Back to Journals » International Journal of General Medicine » Volume 14
Clinical Characteristics and Risk Factors for Pleural Effusion in Patients with Multiple Myeloma
Authors Kang Y , Hou ZL, Yang GZ , Wang XJ, Chen WM, Shi HZ
Received 10 January 2021
Accepted for publication 12 February 2021
Published 25 February 2021 Volume 2021:14 Pages 649—657
DOI https://doi.org/10.2147/IJGM.S300337
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yu Kang,1 Zi-Liang Hou,2 Guang-Zhong Yang,3 Xiao-Juan Wang,1 Wen-Ming Chen,3 Huan-Zhong Shi4
1Department of Internal Medicine, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Beijing Luhe Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Department of Hematology, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China; 4Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Huan-Zhong Shi; Wen-Ming Chen Email [email protected]; [email protected]
Purpose: Pleural effusion (PE) is prevalent in “real-life” populations of multiple myeloma (MM), a common hematologic malignancy. Development of PE likely has prognostic implications. The aim of this study was to investigate the characteristics and identify risk factors for occurrence of PE in MM.
Patients and Methods: We reviewed electronic medical records of 907 patients diagnosed with MM.
Results: Incidence of PE in MM patients was 42.7%. Small and bilateral PE in most cases. PE developed in all MM subtypes, the median time from diagnosis of multiple myeloma to pleural effusion was 6.8 months (range 0.8– 33.6 months). Patients with PE showed worse survival than those without PE (unadjusted hazard ratio with 95% confidence interval: 2.249 [1.774– 2.852]). No difference in survival was found between patients with small PE and those with moderate to large PE (unadjusted HR, 1.402; 95% CI, 1.037– 1.896). Plasma cell proportion (OR, 1.373; 95% CI, 1.153– 1.634; P = 0.009) and amyloidosis (OR, 1.791; 95% CI, 1.408– 2.279; P = 0.024) were risk factors for the occurrence of PE at the initial diagnosis of MM. Plasma cell proportion (OR, 1.853; 95% CI, 1.451– 2.368; P = 0.038), pneumonia (OR, 1.309; 95% CI, 1.143– 1.498; P = 0.008) and heart failure (OR, 1.815; 95% CI, 1.387– 2.374; P = 0.031) were risk factors for the occurrence of PE at relapse of MM.
Conclusion: The incidence of PE in MM patients is notable and PE can occur in all MM subtypes. PE indicates a poor prognosis, even small amounts of effusion. PE is a problem worthy of attention, especially in patients with high plasma cell proportion, amyloidosis or complicated with pneumonia and heart failure.
Keywords: pleural effusion, multiple myeloma, incidence, risk factors, overall survival
Introduction
Pleural effusion (PE) is a common clinical problem. It is estimated that about 1.5 million people suffer from PE in the United States.1 PE can be caused by a variety of reasons, including disease local to the pleura or systemic condition.2–4 Hematologic malignances are one of the causes of pleural effusion. Appearance of PE portends a poorer prognosis in cancer patients.5,6 It was reported that appearance of PE is associated with clinical response during hematologic malignances treatment.7,8 Evaluation of pleural effusion in patients with hematologic malignances is required.
Multiple myeloma (MM) is the second most frequent hematologic malignancy, and each year over 30,000 new cases are diagnosed in the USA.9,10 In Asian countries, the incidence of MM has increased rapidly during the past two decades, and MM now represents the third most common hematologic disease in South Korea.11,12 It is estimated that incidence of MM patients in China was 27,800 new cases each year and a total of 200 000 cases in China.13 Multiple myeloma is a clonal B-cell malignancy characterized by proliferation of uncontrolled plasma cells in the bone marrow or extramedullary sites, leading to excessive production of immunoglobulins. Anemia, renal failure, hypercalcemia, and lytic bone lesions are the most commonly encountered manifestations of MM.14
In our center, PE is frequently diagnosed in patients with MM. Previous literature reported the PE frequency of 10.7% to 13.9%.15,16 However, according to our observations, the incidence of PE in MM patients is much higher. Hitherto, articles on PE in MM patients were mostly case reports, comprehensive studies of PE in MM patients have not been performed. There is a pressing need to better understand the clinical characteristics and risk factors for PE in patients with MM. Thus, here we aimed to investigate the incidence, distribution, and outcomes of PE in patients with MM, and to investigate risk factors for PE in MM patients.
