Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Clinical Characteristics, Iron Metabolism and Neuroinflammation: New Insight into Excessive Daytime Sleepiness in Parkinson’s Disease
Authors Hu Y, Guo P, Lian TH, Zuo LJ, Yu SY , Liu L, Jin Z, Yu QJ, Wang RD, Li LX, Piao YS, Zhang W
Received 8 August 2020
Accepted for publication 8 March 2021
Published 21 June 2021 Volume 2021:17 Pages 2041—2051
DOI https://doi.org/10.2147/NDT.S272110
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yang Hu,1 Peng Guo,1 Teng-Hong Lian,1 Li-Jun Zuo,1 Shu-Yang Yu,1 Li Liu,2 Zhao Jin,1 Qiu-Jin Yu,1 Rui-Dan Wang,1 Li-Xia Li,2 Ying-Shan Piao,3 Wei Zhang3– 7
1Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, 100070, People’s Republic of China; 2Department of Internal Medicine, Beijing Tiantan Hospital, Capital Medical University, Beijing, 100070, People’s Republic of China; 3Center for Cognitive Neurology, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, 100070, People’s Republic of China; 4China National Clinical Research Center for Neurological Diseases, Beijing, 100070, People’s Republic of China; 5Key Laboratory for Neurodegenerative Disorders of the Ministry of Education, Capital Medical University, Beijing, 100069, People’s Republic of China; 6Center of Parkinson’s Disease, Beijing Institute for Brain Disorders, Beijing, 100053, People’s Republic of China; 7Beijing Key Laboratory on Parkinson’s Disease, Beijing, 100053, People’s Republic of China
Correspondence: Wei Zhang
Center for Cognitive Neurology, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, 100070, People’s Republic of China
Tel +8613911996107
Fax +86-10-59978429
Email [email protected]
Background: To investigate the clinical characteristics, iron metabolism and neuroinflammation in Parkinson’s disease (PD) patients with excessive daytime sleepiness (EDS).
Methods: We studied 379 patients with PD and 30 age-matched controls. All subjects were evaluated by Epworth sleepiness scale (ESS) and a series of rating scales and were divided into PD-EDS and PD-NEDS groups according to ESS score. The concentrations of iron and iron-related proteins and inflammatory cytokines in both cerebrospinal fluid (CSF) and serum were examined.
Results: 1. The occurrence rate of EDS in total PD patients was 16.09%. 2. PD-EDS group had significantly severer disease stages, more severe motor and non-motor features of the disease. 3. In CSF, the concentrations of iron and IL-1β in the PD-EDS group were significantly higher and ferritin concentration was prominently lower when compared with the PD-NEDS group and the control group; ESS score was significantly associated with high concentrations of iron and IL-1β and low concentration of ferritin in the PD group. Iron concentration was positively correlated with IL-1β concentration in the PD-EDS group. 4. In serum, no changes were observed in iron and iron-related proteins and inflammatory cytokines among the three groups.
Conclusion: EDS was a common symptom in PD patients. PD patients with EDS had more severe motor and some non-motor symptoms. Overloaded iron-relevant inflammation in the brain might be an underlying mechanism of PD-EDS.
Keywords: Parkinson disease, excessive daytime sleepiness, clinical features, iron metabolism, inflammation
Background
Excessive daytime sleepiness (EDS) is very common in Parkinson’s disease (PD). Studies report that the prevalence of EDS in PD patients ranges from 16% to 74%.1,2 Dopaminergic treatment, severity stages, other concomitant sleep disturbances, dysautonomia, anxiety, and depression have been reported to be potential risk factors for EDS in PD.3–6 One study demonstrated that EDS was different from poor sleep quality and fatigue.7 A few studies concentrated on apathy, rapid eye movement sleep behavior disorder (RBD) and restless leg symptoms in PD with EDS (PD-EDS) patients. In short, the correlation of EDS with disease stage, motor symptoms, motor complications and non-motor symptoms remains controversial.
Magnetic resonance imaging at 3.0 Tesla suggests that the iron concentrations in substantia nigra (SN) and other nuclei are increased and linked to the severity of motor symptoms in PD.8 Iron-related neurodegenerative disorders can result from both iron accumulation in specific brain regions or defects in its metabolism. Iron-exerted toxicity in the presence of unbound or free iron, and excessive free iron caused damaging effects on many cellular processes and induced neurodegeneration.9 Compared with normal subjects, transferrin concentration was remarkably elevated in PD brains,10 suggesting that iron metabolism disruption in the central nervous system (CNS) participated in the pathogenesis of PD. However, previous studies reported that the concentrations of iron and ferritin in serum or cerebrospinal fluid (CSF) from PD patients were not different from healthy controls.11,12 To date, no research has paid attention to the association between EDS and iron metabolism disruption in both CNS and peripheral systems of PD patients.
