Back to Journals » Breast Cancer: Targets and Therapy » Volume 14
Clinical Characteristics and Survival Outcomes of Patients with De Novo Metastatic Breast Cancer
Authors Almasri H , Erjan A , Abudawaba H, Ashouri K, Mheid S, Alnsour A, Abdel-Razeq H
Received 26 July 2022
Accepted for publication 1 October 2022
Published 29 October 2022 Volume 2022:14 Pages 363—373
DOI https://doi.org/10.2147/BCTT.S383874
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Hanan Almasri,1 Ayah Erjan,1 Hebah Abudawaba,1 Khaled Ashouri,2 Sara Mheid,1 Anoud Alnsour,1 Hikmat Abdel-Razeq2,3
1Department of Radiation Oncology, King Hussein Cancer Center, Amman, Jordan; 2Department of Internal Medicine, King Hussein Cancer Center, Amman, Jordan; 3Department of Internal Medicine, School of Medicine, University of Jordan, Amman, Jordan
Correspondence: Hikmat Abdel-Razeq, Department of Internal Medicine, King Hussein Cancer Center, 202 Queen Rania Al-Abdulla St., P.O. Box 1269, Al-Jubeiha, Amman, 11941, Jordan, Tel +96265300460, Email [email protected]
Purpose: Though less than 5% of patients with breast cancer present with De Novo Metastasis (dnMBC) in Western societies, this percentage may reach 30% in developing countries. In this study, we present survival outcomes of patients diagnosed with dnMBC treated at a tertiary center in a developing country.
Patients and Methods: Using hospital-based database, consecutive patients with dnMBC diagnosed between 2013 and 2017 were identified. Demographic data, tumor characteristics, types of treatment, and survival data were retrospectively collected.
Results: A total of 435 patients were included; median age (range) at time of diagnosis was 51 (24– 85) years. Most of the tumors expressed hormone receptors (81% Estrogen Receptor positive, 77% Progesterone Receptor positive). Human epidermal growth factor receptor-2 (HER2) overexpression was reported in 134 (30.9%) patients, while only 24 (5.5%) had Triple Negative (TN) disease. Bone, lung and liver were the most common sites of metastasis involved in 70.6%, 36.1%, and 32.0%, respectively. The median Overall Survival (OS) for all patients was 38 months, and 5-year OS was 32.6%. On univariate analysis, high tumor grade, advanced T-stage, TN-disease and metastasis to multiple sites, but not HER2 status, were associated with poor OS. On multivariate analysis, high tumor grade (Hazard Ratio =1.6, p=0.002), advanced T-stage (Hazard Ratio=1.6, p=0.003), and triple negative status (Hazard Ratio= 2.1, p=0.008) predicted poor OS.
Conclusion: The overall survival of patients with dnMBC remains poor. Better understanding of the disease behavior and factors affecting survival is required for optimal utilization of available regimens and new drugs to hopefully improve patients’ outcomes.
Keywords: overall survival, subtype, developing countries
Introduction
Despite the advances attained in the early detection of breast cancer, it is estimated that approximately 3–6% of newly diagnosed breast cancer patients in developed countries present with distant metastatic disease at the time of diagnosis. In developing countries; however, the estimated incidence ranges between 10 to 30%.1,2 This entity is also referred to as De Novo Metastatic Breast Cancer (dnMBC) which is distinct from recurrent metastatic breast cancer (rMBC) that presents initially localized, then progresses to distant metastasis at a later time.