Patients and Methods
Study Population and Data Collection
This was a retrospective, single-center study. We identified a total of the 907 patients who were diagnosed with multiple myeloma were admitted to Beijing Chao-yang Hospital between January 01, 2000, and December 31, 2017. There were 46 patients without computed tomography (CT) of the chest, which were excluded from the study. Among the enrolled 861 MM patients, 528 (61.3%) were newly diagnosed, and 208 (24.2%) were relapsed multiple myeloma (Figure 1).
 |
Figure 1 Flow chart of study population. Abbreviations: MM, multiple myeloma; PE, pleural effusion; CT, computed tomography. |
The detailed medical history, clinical presentation, laboratory results, and imaging data from all patients were extracted from the electronic medical records. We studied various aspects of PE associated with MM. The study protocol was approved by the Institutional Review Board for Human Studies of Beijing Chaoyang Hospital, Beijing, China. The study was carried out in conformity to the Declaration of Helsinki. The review board exempted the acquisition of informed consent because this was a retrospective study. Patients’ data confidentiality was fully respected during data collection and the preparation of the manuscript.
Diagnosis and Evaluations
The International Myeloma Working Group (IMWG) criteria for the diagnosis of MM were adopted. The survival of all enrolled patients was followed up to December 31, 2018, or until death. Patients were categorized according to the Durie-Salmon staging system (DS) and the International Staging System (ISS).17,18 Definition of relapsed multiple myeloma based on the International Myeloma Working Group relapse criteria for multiple myeloma.19,20
Chest CT was performed to evaluate PE. In our center, the chest CT scans were routinely examined when the patients were initially admitted, and were performed before new course of chemotherapy, or when fever, cough, expectoration, dyspnea, and other respiratory symptoms appeared, as determined by two hematologists and pulmonologists. Malignant pleural effusions (MPE), which are diagnosed based on malignant cells in the pleura or the pleural fluid.
The size of effusion was evaluated on CT scans according to the CT imaging features with anteroposterior quartile and maximum anteroposterior depth measured at the midclavicular line as described by Moy et al. The first anteroposterior-quartile effusions were small, second quartile effusions were moderate, and third and fourth quartile effusions were large.21 Evaluation of PE was done by radiologists and pulmonologists. For statistical considerations, data were analyzed based on the first identified PE in each patient.
Statistical Analysis
Categorical variables were described using counts and percentages, and groups were compared using a chi-square test or Fisher’s exact probability test. Continuous variables were presented as means and standard deviations, and significant differences between two groups were determined with a Student’s t-test. For non-normally distributed data, median and interquartile ranges were used to describe the features, while comparisons of the two sets were performed using a Mann–Whitney U-test. Survival rate was calculated using the Kaplan–Meier (KM) method. The median duration of the follow-up and its 95% CI were calculated using the reverse KM method. To determine the factors associated with the occurrence of pleural effusion in MM, logistic regression analysis was performed. The odds ratios (OR) with 95% confidence intervals (CI) were presented. The statistical analysis of data was performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA) and R software (version 3.5.1) with the corresponding R packages. All tests were two-sided, and a value of P < 0.05 was considered statistically significant.
Results
Incidence and Distribution of Pleural Effusion in Multiple Myeloma Patients
A total of 368 patients had pleural effusion occurred during the course of treatment of MM identified on CT out of 861 patients with available CT data, yielding the incidence of 42.74%. The median follow-up time of the study population was 44.9 months (95% CI, 42.6–48.8). Characteristics of patients with and without pleural effusion are summarized in Table 1. There were no significant differences in age, sex, myeloma subtypes, and staging distribution between 861 included patients with available CT data and 46 patients without chest CT data (Supplementary Table 1).