The role of inflammation in PD has been suggested by increasing evidence showing microglial activation and inflammatory cytokines production from in vivo and postmortem studies.13,14 Inflammation in the SN served as a driving force for dopaminergic cell death and played a pivotal role on PD progression.15 The dead neurons released iron into the extracellular domain and provoked neuroinflammation by way of activating microglia,16 aggravating the deterioration of PD motor symptoms. Recent investigations showed that inflammation besides SN has a relationship with non-motor symptoms of PD.17 For example, patients with EDS had higher concentrations of C-reactive protein.18 Meanwhile, peripheral inflammation potentially participated in the pathogenesis of non-motor symptoms, such as fatigue and cognitive impairment.18 However, the relationship among EDS, iron metabolism disruption and inflammation in PD remains unclear.
In this study, we firstly assessed EDS, motor symptoms and non-motor symptoms in PD patients recruited by using the Epworth sleepiness scale (ESS) and related rating scales. Additionally, we detected concentrations of iron and iron-related proteins and inflammatory cytokines in both CSF and serum. Finally, we analyzed the relationships among ESS score and the concentrations of the above factors in both CSF and serum.
Methods
Subjects
PD Patients
We consecutively recruited 379 PD patients from the Department of Geriatrics and the Department of Neurology, Beijing Tiantan Hospital, Capital Medical University. We diagnosed patients with PD according to Movement Disorder Society Clinical Diagnostic Criteria for Parkinson Disease.19 Clinical information and levodopa equivalent daily dose (LEDD) of PD patients were recorded when they were recruited in this study. Approximately 42.48% of the recruited PD patients in this study are drug-naive and newly diagnosed.
Exclusion criteria were a diagnosis of anemia, hepatopathy, heart failure, pulmonary diseases and chronic renal disease and a history of blood donation. Female patients who had not been through menopause were excluded in this study. Patients who had taken iron supplements were excluded.
Control Subjects
A total of 30 normal control subjects were consecutively recruited according to the following criteria: (1) no neurological symptom or signs; (2) no essential tremor, PD and related disorders; (3) magnetic resonance imaging of the head was normal; (4) no other diseases influencing patients’ sleep; (5) no systemic infectious diseases or autoimmune diseases; (6) no hallucination or other neuropsychiatric symptoms; (7) no heavy drinking or drugs abuse; (8) no iron supplement taking; and (9) no sleep disturbance.
The exclusion criteria were also applied to the control group.
Clinical Features and Assessments
EDS
EDS of PD patients was identified and quantified by ESS. This rating scale is a 24-point scale containing 8 questions with each score ranging from 0 to 3. ESS ≥10 was regarded as EDS.20
Demographic Variables, Motor Symptoms and Non-Motor Symptoms
We recorded PD patients’ demographics, including age, age at disease onset, gender, disease duration and LEDD. We assessed motor symptoms using the Unified Parkinson’s Disease Rating Scale (UPDRS) III. The component scores for motor symptoms were as follows: (1) tremor score: the sum of UPDRS items 20 and 21; (2) rigidity score: UPDRS item 22; (3) bradykinesia score: the sum of UPDRS items 23, 24, 25, 26 and 31; and (4) postural and gait abnormalities score: the sum of UPDRS items 27, 28, 29 and 30. The wearing-off phenomenon was evaluated using the Wearing-off Questionnaire-9 (WOQ-9), and dyskinesia was evaluated using the sum of UPDRS items 32, 33, 34 and 35.
Motor phenotype was determined as either TD phenotype or PIGD phenotype following the classification algorithm.21 According to the original classification methods, the ratio of the mean UPDRS tremor scores (8 items) to the mean UPDRS PIGD scores (5 items) was used to define TD phenotype (ratio ≥1.5), PIGD phenotype (ratio ≤ 1), and indeterminate phenotype (ratios > 1.0 and < 1.5).
The following scales were used to evaluate non-motor symptoms: Pittsburgh Sleep Quality index (PSQI), Rapid Eye Movement (REM) Sleep Behavior Disorder (RBD) Screening Questionnaire (RBDSQ), Scale For Outcomes in PD For Autonomic Symptoms (SCOPA-AUT), Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Hamilton Depression (HAMD) Scale −24 items, Hamilton Anxiety (HAMA) Scale −14 items, Fatigue Severity Scale (FSS), Modified Apathy Evaluation Scale (MAES) and Restless Leg Syndrome Rating Scale (RLSRS).
This study has been approved by the Institutional Review Board (IRB) in Beijing Tiantan Hospital, Capital Medical University (KY2013-003-03). This study met the guidelines of the Capital Medical University, which abide by the Helsinki Declaration on ethical principles for medical research involving human subjects. Written informed consent has been provided by the participants. The ethical statements cover all of these requirements.
CSF and Serum Samples Collection
Drug withdrawal was made for 12–14 hours if patients’ condition permitted. Lumbar puncture (3 mL CSF) and blood draw (2 mL) were performed between 7.00 and 10.00 a.m. under fasting condition. Testing samples were centrifuged immediately at 3000 rpm for 10 minutes. An average of 0.5 mL CSF and serum were respectively aliquoted and stored at −80°C. Disposable blood collecting needle was from Becton Dickinson (Franklin Lakes, New Jersey, USA), Lumbar puncture package was from SPETCH (Foshan, China).