In Jordan, breast cancer remains the most common cancer among females accounting for 39.7% of all newly diagnosed cancers.3 At our institution, a tertiary referral center, the percentage of breast cancer cases diagnosed with dnMBC has been relatively high, but constant throughout the last few years ranging between 14 to 19% of all new breast cancer cases.4
Although some studies have demonstrated a superior prognosis of dnMBC compared to rMBC,5–7 the Overall Survival (OS) of this patient population remains poor.1 The difference in prognosis between dnMBC and rMBC suggests the presence of inherent differences between the two entities. Factors that contribute to this disparity may be related to biologic and molecular differences. One theory is that rMBC arises from resistant cell clones that have survived previous anticancer treatments due to intertumor heterogeneity and differential drug sensitivity. This poses a clinical challenge for treatment of rMBC cases that is not present in dnMBC as these are still treatment naïve.8,9 Nevertheless, the treatment of dnMBC remains largely palliative.10 The recent introduction of many new anti-cancer drugs has significantly improved patients’ outcomes. In addition to targeting hormone receptors (HR), drugs that are directed at downstream elements of the molecular pathways have been developed to overcome endocrine resistance. Of these, mammalian target of rapamycin (mTOR) inhibitors have shown clinical benefit in prolonging Progression-Free Survival (PFS) in postmenopausal women with HR positive/Human epidermal growth factor receptor-2 (HER-2) negative tumors after progression on non-steroidal aromatase inhibitors.11 More recently, the introduction of cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors as first line treatment in pre- and postmenopausal patients has revolutionized the management of advanced HR positive/HER-2 negative metastatic breast cancer.12,13 In fact, the updated results of the Monaleesa-3 Phase III randomized clinical trial have shown prolongation of OS with the use of ribociclib in addition to fulvestrant in postmenopausal women in first- and second-line setting.14
Additionally, a prolonged PFS has been observed with the addition of pertuzumab to trastuzumab and docetaxel in patients with HER-2 positive disease.15 The benefit of addition of pertuzumab has translated into improved OS increment that was maintained after a median of more than 8 years of follow-up.16 Trastuzumab emtansine has also shown clinical benefit in HER-2 positive patients as a second line treatment.17 In triple negative advanced breast cancer; however, there has been a paucity of agents apart from conventional chemotherapy, until the very recent introduction of pembrolizumab in the first line setting for treatment of this group.18 Likewise, improved OS has been observed with the addition of atezolizumab to nab-paclitaxel in the PD-L1 positive population.19 Data on de novo metastatic breast cancer are limited due to the heterogeneity of the disease and the fact that most of the published literature analyzes both rMBC and dnMBC together. Therefore, a better understanding of the disease behavior and factors affecting survival is required for optimal utilization of available regimens and new drugs to improve patient outcomes.
Several published studies have examined factors that might affect the outcome of dnMBC. A population-based Italian study has shown that the prognosis of dnMBC has been improving over time particularly in the last decade with the introduction of novel drugs. In the same study, poor tumor characteristics including triple negative receptors, node positivity, and high Ki67 were predictive of worse survival.20 Results from another retrospective study have shown that molecular subtype was predictive of prognosis. Triple negative disease harbored the worst prognosis compared to other subtypes, while the outcome of HER-2 positive disease was not necessarily worse despite the apparent aggressive presentation; a finding that is explained by the advances in anti-HER2 therapeutic targets.21 Some sites of distant metastasis, namely, brain and liver predicted poorer outcome compared to other sites in a SEER database analysis study.22
Whether similar outcomes are expected in a developing country is unknown. A study from Brazil has demonstrated an inferior OS compared to high income countries. Delay in diagnosis and poor access to health care and new anti-cancer drugs, may represent some of the contributing factors for patients treated in developing countries.23
In this study, we aimed to review the outcomes of breast cancer patients diagnosed with de novo metastatic disease treated at our institution to identify potential clinico-pathological and treatment-related factors that affect their prognosis. We also aimed to identify any differences in the outcomes of a developing country compared to published data from high-income countries.
Materials and Methods
We retrospectively reviewed the medical records of metastatic breast cancer patients diagnosed in the period between 2013 and 2017 inclusive, who received their full treatment at King Hussein Cancer Center. Patients with missing follow-up data were excluded. All diagnoses were confirmed by central pathology review. Demographic variables (age, sex), tumor-related variables (histology, grade, primary tumor size, T and N stage, HR status, HER2 over-expression/amplification status, and sites of metastatic disease), and systemic or radiotherapy treatments received were collected into a database. Follow-up data including date of disease progression, vital status, and date of death were collected from the hospital database and national registry. The study was approved by King Hussein Cancer Center Institutional Review Board.
Patients were stratified with regard to age into two categories: those who were ≤ 50 years old and those who were >50 at the time of diagnosis. Histologic subtypes were recorded as invasive ductal carcinoma (IDC), invasive lobular carcinoma (ILC), or others. Grade was extracted from pathology report and recorded as GI, GII, or GIII. Lymph node (LN) status was recorded as positive or negative based on radiological or histological findings.
Hormone receptor status was recorded from pathology reports where immunohistochemical stains for estrogen receptor (ER) /progesterone receptor (PR) were performed on formalin fixed paraffin embedded sections using automated immunostaining method. Any staining of 1% or more was considered positive. HER-2 results were reported according to the ASCO/CAP reporting guidelines. Score 3+ is considered positive whereas Score 2+ is reported as equivocal and reclassified using in situ-hybridization method.24
The American Joint Committee on Cancer (AJCC) 8th edition staging system was used to determine the clinical or pathological T and N stages. The sites of metastasis were recorded based on clinical findings and imaging studies for each patient.25 Patients received treatment according to institutional clinical practice guidelines.