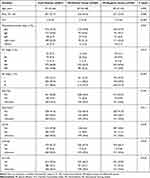 |
Table 1 Characteristics of Patients with Multiple Myeloma |
The median time from diagnosis of multiple myeloma to pleural effusion was 6.8 months (range 0.8–33.6 months), 56.3% patients developed PE in the first year following the initial diagnosis of MM. PE developed in all myeloma subtypes, the distribution of myeloma types in patients with PE was similar to patients without PE. While 321 (87.2%) patients developed PE presented with DS stage III, 213 (57.9%) patients presented with ISS stage III. The distribution of myeloma staging using the DS and ISS was significantly different between the PE-negative and PE-positive group (P = 0.015, P < 0.001, respectively). The characteristics of patients developed PE are shown in Table 1.
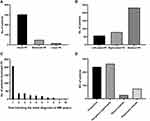
The distribution features of pleural effusion in multiple myeloma patients are shown in Figure 2. 15.5% of PEs were left-sided, 21.5% were right-sided, while both sides were affected in 63.0% of cases. In either unilateral or bilateral effusion, 82.6% of PEs were of small size, while moderate and large sizes were present in 13.0% and 4.3% of cases, respectively. Thirteen (38.2%) malignant pleural effusions (MPE) was confirmed in 34 patients who had undergone thoracentesis, 7 (53.8%) MPE was of small size. Pneumonia and pleural hypertrophy were frequently seen in patients with PE.
Outcomes of Pleural Effusion and Overall Survival
During the median follow-up period of 44.9 months (95% CI, 42.6–48.8), the complete disappearance of PE was observed in 23 (6.25%) of the patients. Decreased PE was noted in 39 (10.6%) patients, including PE decreased then reoccurred in 9 patients. PE persisted in 138 (37.5%) patients, while PE increased in 69 (18.8%) patients. The response was not documented in the remaining 99 patients. In this study, 78/368 (19.8%) patients with PE experienced dyspnea.
Thirty-four (9.24%) patients underwent thoracentesis, 15 (44.1%) patients underwent thoracentesis because of intolerable dyspnea. The median time from the initial PE was found to the first thoracentesis was 1.0 months (range, 0–7.5 months).
One hundred and eighty-four (32.1%) patients died during the follow-up duration. The median overall survival (OS) was 82.4 months (95% CI, 72.5–89.6), and the 5-year OS rates were 62.0% (95% CI, 56.9%-67.5%). Kaplan–Meier (KM) curves of overall survival in MM patients following the initial diagnosis of MM are showed in Figure 3. Patients with PE showed significantly worse survival since the initial diagnosis of MM than those without PE (unadjusted HR, 2.249; 95% CI, 1.774–2.852). No difference in survival was found between patients with small PE and those with moderate to large PE (unadjusted HR, 1.402; 95% CI, 1.037–1.896).
Risk Factors for Pleural Effusion in Multiple Myeloma Patients
We explored the risk factors for pleural effusion using logistic regression, as shown in Table 2. Our study investigated patients who presented pleural effusion at the time of initial MM diagnosis or at the time of relapse. Fifty-two (9.8%) presented pleural effusion at the time of initial MM diagnosis among 528 patients who were newly diagnosed in our center. Among the 208 patients who were relapsed, 143 (68.8%) presented pleural effusion at the time of relapse. Input variables for logistic regression analysis were selected from significant variables obtained from the univariate analysis and variables related to the occurrence of PE that were reported in the previous literature, the final model contains 13 variables.
 |
Table 2 Risk Factors for the Occurrence of Pleural Effusion in MM Patients* |
Clonal plasma cell proportion in the bone marrow at the time of initial MM diagnosis (OR, 1.373; 95% CI, 1.153–1.634; P = 0.009) and amyloidosis (OR, 1.791; 95% CI, 1.408–2.279; P = 0.024) were independent risk factors for the occurrence of pleural effusion at the initial diagnosis of MM. Clonal plasma cell proportion in the bone marrow at the time of MM relapse (OR, 1.853; 95% CI, 1.451–2.368; P = 0.038), pneumonia (OR, 1.309; 95% CI, 1.143–1.498; P = 0.008) and heart failure (OR, 1.815; 95% CI, 1.387–2.374; P = 0.031) were independent risk factors for the occurrence of pleural effusion at relapse of MM.