Assay for the Concentrations of Iron and Iron-Related Proteins in CSF and Serum
Enzyme Linked Immunosorbent Assay method was applied to determine the concentrations of iron and iron-related proteins, including transferrin, ferritin and lactoferrin in CSF and serum. Iron: kit Ab83366 (Abcam Company, Cambridge, UK), transferrin: kit Ab108911 (Abcam Company, Cambridge, UK), ferritin: kit Ab108837 (Abcam Company, Cambridge, UK), lactoferrin: kit E01L0224 (Shanghai Lanji Biological Limited Company, Shanghai, China).
Assay for the Concentrations of Inflammatory Cytokines in CSF and Serum
Chemical colorimetric method was used to measure the concentrations of NO and H2O2. NO: kit A012, H2O2: kit A064 (Nanjing Jiancheng Biological Engineering Research Institute, Nanjing, China).
Enzyme Linked Immunosorbent Assay was applied to measure the concentrations of inflammatory cytokines, including IL-1β, TNF-α and PGE2 both in serum and CSF. IL-1β: kit 1R040 (RB Company, Shanghai, China), TNF-α: kit1R350 (RB Company, Shanghai, China), PGE2: kit CSB-E07965h (CUSABIO Company, Wuhan, China).
Data Analyses
SPSS Statistics 20.0 (IBM Corporation, New York, USA) was used for statistical calculation. Continuous variables, if they were normally distributed, were reported as mean± SD values, and were compared by two-tailed t-test. If they were not normally distributed, they were reported as mean (range interquartile), and were compared by non-parametric test. Discrete variables were compared by Chi square test.
Pearson correlation was performed between ESS score and the concentrations of iron and iron-related proteins and the concentrations of inflammatory cytokines in CSF and serum, between the concentrations of iron and iron-related proteins and inflammatory cytokines in CSF and serum in PD-EDS group. To further explore significant correlations between ESS score and motor and non-motor symptoms, and the concentrations of iron, ferritin and IL-1β in CSF, logistic regression model was carried out. P < 0.05 was considered significant.
Results
The Frequency of PD with EDS
A total of 379 PD patients completed the evaluation of motor and non-motor symptoms. Sixty-one out of 379 PD patients (16.095%) had EDS (ESS score ≥ 10), while 318 PD patients (83.905%) did not have EDS (NEDS) (ESS score < 10). The average ESS scores of PD-EDS and PD-NEDS groups were 11.803 ± 2.372 and 3.327 ± 2.460, respectively.
Demographic Variables, Motor Symptoms, Non-Motor Symptoms and Dopaminergic Medication Usage of PD-NEDS and PD-EDS Groups
The PD-EDS group had dramatically more advanced Hoehn-Yahr (H-Y) stage, larger numbers of wearing-off, and scored higher for dyskinesia than the PD-NEDS group (Table 1).
 |
Table 1 Clinical Variables of PD-NEDS and PD-EDS Groups |
The PD-EDS group had significantly higher scores on the scales of SCOPA-AUT, HAMD, HAMA, FSS and RLSRS than the PD-NEDS group, indicating that the PD-EDS group had significantly severer sleep disorders, autonomic dysfunctions, depression, anxiety, fatigue, and restless leg symptoms than the PD-NEDS group (Table 1). The median score of HAMD and HAMA in our PD patients is 12.00 (6.00~18.00) and 10.00 (5.00~17.00). PD patients who have higher scores of HAMD and HAMA in our study mostly had mild depression and anxiety, and were firstly informed to maintain good mood, intensify communication with others and take no medicine.
The majority of our patients were drug-naive patients with neither L-dopa nor a dopamine agonist (42.480%), about 30.343% were on L-dopa monotherapy, about 13.720% on a dopamine agonist monotherapy, about 12.929% were receiving L-dopa and a single dopamine agonist; and 0.528% received multiple dopamine agonists in combination with L-dopa. There were no significant differences for the dopaminergic medication usage in the PD-EDS and PD-NEDS groups (Table 1). In this study, the dopamine receptor agonists that PD patients used were pramipexole and piribedil. For statistical comparison of the prevalence of EDS in patients with different dopamine agonists therapy, the patients with one dopamine agonist in combination with L-dopa and patients with a dopamine agonist monotherapy were considered. However, there were no significant differences for the prevalence of EDS in the usage of pramipexole and piribedil (Table 2).
 |
Table 2 The Prevalence of EDS in Different Dopamine Agonist Therapy |
The Relationship Among PD-EDS, Iron Metabolism and Inflammation in CNS
In this study, a total of 69 PD patients (PD-EDS group: 16 cases, PD-NEDS group: 53 cases) and 30 controls had CSF samples collected and completed the tests. The concentrations of iron and iron-related proteins in CSF were compared among control, PD-NEDS and PD-EDS groups (Table 3). We found that the concentration of iron in CSF in the PD-EDS group was significantly elevated compared with both control and PD-NEDS groups. Further analyses indicated that ESS score was increased as iron concentration was elevated in CSF in the PD group. The concentration of ferritin in CSF in the PD-EDS group was significantly reduced compared with that in PD-NEDS and control groups. Further analyses revealed that ESS score was enhanced as ferritin concentration in CSF was reduced in the PD group.