Statistical Analysis
The number and proportion of the categorical variables and the mean and standard deviation (SD) of the continuous explanatory variables were reported. Survival was plotted using the Kaplan-Meier method and compared using Log rank tests. Multivariate analysis was performed using Cox Regression Method. Variables with p-value of ≤ 0.05 on Log rank test were included in the multivariate analysis.
OS was defined as the time from diagnosis to death of any cause or date of last follow-up. PFS was defined as the time from diagnosis to disease progression or death of any cause. The outcomes of interest in the study are median and 5-year OS and PFS for the whole cohort. Overall survival and PFS were also compared between groups stratified by age, tumor grade, T-stage, nodal status, hormone receptor and HER-2 status and sites of distant metastasis. All reported P-values were two-sided, and the significance level was set at ≤ 0.05. Statistical analysis was performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Results
A total of 435 patients were included in the analysis. Baseline characteristics are shown in Table 1. Median age at diagnosis was 51 years (range: 24–85 years). The cohort included 432 females and 3 males. IDC was the predominant histology constituting 85.5% (n=372) of all histologies, followed by ILC which accounted for 8.5% (n=37). Around half (n=201, 46.2%) of the tumors were high-grade tumors (G-III). Advanced T-stage (T3 and T4) was found in 205 (47.2%) patients while 322 (74.0%) had regional lymph node involvement. Mean tumor size was 5.4 cm (SD=2.9). Majority of the patients had HR positive tumors (ER positive in 81.4%, PR positive in 77.2%) while HER2 overexpression was reported in 134 (30.9%) and 24 (5.5%) were Triple Negative (TN).
 |
Table 1 Demographic, Tumor-Related, and Treatment Characteristics of Metastatic Breast Cancer Patients, n=435 |
The most common site of distant metastasis was bone (n=307, 70.6%) followed by lung (n= 157, 36.1%) and liver (n=139, 32.0%). Bone as the only site of metastasis was reported in 82 (18.9%) patients. Central nervous system (CNS) metastasis was reported in 104 (23.9%) (Table 2).
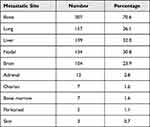 |
Table 2 Number and Percentage of Patients Presenting with Metastasis to Specific Organ Sites |
The median OS for all patients was 38 months, and 5-year OS was 32.6%. On univariate analysis, no difference in OS was observed when comparing patients based on age group (≤ 50 vs > 50; OS 30.1% vs 34.9%, log rank p=0.995). Worse OS was seen in Grade III tumors in comparison with Grade I/II tumors (5-year OS: 19.1% vs 45.7%, log rank p<0.001). Likewise, patients with advanced T-stage (T3 and T4) showed significantly inferior OS compared to those who presented with T1 and T2 tumors (5-year OS: 26.5% vs 46.9%, log-rank p<0.001). A trend toward better 5-year-OS was observed in patients who presented with uninvolved regional lymph nodes compared to those with involved regional lymph nodes, (OS=41.7% vs 31.7%, log rank p=0.105).
While Triple Negative (TN) status predicted a significantly lower OS compared to other subtypes (5-year OS 0% in TN vs 35.0% in non-TN, log rank p<0.001), comparing OS in patients with or without HER-2 overexpression did not show a significant difference (5-year OS 32.9% with HER-2 overexpression vs 33.4% without it, log rank p=0.341).
The presence of multiple metastatic sites was associated with poorer OS compared to metastasis to a single organ (5-year OS: 24.1% for multiple vs 50.0% for single organ metastasis, log rank p<0.001). Patients with metastatic disease confined to the bones demonstrated statistically significant superior overall survival compared to those who had additional metastatic sites (OS= 55.9% vs 28.1%, log rank p<0.001) (Figure 1).
The median PFS for the whole cohort was 15.2 months. Factors predicting worse PFS were similar to those predicting worse OS including TN status (log rank p<00.1), high grade (log rank p=0.002), T-stage (III/IV) (log rank p<0.001), regional nodal involvement (log rank p<0.001), and more than one site of metastasis (log rank p<0.001). Better PFS was observed in patients who had metastasis to the bone alone compared to those who had additional sites of metastasis (Median 19.7 months vs 13.9 months, log rank p=0.001). HER-2 status did not predict a significant difference in PFS (Figure 2).
