. Simner PJ, Miller S, Carroll KC. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clinical Infect Dis. 2018;66(5):778–788. doi:10.1093/cid/cix881
22. Geens T, Desplanques A, Van Loock M, et al. Sequencing of the Chlamydophila psittaci ompA gene reveals a new genotype, E/ B and the need for a rapid discriminatory genotyping method. J Clin Microbiol. 2005;43(5):2456–2461. doi:10.1128/jcm.43.5.2456-2461.2005
23. Burt SA, Röring RE, Heijne M. Chlamydia psittaci and C. avium in feral pigeon (Columba livia domestica) droppings in two cities in the Netherlands. Vet Q. 2018;38(1):63–66. doi:10.1080/01652176.2018.1482028
24. Radomski N, Einenkel R, Müller A, Knittler MR. Chlamydia-host cell interaction not only from a bird’s eye view: some lessons from Chlamydia psittaci. FEBS Lett. 2016;590(21):3920–3940. doi:10.1002/1873-3468.12295
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.