 |
Table 3 The Concentrations of Iron and Iron-Related Proteins and Inflammatory Cytokines in CSF or Serum from Control, PD-NEDS and PD-EDS Groups |
The concentrations of inflammatory cytokines in CSF were compared among the control, PD-NEDS and PD-EDS groups (Table 3). It was observed that IL-1β concentration in CSF in the PD-EDS group was significantly increased compared with that in PD-NEDS and control groups. TNF-α concentrations in CSF in PD-NEDS and PD-EDS groups were all significantly reduced when compared with controls. Further analyses showed that ESS scored higher as IL-1β concentration in CSF elevated in patients with PD.
The correlations of the concentration of iron and iron-related proteins with inflammatory cytokines in CSF were analyzed. Data showed that the concentration of iron increased as IL-1β concentration in CSF elevated in the PD-EDS group (r = 0.914, P = 0.004) (Table 4). The iron concentration did not correlate significantly with IL-β concentration in CSF in the PD-NEDS group.
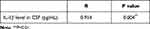 |
Table 4 Correlation Between Iron Level in CSF and IL-1β Level in CSF from PD-EDS Group |
Influencing Factors of ESS for PD Patients
Logistic regression model was carried out, we put ESS score as a dependent variable, whereas concentrations of iron, ferritin and IL-1β in CSF, motor subtype, H-Y stage and the score of MoCA were independent variables.
Data showed that the concentrations of iron, ferritin and IL-1β in CSF, Hoehn-Yahr stage and TD phenotype were the influencing factors of ESS in PD patients (regression coefficient = 2.787, −0.347, 0.080, P < 0.05) (Table 5).
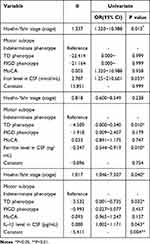 |
Table 5 Influencing Factors for EDS in PD Group |
The Relationship Among PD-EDS, Iron Metabolism and Inflammatory Cytokines in Peripheral System
In this study, a total of 166 PD patients (PD-EDS group: 27 cases, PD-NEDS group: 139 cases) and 30 controls had blood samples collected and completed the tests. First of all, the relationship between PD-EDS and the concentrations of iron and transferrin, ferritin and lactoferrin in serum was studied (Table 3). The results showed that iron and transferrin concentrations in serum in PD-NEDS and PD-EDS groups were all prominently decreased when compared with the control group. The concentrations of lactoferrin and ferritin of serum in PD-EDS and PD-NEDS groups were all strikingly elevated when compared with the control group. The differences in serum concentrations of iron, transferrin, lactoferrin and ferritin between PD-NEDS and PD-EDS groups have no statistical significance. Further study showed no significant correlation between ESS scores and the concentrations of iron and iron-related proteins in PD patients (P > 0.05).
Next, analyses of the correlations between EDS scores and the serum concentrations of NO, H2O2, IL-1β, PGE2 and TNF-α in PD patients were performed. The serum concentrations of the inflammatory cytokines were compared among the control, PD-NEDS and PD-EDS groups (Table 3). It was found that serum concentration of PGE2 in the PD-NEDS group was strikingly decreased when compared with the control group.
Finally, correlation of the concentrations of iron and iron-related proteins with inflammatory cytokines in serum were analyzed and no correlation was found (P > 0.05).
Discussion
As we observed, the occurrence rate of EDS in PD patients was 16.09%, which was consistent with the result from a previous study,22 demonstrating that EDS was very common in PD patients. In this investigation, compares with the PD-NEDS group, the PD-EDS group has more advanced H-Y stage and more severe motor symptoms, suggesting that PD-EDS patients exhibited a mode of more widespread neurodegenerative progression in SN.23 Motor complications were common in advanced PD patients. Previous studies reported that the cumulative levodopa dose, female sex and younger age of onset were associated with the development of motor complications.24 This study found that PD-EDS group had significantly more numbers of wearing-off and higher score of dyskinesia than PD-NEDS group. One prospective study recruited 21 drug-naive PD patients at baseline and followed up for a mean of 2.6 ± 1.3 years, and found that the median time to development of motor complications after initiation of levodopa therapy was 6 months. It revealed that the incidence of motor complications after initiating levodopa was independent of the initial treatment, it was associated with levodopa daily dose and disease progression, but not with the duration of levodopa therapy.25 In this study, we found no significance for LEDD between the PD-EDS and PD-NEDS groups, but more advanced H-Y stage in the PD-EDS group, implying that disease progression might play an important role in the motor complications in PD-EDS patients. In logistic regression, this study found that EDS was significantly and negatively related to TD-phenotype, and was not associated with PIGD-phenotype. A previous study found that EDS was associated with higher PIGD score26 because of prediction of more rapidly progressive disability in PD. Yet, our study differed from that study. Tremor in PD appeared at rest and disappeared during sleep. It might be that patients in our study had lighter symptoms and were in a relatively early stage.