On Multivariate analysis, factors associated with poorer OS were high tumor grade (Hazard ratio=1.64, p=0.0016), advanced T-stage (Hazard ratio=1.59, p=0.0029) and TN status (Hazard ratio=2.07, p=0.0084). Likewise, multivariate analysis revealed worse PFS in patients with high-grade tumors (Hazard ratio=1.56, p=0.0012), those who had advanced T-stage (Hazard ratio= 1.31, p=0.0436, those who had involved regional lymph nodes (Hazard ratio=1.71, p=0.0052), and those who had TN disease (Hazard ratio=2.31, p=0.0026) (Table 3).
Discussion
In our study, we reviewed a cohort of patients diagnosed with dnMBC between 2013 and 2017 and treated at a tertiary cancer center. The median OS for the whole cohort was 38 months and the 5-year OS was 32.6%. We were able to identify several prognostic factors that are predictive of survival including stage of the local tumor, nuclear grade, molecular subtype, metastasis to multiple organs, and metastasis to bone alone.
Our findings suggest a similar median OS as previously published studies from developed countries. A study by den Brok et al reported a median OS of 34 months for patients with dnMBC treated in British Columbia in the period between 2001 and 2009.7 Likewise, Dawood et al reported median OS of 39.2 months for a cohort of 643 dnMBC patients treated at MD Anderson Cancer center in the United States between 1992 and 2007.6 Reports from developing countries have demonstrated inferior outcomes compared with developed countries. Bhoo-Pathy et al’s study reviewed 856 Asian patients with dnMBC and showed a median survival of 21 months for patients treated in the period between 2006 and 2010.26 Similarly, in a population-based retrospective study from Brazil including patients treated between 1995 and 2011, Soares et al reported a median OS of 20 months.23 We believe that our results are more comparable to those observed in developed countries. This is probably because our cohort represents a group of patients who were treated at a leading tertiary cancer center with unified clinical practice guidelines that incorporate most of the up-to-date drugs and are based on international guidelines.
We analyzed multiple clinicopathological variables to identify factors that are predictive of prognosis. Of demographic factors, patient’s age did not seem to affect OS. The effect of patient’s age on OS in dnMBC has been inconsistent in previous studies. Data published by Zeichner et al, revealed no difference in outcome between patients older or younger than 50 years of age.27 On the other hand, Dawood et al’s study found age younger than 50 at the time of diagnosis to be predictive of better OS.6
Of tumor-related variables, we found that tumors presenting with advanced T stage, regional nodal involvement, and high nuclear grade were associated with worse survival compared with low grade, smaller tumors or those without lymph node metastasis. In a study by Cortesi et al, heavy nodal involvement (≥4 lymph nodes) was associated with worse OS.20 This finding is interesting as despite the fact that distant metastasis is present, low tumor burden locally appears to still affect the prognosis. The question of whether or not locoregional therapy is beneficial in such cases remains controversial. In a large series published by Iwase et al reviewing 1981 patients treated between 1995 and 2017 at MD Anderson Cancer Center, better OS was observed in tumors with low nuclear grade and in patients who received locoregional treatment.28
In addition, we found that molecular subtype largely affects the prognosis with the worst being the TN subtype. On the contrary, no difference was observed in HER-2 positive versus negative subtypes. This is in line with observations of several other studies. Wu et al demonstrated a Hazard Ratio of 2.5 (CI: 2.296–2.800, P<0.001) for worse OS in patients with triple negative disease compared with HR positive disease.22 Likewise, Press et al found the mortality risk to be increased in TN subtype (Hazard Ratio = 2.02, 95% CI 1.89–2.16) and reduced in HR positive/HER2neu positive subtype (Hazard Ratio = 0.83, 95% CI 0.78–0.88) when compared to HR positive/HER-2 negative subtype.21 Limited treatment options in TN disease compared to the recent advances and the availability of novel medications for HER-2 positive disease may explain this finding. Without anti-HER2 directed therapy, the outcome of metastatic HER-2 positive disease would be extremely poor as the addition of trastuzumab to chemotherapy has been shown to reduce the relative risk of death by 20% in a randomized clinical trial by Slamon et al.29 Improvement of OS over time after the introduction of trastuzumab