Discussion
To the best of our knowledge, this was the largest series dealing with PE in patients with MM. The noteworthy finding in this study was the incidence of PE in MM patients. Namely, the incidence of PE in MM patients was 42.7%, which is much higher than the data from prior reports. Lower incidence in other studies may reflect the fact that most previous studies were in the form of case reports, and only two studies mentioned the frequency of PE in the range between 10.7% and 13.9%;15,16 however, there were no detailed evaluations. Moreover, a higher incidence of PE in our series may be due to the meticulous attention paid to detecting PE in our hospital. Beijing Chao-Yang Hospital not only has the Beijing Institute of Respiratory Diseases, well recognized at the national level, but also houses one of the largest myeloma treatment centers in China. Our study suggested that the incidence of PE in MM patients is actually notable, and it is likely that MM is often overlooked in patients with PE in other centers.
We noted that patients with PE showed worse survival than those without PE. The statistical calculation is hinging on the presence or absence of pleural effusion, whatever the clearly etiologies of PEs. It is just the presence of PE has an impact on prognosis, which is why it is very important to identify it. Pleural effusion is almost always a manifestation of one or more underlying primary conditions. It is possible that presence of effusion is a marker of poor prognosis by virtue of the severe tumor burden or worse host comorbidity status.
Hitherto, the distribution of the PE in MM patients has not been studied. Previous studies reported inconsistent and unclear data on incidence of PE among different subtypes of MM. A higher prevalence of IgG and IgA subtypes was reported in previous case reports.22–25 Here we found that PE occurred in all classes of myeloma subtypes. We noted that majority of PEs in our study were of small size. It is noteworthy that even small PE, its presence is an important prognostic factor of worse survival. No difference in survival was found between patients with small PE and those with moderate to large PE. In addition, MPE is not always massive, given that we found that in more than 50% MPEs was confirmed in 34 patients who had undergone thoracentesis in our study were of small size. Small PE might represent an early phase of malignant PE or severe comorbid disease. We suggested that more attention should be paid to pleural effusion, even small amounts of pleural effusion.
Another important aspect of our study was related to identification of risk factors associated with the occurrence of PE. Through logistic regression analysis, we demonstrated that plasma cell proportion was an independent risk factor for the occurrence of PE either at the initial diagnosis of MM or at the time of MM relapse. This association remained significant after adjustment for age, sex, M-protein types, the ISS or DS stage, fluorescence in situ hybridization (FISH) test, presence of heart disease or renal failure, history of tuberculous and hypoproteinemia. MM is a malignancy of the B-cell lineage, characterized by the accumulation of clonal plasma cells in the bone marrow, leading to excessive production of immunoglobulins. High proportion of clonal plasma cells in the bone marrow indicates greater severity of the disease.26,27 It is supposed that a factor of the development of PE in MM patient is the production of large quantities of immunoglobulins, which leads to high colloid osmotic pressure of the fluid.28
Additionally, in this study, amyloidosis was found to be an independent risk factor for the occurrence of PE at the initial diagnosis of MM. In amyloidosis, amyloid protein is derived from immunoglobulin light chains, and most often involves the kidneys and the heart. Direct pleural amyloidosis can also lead to pleural effusions. Sunny et al reported a case of exudate and amyloidosis in thoracoscopic pleural biopsy.29 Pneumonia and heart failure were found to be significant independent predictors for the occurrence of pleural effusion at relapse by logistic regression. In our study, more than 65% of patients experienced pneumonia at the onset of pleural effusion in our study. For these MM patients undergoing chemotherapy, infection does play a role in the development of PE. In addition, many of MM patients have heart failure. The capillary hydrostatic pressure might be increased lead to the occurrence of PE. Multiple myeloma can have direct and indirect detrimental effects on cardiac function. Several drug classes used in the treatment of MM are known to increase the risk of cardiac events, such as doxorubicin, anthracycline, lenalidomide, pomalidomide, carfilzomib and bortezomib.30