In this study, PD-EDS patients had significantly more severe non-motor symptoms, indicated by higher scores of SCOPA-AUT, HAMA, HAMD, FSS and RLSRS. The autonomic system controls alertness via a mechanism relating to circadian rhythm,27 and thus autonomic symptoms were a line of clinical variables significantly associated with EDS.28 It implied that the Braak’s stage involved structures related to both dysautonomia and EDS were severely and simultaneously compromised in PD-EDS patients. A previous study showed that the serotoninergic system in raphe nucleus and noradrenergic system in locus ceruleus were involved in the control of mood.28 Interestingly, another study reported that EDS in PD was commonly associated with the dysfunction of raphe nuclei.29 Thus, there might be a biological substrate for the close association between EDS and anxiety and depression in PD patients. Our previous investigation revealed that decrease of serotonin concentration in CSF was associated with fatigue.30 Since 5-hydroxytryptamine governed sleep-wake behavior, an imbalance was correlated to both EDS and fatigue.31 Therefore, these findings suggested that EDS and fatigue in PD might share a common dysfunction in pathophysiology and serotoninergic systems. RLS was considered to be related to dysfunction of the central dopaminergic system.32 We recently found that the CSF concentration of dopamine in PD with RLS was much lower than in PD patients with no RLS.33 It was also reported that the depletion of dopamine caused EDS.34 Therefore, EDS and RLS might both be related to the low brain dopamine concentration.
Further, we investigate the mechanism of EDS in PD. Abnormal iron accumulation in the brains of PD patients has been regularly reported since 1922. In the brains of PD patients, elevated iron concentrations in nigra and lateral globus pallidus were observed,35 and excessive free iron enhanced α-synuclein aggregation and thus promoted the formation of Lewy bodies.36 There are a few studies that investigated the iron level in CSF or serum and they have no definite conclusions. One study reported that increased CSF iron concentration was correlated with oxidative stress in PD patients.37,38 Another meta-analysis illustrated that there is no difference in CSF iron concentration between PD patients and controls.39 However, no study has established the relationship between CSF iron level and EDS in PD. This study found that CSF iron concentration was strikingly increased and CSF ferritin concentration in the PD-EDS group was obviously decreased compared with the PD-NEDS group. Logistic regression showed that CSF iron concentration was positively and CSF ferritin concentration was negatively related to EDS. Ferritin was the main iron storage cellular protein, and iron bound to ferritin was considered non-toxic. However, lower ferritin level was incapable of bearing the excessive iron in the brain, resulting to iron accumulation and neuronal death. Therefore, excessive iron accumulation and iron metabolism disruption in brain might take part in the development of EDS in PD patients.
Iron and its metabolism participate in the progression of PD pathology.40 Serum iron was transported into the brain across the blood–brain barrier (BBB). The widely known mechanism accounting for excessive iron penetration into brain involves the binding of iron-loaded large molecules, mainly transferrin, to its receptor and its translocation to the intracellular compartment.41 In a similar manner, lactoferrin-bound iron could bind to lactoferrin receptors, and contribute to iron transport through the plasma membrane. Meanwhile, a broken or leaky BBB might provide the possibility allowing elevated iron into the brain42 via lactoferrin receptors. In the current study, compared with the control group, both the PD-NEDS and PD-EDS groups had significantly decreased concentrations of iron and transferrin, and prominently increased concentrations of lactoferrin and ferritin in serum. A previous study has reported that serum ferritin was upregulated in the circulation of PD patients.43 Another study has suggested a progressive partitioning of iron to the SN pars compacta of the brain from the peripheral system in PD patients,44 suggesting that there was a disruption of the normal homeostasis between peripheral system and brain iron, in favor of an accumulation of iron in the brain.
In the brain, inflammation played a crucial part in the development of PD, and it was involved in several non-motor symptoms, such as pure apathy45 and cognitive impairment.46 One study demonstrated elevated IL-1β concentration in hypothalamus mediated sleep disorders in rats with rotenone-induced parkinsonism.47
In the study, the correlation of EDS and inflammation in PD patients was investigated. We found that the PD-EDS group had significantly increased CSF IL-1β concentration when compared with the PD-NEDS and the control groups, and ESS score was significantly correlated to CSF IL-1β concentration in PD patients. Furthermore, it was demonstrated that IL-1β concentration in CSF was the influencing factor of ESS for PD patients by using logistic regression. Thus, inflammation might play a pivotal role on EDS in PD patients. PD-EDS and PD-NEDS groups showed no difference in other inflammatory cytokines, implying that IL-1β might be a potential inflammatory indicator for EDS of PD. We also found that the TNF-α concentration in CSF in the PD-NEDS and PD-EDS groups were significantly reduced, but there was no difference between the two groups. The decreased CSF concentration of TNF-α in the PD-EDS and PD-NEDS groups might be related to the increased usage of TNF-α in the brain or because TNF-α was bound to the brain. This hypothesis was sustained by another study which found higher brain TNF-α concentration in PD patients than in controls.48
A previous study found that microglial activation was correlated with elevated iron concentration in SN.49 Meanwhile, excessive neuroinflammatory cytokines generated by microglia led to dysregulation of iron.50 In this study, CSF iron concentration was positively related to IL-1β concentration in the PD-EDS group. We hypothesize the elevated brain iron concentration might promote microglial activation, robustly produce IL-1β, and evoke neuronal death in EDS-associated regions, leading to the occurrence of EDS in PD patients.