has also been demonstrated in the study published by Iwase et al in HR positive/HER-2 positive dnMBC.28 Whereas, for TN disease, the results reported by denBrok et al showed only a slight difference in OS between patients who did or did not receive systemic treatment, highlighting the limitations of the currently available treatments and the need to employ novel medications in this subgroup. In fact, the introduction of immunotherapy is showing promising results in patients with metastatic triple negative breast cancer. The recently published results of the KEYNOTE-355 randomized phase III trial have shown a statistically significant and clinically meaningful improvement in PFS with the addition of pembrolizumab to chemotherapy in this group of patients.18
We observed improvement in OS in patients with metastasis to a single site compared to those who had multiple organ metastasis. This finding was also observed in a study published by Ren et al where patients with single organ metastasis were found to have better survival compared to those with multi-organ metastasis.30 This area deserves special attention given the growing evidence on the superior outcomes of oligometastatic disease and the potential for cure. This is leading to more inclination for aggressive treatment of patients who harbor a limited number of metastases. Current approaches aim to treat such patients with intention to cure by offering standard treatment in addition to treating metastatic sites with surgery or ablative radiotherapy. In a Phase II prospective study by Trovo et al, a prolonged PFS was observed in patients treated with radical radiotherapy to the metastatic sites in a cohort of 54 patients of whom 40 (74%) had distant metastasis at the time of initial diagnosis.31
Our study is, to our knowledge, the first report from the region that investigated the outcomes of patients with de novo metastatic breast cancer separate from rMBC. Furthermore, the cohort reviewed was diagnosed and treated in recent years, which reflects better on the effect of contemporary treatments on patient outcomes. Some limitations of this study may include the limited number of patients as the data reflect outcomes at a single institution rather than being at a national level.
Conclusion
Although the outcomes of dnMBC are generally poor, identifying prognostic factors is essential for better understanding of the disease behavior. Improved survival may be achieved with optimal utilization of available regimens and new drugs to tailor treatments according to disease characteristics.
Abbreviations
AJCC, American Joint Committee on Cancer; CNS, central nervous system; dnMBC, de novo metastatic breast cancer; ER, estrogen receptor; HER-2, human epidermal growth factor receptor-2; HR, hormone receptors; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; IRB, institutional review board; LN, lymph node; mTOR, mammalian target of rapamycin; OS, overall survival; PFS, progression-free survival; PR, progesterone receptor; rMBC, recurrent metastatic breast cancer; SD, standard deviation; TN, triple negative.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study was approved by the institutional review board (IRB) at King Hussein Cancer center, (Approval ID: 19 KHCC 53). Due to the retrospective nature of the study, the data were collected from the patients’ charts. The informed consent requirement was waived by IRB. The data collected were anonymized and confidentiality was maintained in accordance with the Declaration of Helsinki.
Acknowledgments
The authors would like to acknowledge Ms. Ayat Taqash for performing the statistical analysis for this study and Ms. Rayan Bater for coordination.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi:10.3322/caac.21583
2. Daily K, Douglas E, Romitti PA, Thomas A. Epidemiology of de novo metastatic breast cancer. Clin Breast Cancer. 2021;21(4):302–308. doi:10.1016/j.clbc.2021.01.017
3. Abdel-Razeq H, Mansour A, Jaddan D. Breast cancer care in Jordan. JCO Glob Oncol. 2020;6:260–268. doi:10.1200/JGO.19.00279
4. Abdel-Razeq H, Almasri H, Abdel Rahman F, et al. Clinicopathological characteristics and treatment outcomes of breast cancer among adolescents and young adults in a developing country. Cancer Manag Res. 2019;11:9891–9897. doi:10.2147/CMAR.S229337
5. Lao C, Kuper-Hommel M, Elwood M, Campbell I, Edwards M, Lawrenson R. Characteristics and survival of de novo and recurrent metastatic breast cancer in New Zealand. Breast Cancer. 2021;28(2):387–397. doi:10.1007/s12282-020-01171-3
6. Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010;21(11):2169–2174. doi:10.1093/annonc/mdq220