Since the incidence of PE was higher than expected, these patients with PE actually pose a diagnostic and therapeutic challenge in need of better management approaches. Infection and heart failure are important factors leading to PE.16,31 Also, considering the high prevalence of tuberculosis in China, tuberculosis might have been overlooked in these MM patients with PE. These are possibly treatable causes. On the other hand, in patients underwent thoracentesis, MPE was confirmed in13/34 (38.2%) patients. Previous literature reported the MPE in MM patients frequency of 0.8% to 2.65%.32–34 A part of the effusions in 334 patients who did not receive additional evaluation to determine the cause of PE may be MPE, but they were not identified. Complete disappearance of PE was observed in only 6.5% patients, and most PE persisted or increased in the remaining patients. If PE went undetected or did not be pay attention to, it may result in some patients end up receiving suboptimal treatment. Identify reversible causes of PE or MPE conducive to clinical decision making. In addition, recent evidence suggests that indwelling pleural catheters are safe in hematologic malignancies.35 Therefore, a diagnostic thoracentesis or further evaluation of PE should be performed widely in MM patients.
Though our investigation provided first comprehensive study to evaluate epidemiology, clinical characteristics, risk factors, and prognosis of PE in MM patients, it had a few limitations. First of all, our study was a real-world, single-center, retrospective cohort study, which resulted in incomplete data and inability to control examinations and treatment. Some patients were asymptomatic and may not have been picked up in the absence of routine evaluation. Long follow-up is often associated with missing data, and is likely to have bias errors. Second, few patients underwent thoracentesis, which might have limited the interpretative power on the cause of the effusion. Finally, future research will be required to determine if PE is a sign for therapy change and if more aggressive therapy after the diagnosis of PE can improve prognosis.
Conclusions
The incidence of PE in MM patients is notable and PE can occur in all MM subtypes. PE indicates a poor prognosis, even small amounts of effusion. PE is a problem worthy of attention, especially in patients with high plasma cell proportion, amyloidosis or complicated with pneumonia and heart failure.
Acknowledgments
The authors thank the patients, their families, and all investigators who participated in the study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Light RW. Pleural effusions. Med Clin North Am. 2011;95(6):1055–1070.
2. Light RW. Clinical practice. Pleural effusion. N Engl J Med. 2002;346(25):1971–1977.
3. Sahn SA, Heffner JE. Pleural fluid analysis. In: Light RW, Lee YCG, editors. Textbook of Pleural Diseases.
4. BTS Pleural Guideline Group. Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii4–ii17.
5. Goldstraw P, Crowley J, Chansky K, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714.
6. Ryu JS, Azra M, Lee SK, et al. Prognostic impact of minimal pleural effusion in non-small-cell lung cancer. J Clin Oncol. 2014;32(9):960–967.
7. Nakaya A, Fujita S, Satake A, et al. Clinical significance of dasatinib-induced pleural effusion in patients with de novo chronic myeloid leukemia. Hematol Rep. 2018;10(3):7474.
8. Porkka K, Khoury HJ, Paquette RL, et al. Dasatinib 100 mg once daily minimizes the occurrence of pleural effusion in patients with chronic myeloid leukemia in chronic phase and efficacy is unaffected in patients who develop pleural effusion. Cancer. 2010;116(2):377–386.
9. Röllig C, Knop S, Bornhäuser M, et al. Multiple myeloma. Lancet. 2015;385(9983):2197–2208.
10. Siegel RL, Miller KD, Jemal A, et al. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
11. Chen JH, Chung CH, Wang YC, et al. Prevalence and mortality-related factors of multiple myeloma in Taiwan. PLoS One. 2016;11(12):e0167227.