The relationship of inflammatory cytokines between peripheral system and brain is still unclear. Several peripheral blood leukocyte adhesion molecules have been reported as contributing to a connection between systemic inflammation and neuroinflammation in PD, including macrophage antigen complex-1, lymphocyte function-associated antigen 1, E-selectin, and P-selectin.51 Another study reported that the origin of inflammatory biomarkers in the peripheral system could be by entering the body via gut dysbiosis and translocation.52
The evaluation of EDS on the basis of a subjective scale is one of the limitations in the study, another is the lack of assessment of sleep apnea, which can cause daytime sleepiness and is associated with inflammation.
Conclusions
In summary, EDS was common in PD patients. PD-EDS patients showed more advanced disease stage, and more severe motor and some non-motor symptoms. Iron metabolism disruption in CNS might be associated with PD-EDS through inflammation. This investigation may cast a new light in terms of clinical features and pathogenesis of PD-EDS involving disturbed iron metabolism and related inflammation in the brain.
Funding
This work was supported by The National Key Research and Development Program of China (2016YFC1306000, 2016YFC1306300), National Key R&D Program of China-European Commission Horizon 2020 (2017YFE0118800-779238); The National Natural Science Foundation of China (81571229, 81071015, 30770745), The Key Project of National Natural Science Foundation of China (81030062); The Key Project of Natural Science Foundation of Beijing, China (B) (kz201610025030), The Key Project of Natural Science Foundation of Beijing, China (4161004, kz200910025001), The Natural Science Foundation of Beijing, China (7082032); National Key Basic Research Program of China (2011CB504100); Important National Science & Technology Specific Projects (2011ZX09102-003-01); National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2013BAI09B03); Capital Clinical Characteristic Application Research, China (Z12110700100000, Z121107001012161); Project of Scientific and Technological Development of Traditional Chinese Medicine in Beijing, China (JJ2018-48); High Concentration Technical Personnel Training Project of Beijing Health System, China (2009-3-26); Project of Beijing Institute for Brain Disorders, China (BIBD-PXM2013_014226_07_000084); Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality, China (IDHT20140514); Excellent Personnel Training Project of Beijing, China (20071D0300400076); Beijing Healthcare Research Project, China (JING-15-2); Basic-Clinical Research Cooperation Funding of Capital Medical University, Beijing, China (2015-JL-PT-X04, 10JL49, 14JL15); Natural Science Foundation of Capital Medical University, Beijing, China (PYZ2018077); Youth Research Funding, Beijing Tiantan Hospital, Capital Medical University, Beijing, China (2015-YQN-14, 2015-YQN-15, 2015-YQN-17).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Arnulf I. Excessive daytime sleepiness in parkinsonism. Sleep Med Rev. 2005;9:185–200. doi:10.1016/j.smrv.2005.01.001
2. Hobson DE, Lang AE, Martin WR, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden-onset sleep in parkinson disease: a survey by the canadian movement disorders group. JAMA. 2002;287:455–463. doi:10.1001/jama.287.4.455
3. Videnovic A, Golombek D. Circadian and sleep disorders in parkinson’s disease. Exp Neurol. 2013;243:45–56. doi:10.1016/j.expneurol.2012.08.018
4. Breen DP, Williams-Gray CH, Mason SL, Foltynie T, Barker RA. Excessive daytime sleepiness and its risk factors in incident parkinson’s disease. J Neurol Neurosurg Psychiatry. 2013;84:233–234. doi:10.1136/jnnp-2012-304097
5. Goldman JG, Ghode RA, Ouyang B, Bernard B, Goetz CG, Stebbins GT. Dissociations among daytime sleepiness, nighttime sleep, and cognitive status in parkinson’s disease. Parkinsonism Relat Disord. 2013;19:806–811.
6. Kurtis MM, Rodriguez-Blazquez C, Martinez-Martin P. Relationship between sleep disorders and other non-motor symptoms in parkinson’s disease. Parkinsonism Relat Disord. 2013;19:1152–1155.