7. den Brok WD, Speers CH, Gondara L, Baxter E, Tyldesley SK, Lohrisch CA. Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed. Breast Cancer Res Treat. 2017;161(3):549–556. doi:10.1007/s10549-016-4080-9
8. Seltzer S, Corrigan M, O’Reilly S. The clinicomolecular landscape of de novo versus relapsed stage IV metastatic breast cancer. Exp Mol Pathol. 2020;114:104404. doi:10.1016/j.yexmp.2020.104404
9. Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol. 2015;12(7):381–394. doi:10.1038/nrclinonc.2015.73
10. Caswell-Jin JL, Plevritis SK, Tian L, et al. Change in survival in metastatic breast cancer with treatment advances: meta-analysis and systematic review. JNCI Cancer Spectr. 2018;2(4):pky062. doi:10.1093/jncics/pky062
11. Yardley DA, Noguchi S, Pritchard KI, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis [published correction appears in Adv Ther. 2014;31(9):1008-9]. Adv Ther. 2013;30(10):870–884. doi:10.1007/s12325-013-0060-1
12. Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer [published correction appears in Ann Oncol. 2019 Nov 1;30(11):1842]. Ann Oncol. 2018;29(7):1541–1547. doi:10.1093/annonc/mdy155
13. Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised Phase 3 trial. Lancet Oncol. 2018;19(7):904–915. doi:10.1016/S1470-2045(18)30292-4
14. Slamon DJ, Neven P, Chia S, et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival [published correction appears in Ann Oncol. 2021;32(10):1307]. Ann Oncol. 2021;32(8):1015–1024. doi:10.1016/j.annonc.2021.05.353
15. Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–119. doi:10.1056/NEJMoa1113216
16. Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–530. doi:10.1016/S1470-2045(19)30863-0
17. Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial [published correction appears in Lancet Oncol. 2017 Aug;18(8):e433][published correction appears in Lancet Oncol. 2018;19(12):e667]. Lancet Oncol. 2017;18(6):732–742. doi:10.1016/S1470-2045(17)30312-1
18. Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–1828. doi:10.1016/S0140-6736(20)32531-9
19. Emens LA, Adams S, Barrios CH, et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis [published correction appears in Ann Oncol. 2021 Aug 2; [published correction appears in Ann Oncol. 2021;32(12):1650]. Ann Oncol. 2021;32(8):983–993. doi:10.1016/j.annonc.2021.05.355
20. Cortesi L, Toss A, Cirilli C, et al. Twenty-years experience with de novo metastatic breast cancer. Int J Cancer. 2015;137(6):1417–1426. doi:10.1002/ijc.29503
21. Press DJ, Miller ME, Liederbach E, Yao K, Huo D. De novo metastasis in breast cancer: occurrence and overall survival stratified by molecular subtype. Clin Exp Metastasis. 2017;34(8):457–465. doi:10.1007/s10585-017-9871-9
22. Wu SG, Li H, Tang LY, et al. The effect of distant metastases sites on survival in de novo stage-IV breast cancer: a SEER database analysis. Tumour Biol. 2017;39(6):1010428317705082. doi:10.1177/1010428317705082
23. Soares LR, Freitas-Junior R, Curado MP, Paulinelli RR, Martins E, Oliveira JC. Low overall survival in women with de novo metastatic breast cancer: does this reflect tumor biology or a lack of access to health care? JCO Glob Oncol. 2020;6:679–687. doi:10.1200/JGO.19.00408
24. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi:10.1200/JCO.2013.50.9984
25. Giuliano AE, Edge SB, Hortobagyi GN. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol. 2018;25(7):1783–1785. doi:10.1245/s10434-018-6486-6
26. Bhoo-Pathy N, Verkooijen HM, Tan EY, et al. Trends in presentation, management and survival of patients with de novo metastatic breast cancer in a Southeast Asian setting. Sci Rep. 2015;5:16252. doi:10.1038/srep16252
27. Zeichner SB, Herna S, Mani A, et al. Survival of patients with de-novo metastatic breast cancer: analysis of data from a large breast cancer-specific private practice, a university-based cancer center and review of the literature. Breast Cancer Res Treat. 2015;153(3):617–624. doi:10.1007/s10549-015-3564-3
28. Iwase T, Shrimanker TV, Rodriguez-Bautista R, et al. Changes in overall survival over time for patients with de novo metastatic breast cancer. Cancers. 2021;13(11):2650. doi:10.3390/cancers13112650
29. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi:10.1056/NEJM200103153441101
30. Ren Z, Li Y, Hameed O, Siegal GP, Wei S. Prognostic factors in patients with metastatic breast cancer at the time of diagnosis. Pathol Res Pract. 2014;210(5):301–306. doi:10.1016/j.prp.2014.01.008
31. Trovo M, Furlan C, Polesel J, et al. Radical radiation therapy for oligometastatic breast cancer: results of a prospective phase II trial. Radiother Oncol. 2018;126(1):177–180. doi:10.1016/j.radonc.2017.08.032
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