12. Park HJ, Park EH, Jung KW, et al. Statistics of hematologic malignancies in Korea: incidence, prevalence and survival rates from 1999 to 2008. Korean J Hematol. 2012;47(1):28–38.
13. Lu J, Lu J, Chen W, et al. The Chinese medical doctor association hematology branch. Clinical features and treatment outcome in newly diagnosed Chinese patients with multiple myeloma: results of a multicenter analysis. Blood Cancer J. 2014;4:e239. doi:10.1038/bcj.2014.55
14. Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–2972.
15. Wang Z, Xia G, Lan L, et al. Pleural effusion in multiple myeloma. Intern Med. 2016;55(4):339–345.
16. Byun JM, Kim KH, Choi IS, et al. Pleural effusion in multiple myeloma: characteristics and practice patterns. Acta Haematol. 2017;138(2):69–76.
17. Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91(7):719–734.
18. Rajkumar SV. Myeloma today: disease definitions and treatment advances. Am J Hematol. 2016;91(1):90–100.
19. Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9.
20. Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International myeloma workshop consensus panel 1. Blood. 2011;117:4691–4695.
21. Moy MP, Levsky JM, Berko NS, et al. A new, simple method for estimating pleural effusion size on CT scans. Chest. 2013;143(4):1054–1059.
22. Juan NR. Pleural effusion in multiple myeloma. Chest. 1994;105:622–624.
23. Kamble R, Wilson CS, Fassas A, et al. Malignant pleural effusion of multiple myeloma: prognostic factors and outcome. Leuk Lymphoma. 2005;46(8):1137–1142.
24. Zhang LL, Li YY, Hu CP, et al. Myelomatous pleural effusion as an initial sign of multiple myeloma - a case report and review of literature. J Thorac Dis. 2014;6(7):E152–E159.
25. Saidane O, Slouma M, Haouet S, et al. Cutaneous and pleural involvement in a patient with multiple myeloma. BMJ Case Rep. 2015;201.
26. Rajkumar SV. Multiple myeloma: 2018 update on diagnosis, risk-stratification, and management. Am J Hematol. 2018;93(8):981–1114.
27. Gerecke C, Fuhrmann S, Strifler S, et al. The diagnosis and treatment of multiple myeloma. Dtsch Arztebl Int. 2016;113(27–28):470–476.
28. Chetan BP, Neeraj G, Rakesh CG, et al. Varna Indushekar Myelomatous pleural effusion-thoracoscopic evaluation of a rare entity. Lung India. 2015;32(5):505–507.
29. George S, Ravindran M, Anandan PT, et al. Primary systemic amyloidosis: a rare cause for pleural effusion. Respir Med Case Rep. 2014;13:39–42.
30. Kistler K, Rajangam K, Faich G. Cardiac event rates in patients with newly diagnosed and relapsed multiple myeloma in US clinical practice. Blood. 2012;120:2916.
31. Dipenkumar M, Hyejeong J, Seongho K, et al. Incidence, etiology, and outcome of pleural effusions in allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2016;91(9):E341–E347.
32. Kim YJ, Kim SJ, Min K, et al. Multiple myeloma with myelomatous pleural effusion: a case report and review of the literature. Acta Haematol. 2008;120(2):108–111.
33. Arora P, Gupta SK, Mallik N, et al. Flow cytometry in diagnosis of myelomatous pleural effusion: a case report. Indian J Hematol Blood Transfus. 2016;32(Suppl 1):138–342.
34. Mangla A, Agarwal N, Kim GJ, et al. Primary malignant myelomatous pleural effusion. Clin Case Rep. 2016;4(8):803–806.
35. Vakil E, Jimenez CA, Faiz SA, et al. Pleural effusions in hematologic malignancies and their management with indwelling pleural catheters. Curr Opin Pulm Med. 2018;24(4):384–391.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.