7. Höglund A, Broman JE, Pålhagen S, Fredrikson S, Hagell P. Is excessive daytime sleepiness a separate manifestation in parkinson’s disease? Acta Neurol Scand. 2015;132:97–104. doi:10.1111/ane.12378
8. Wallis LI, Paley MN, Graham JM, et al. Mri assessment of basal ganglia iron deposition in parkinson’s disease. J Magn Reson Imaging. 2008;28:1061–1067. doi:10.1002/jmri.21563
9. Sian-Hülsmann J, Mandel S, Youdim MB, Riederer P. The relevance of iron in the pathogenesis of parkinson’s disease. J Neurochem. 2011;118:939–957. doi:10.1111/j.1471-4159.2010.07132.x
10. Mariani S, Ventriglia M, Simonelli I, et al. Fe and cu do not differ in parkinson’s disease: a replication study plus meta-analysis. Neurobiol Aging. 2013;34:632–633. doi:10.1016/j.neurobiolaging.2012.05.015
11. Hozumi I, Hasegawa T, Honda A, et al. Patterns of levels of biological metals in csf differ among neurodegenerative diseases. J Neurol Sci. 2011;303:95–99. doi:10.1016/j.jns.2011.01.003
12. Farhoudi M, Taheraghdam A, Farid GA, et al. Serum iron and ferritin level in idiopathic parkinson. Pak J Biol Sci. 2012;15:1094–1097. doi:10.3923/pjbs.2012.1094.1097
13. Ha D, Stone DK, Mosley RL, Gendelman HE. Immunization strategies for parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S218–S221.
14. Fellner L, Jellinger KA, Wenning GK, Stefanova N. Glial dysfunction in the pathogenesis of α-synucleinopathies: emerging concepts. Acta Neuropathol. 2011;121:675–693. doi:10.1007/s00401-011-0833-z
15. Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi:10.1038/nrn2038
16. Zhang W, Phillips K, Wielgus AR, et al. Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of parkinson’s disease. Neurotox Res. 2011;19(1):63–72. doi:10.1007/s12640-009-9140-z
17. Lindqvist D, Hall S, Surova Y, et al. Cerebrospinal fluid inflammatory markers in parkinson’s disease–associations with depression, fatigue, and cognitive impairment. Brain Behav Immun. 2013;33:183–189. doi:10.1016/j.bbi.2013.07.007
18. Lindqvist D, Kaufman E, Brundin L, Hall S, Surova Y, Hansson O. Non-motor symptoms in patients with parkinson’s disease - correlations with inflammatory cytokines in serum. PLoS One. 2012;7:e47387. doi:10.1371/journal.pone.0047387
19. Postuma RB, Berg D, Stern M, et al. Mds clinical diagnostic criteria for parkinson’s disease. Mov Disord. 2015;30:1591–1601. doi:10.1002/mds.26424
20. Johns MW. A new method for measuring daytime sleepiness: the epworth sleepiness scale. Sleep. 1991;14:540–545. doi:10.1093/sleep/14.6.540
21. Jankovic J, McDermott M, Carter J, et al. Variable expression of parkinson’s disease: a base-line analysis of the datatop cohort. The parkinson study group. Neurology. 1990;40:1529–1534. doi:10.1212/wnl.40.10.1529
22. Tholfsen LK, Larsen JP, Schulz J, Tysnes OB, Gjerstad MD. Development of excessive daytime sleepiness in early parkinson disease. Neurology. 2015;85:162–168. doi:10.1212/WNL.0000000000001737
23. Kumar S, Bhatia M, Behari M. Excessive daytime sleepiness in parkinson’s disease as assessed by epworth sleepiness scale (ess). Sleep Med. 2003;4:339–342. doi:10.1016/s1389-9457(03)00105-9
24. Scott NW, Macleod AD, Counsell CE. Motor complications in an incident parkinson’s disease cohort. Eur J Neurol. 2016;23:304–312. doi:10.1111/ene.12751
25. Cilia R, Akpalu A, Sarfo FS, et al. The modern pre-levodopa era of parkinson’s disease: insights into motor complications from sub-saharan africa. Brain. 2014;137:2731–2742. doi:10.1093/brain/awu195
26. Amara AW, Chahine LM, Caspell-Garcia C, et al. Longitudinal assessment of excessive daytime sleepiness in early parkinson’s disease. J Neurol Neurosurg Psychiatry. 2017;88:653–662. doi:10.1136/jnnp-2016-315023
27. Buijs RM, Escobar C, Swaab DF. The circadian system and the balance of the autonomic nervous system. Handb Clin Neurol. 2013;117:173–191. doi:10.1016/B978-0-444-53491-0.00015-8
28. Simuni T, Caspell-Garcia C, Coffey C, et al. Correlates of excessive daytime sleepiness in de novo parkinson’s disease: a case control study. Mov Disord. 2015;30:1371–1381. doi:10.1002/mds.26248
29. Schwartz JR, Roth T. Neurophysiology of sleep and wakefulness: basic science and clinical implications. Curr Neuropharmacol. 2008;6:367–378. doi:10.2174/157015908787386050
30. Zuo LJ, Yu SY, Hu Y, et al. Serotonergic dysfunctions and abnormal iron metabolism: relevant to mental fatigue of parkinson disease. Sci Rep. 2016;6:19. doi:10.1038/s41598-016-0018-z
31. Foglieni B, Ferrari F, Goldwurm S, et al. Analysis of ferritin genes in parkinson disease. Clin Chem Lab Med. 2007;45:1450–1456. doi:10.1515/CCLM.2007.307
32. Suzuki K, Miyamoto M, Miyamoto T, et al. Nocturnal disturbances and restlessness in parkinson’s disease: using the Japanese version of the parkinson’s disease sleep scale-2. J Neurol Sci. 2012;318:76–81. doi:10.1016/j.jns.2012.03.022
33. Piao YS, Lian TH, Hu Y, et al. Restless legs syndrome in parkinson disease: clinical characteristics, abnormal iron metabolism and altered neurotransmitters. Sci Rep. 2017;7:10547.
34. Bhalsing K, Suresh K, Muthane UB, Pal PK. Prevalence and profile of restless legs syndrome in parkinson’s disease and other neurodegenerative disorders: a case-control study. Parkinsonism Relat Disord. 2013;19:426–430.
35. Dusek P, Jankovic J, Le W. Iron dysregulation in movement disorders. Neurobiol Dis. 2012;46:1–18. doi:10.1016/j.nbd.2011.12.054
36. Crichton RR, Dexter DT, Ward RJ. Brain iron metabolism and its perturbation in neurological diseases. J Neural Transm. 2011;118:301–314. doi:10.1007/s00702-010-0470-z
37. Qureshi GA, Qureshi AA, Memon SA, Parvez SH. Impact of selenium, iron, copper and zinc in on/off parkinson’s patients on l-dopa therapy. J Neural Transm Suppl. 2006;229–236. doi:10.1007/978-3-211-33328-0_24
38. Jiao J, Guo H, He Y, Wang J, Yuan J, Hu W. Meta-analysis of the association between serum iron levels and parkinson’s disease: evidence from 11 publications. Brain Res. 2016;1646:490–493. doi:10.1016/j.brainres.2016.06.044
39. Genoud S, Senior AM, Hare DJ, Double KL. Meta-analysis of copper and iron in parkinson’s disease brain and biofluids. Mov Disord. 2020;35:662–671. doi:10.1002/mds.27947
40. Logroscino G, Marder K, Graziano J, et al. Altered systemic iron metabolism in parkinson’s disease. Neurology. 1997;49:714–717. doi:10.1212/wnl.49.3.714
41. Kaur D, Andersen J. Does cellular iron dysregulation play a causative role in parkinson’s disease? Ageing Res Rev. 2004;3:327–343. doi:10.1016/j.arr.2004.01.003
42. Oestreicher E, Sengstock GJ, Riederer P, Olanow CW, Dunn AJ, Arendash GW. Degeneration of nigrostriatal dopaminergic neurons increases iron within the substantia nigra: a histochemical and neurochemical study. Brain Res. 1994;660:8–18. doi:10.1016/0006-8993(94)90833-8
43. Pretorius E, Swanepoel AC, Buys AV, Vermeulen N, Duim W, Kell DB. Eryptosis as a marker of parkinson’s disease. Aging. 2014;6:788–819. doi:10.18632/aging.100695
44. Costa-Mallen P, Gatenby C, Friend S, et al. Brain iron concentrations in regions of interest and relation with serum iron levels in parkinson disease. J Neurol Sci. 2017;378:38–44. doi:10.1016/j.jns.2017.04.035
45. Wang F, Yu SY, Zuo LJ, et al. Excessive iron and α-synuclein oligomer in brain are relevant to pure apathy in parkinson disease. J Geriatr Psychiatry Neurol. 2016;29:187–194. doi:10.1177/0891988716632918
46. Yu SY, Zuo LJ, Wang F, et al. Potential biomarkers relating pathological proteins, neuroinflammatory factors and free radicals in pd patients with cognitive impairment: a cross-sectional study. BMC Neurol. 2014;14:113. doi:10.1186/1471-2377-14-113
47. Yi PL, Tsai CH, Lu MK, Liu HJ, Chen YC, Chang FC. Interleukin-1beta mediates sleep alteration in rats with rotenone-induced parkinsonism. Sleep. 2007;30:413–425. doi:10.1093/sleep/30.4.413
48. Nagatsu T, Sawada M. Inflammatory process in parkinson’s disease: role for cytokines. Curr Pharm Des. 2005;11:999–1016. doi:10.2174/1381612053381620
49. Hunter RL, Liu M, Choi DY, Cass WA, Bing G. Inflammation and age-related iron accumulation in f344 rats. Curr Aging Sci. 2008;1:112–121. doi:10.2174/1874609810801020112
50. Wang J, Song N, Jiang H, Wang J, Xie J. Pro-inflammatory cytokines modulate iron regulatory protein 1 expression and iron transportation through reactive oxygen/nitrogen species production in ventral mesencephalic neurons. Biochim Biophys Acta. 2013;1832:618–625. doi:10.1016/j.bbadis.2013.01.021
51. Chiang P-L, Chen H-L, Lu C-H, et al. White matter damage and systemic inflammation in parkinson’s disease. BMC Neurosci. 2017;18(1):48. doi:10.1186/s12868-017-0367-y
52. Caggiu E, Arru G, Hosseini S, et al. Inflammation, infectious triggers, and parkinson’s disease. Front Neurol. 2019;10:122. doi:10.3389/fneur.2019.00122
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
