Back to Journals » Infection and Drug Resistance » Volume 15
Clinical Characteristics and Risk Factors for in-Hospital Mortality in 240 Cases of Infective Endocarditis in a Tertiary Hospital in China: A Retrospective Study
Authors Zhang X , Jin F, Lu Y , Ni F, Xu Y, Xia W
Received 22 February 2022
Accepted for publication 23 May 2022
Published 18 June 2022 Volume 2022:15 Pages 3179—3189
DOI https://doi.org/10.2147/IDR.S362601
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xiaohui Zhang,1,2,* Fei Jin,1,2,* Yanfei Lu,1,2 Fang Ni,1,2 Yuqiao Xu,1,2 Wenying Xia1,2
1Department of Laboratory Medicine, Jiangsu Province Hospital and Nanjing Medical University First Affiliated Hospital, Nanjing, People’s Republic of China; 2Branch of National Clinical Research Center for Laboratory Medicine, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuqiao Xu; Wenying Xia, Department of Laboratory Medicine, Jiangsu Province Hospital and Nanjing Medical University First Affiliated Hospital, Street No. 300, Guangzhou, 210029, People’s Republic of China, Tel +8625-6830-6146 ; +8625-6830-6287, Fax +8625-8372-4440, Email [email protected]; [email protected]
Purpose: This study aimed (i) to investigate the clinical characteristics and risk factors related to in-hospital mortality in patients with infective endocarditis (IE) and (ii) to compare the differences in three age groups.
Methods: A total of 240 IE cases diagnosed using the modified Duke criteria between January 2016 and December 2019 were included and retrospectively studied. Patients were stratified into three age groups: < 50 y, 50– 65 y, and > 65 y.
Results: The mean age of the patients was 51 ± 14 y, and 154 patients (64.2%) were male. In addition, 136 (56.7%) patients with IE had no previous cardiac disease. Congenital heart disease (CHD, 21.3%) was the most common underlying heart disease, followed by rheumatic heart disease (RHD, 8.8%). Streptococcus was found in 55 (22.9%) patients and was the most common causative pathogen, comprising 52.9% of all positive blood cultures. Echocardiography showed the presence of vegetations in 88.3% of cases and the predominant involvement of the left heart valves. Fever and cardiac murmur were the most frequent presentations, with no significant differences among age groups. Compared with younger patients, elderly patients had a lower operation rate and higher in-hospital mortality. The independent risk factors of in-hospital mortality were age > 65 y, intracranial infection, splenic embolization, cerebral hemorrhage, NYHA class III–IV, and prosthetic valve infection.
Conclusion: CHD replaces RHD as the most common underlying heart disease in IE patients. Patients without previous cardiac disease are at increased risk of IE. Streptococcus is still the primary causative pathogen of IE. Elderly patients present with more comorbidities and complications, in addition to a more severe prognosis than younger patients. Age older than 65 y, intracranial infection, splenic embolization, cerebral hemorrhage, NYHA class III–IV, and prosthetic valve infection showed poorer in-hospital outcomes.
Keywords: infective endocarditis, clinical characteristics, risk factors, elderly patients
Introduction
Infective endocarditis (IE) is the infection of the heart valve, the endocardial surface, or the septum and accompanying systemic pathological process.1 The overall morbidity of IE in developed countries ranges between 30 and 100 episodes per million individuals annually.2 However, the incidence of IE in recent years has increased with the application of prosthetic valve replacement, indwelling cardiac devices, and various endovascular treatment technologies.3 IE is a life-threatening disease with high mortality rates of 14–22% in-hospital and reaching 51% at 10 years despite advances in medical and surgical care.4 The prognosis for patients with IE is strongly dependent on early diagnosis and prompt treatment. However, the clinical presentation of IE can be highly variable and nonspecific, which potentially leads to a significant delay in diagnosis, thus negatively affecting patient prognosis.5 The epidemiological characteristics of IE have recently changed owing to the evolution of medical practice and demographic changes.6 Moreover, the incidence of IE in elderly patients has increased because of the aging general population and the increased proportion of comorbidities.4,7,8 Age is currently included as a predictor in several risk scores and is regarded as an important independent predictor of mortality in patients with IE.7,9,10
Therefore, the objectives of this study are as follows: (1) to analyze the clinical features, echocardiogram, and microbiological profile of IE and compare the three age groups and (2) to explore the prognostic risk factors of in-hospital mortality.
Materials and Methods
Patients
This study was conducted at the First Affiliated Hospital of Nanjing Medical University, a tertiary general hospital with 4200 licensed beds in East China. Patients diagnosed with definite IE in accordance with the modified Duke criteria11 between January 2016 and December 2019 were included (Figure 1). Incomplete case records, nonhospitalization, indefinite diagnosis, or untreated IE comprised the exclusion criteria for this study. Ultimately, 240 inpatients were enrolled.
 |
Figure 1 Definite diagnosis of IE according to the modified Duke criteria. |
Data Extraction and Study Design
Data were retrieved from electronic medical records. The collected data included the demographic information, clinical features, predisposing factors, pathogenic microorganisms in the blood, echocardiographic findings, complications, treatments, and prognoses (in-hospital and one-year mortality) of the patients. The patients were classified into three groups by age. For each age group, the number of patients was as follows: under 40 y, 104 (43.3%); 50–65 y, 103 (42.9%); over 65 y, 33 (13.8%). To explore the risk factors of in-hospital mortality, we subdivided the patients into the survival group and the death group.
Definition
For a definitive diagnosis of IE, the ESC guidelines for pathological and clinical criteria were used as reference. Although some patients with negative blood cultures failed to meet the clinical criteria, IE patients confirmed by intraoperative exploration and postoperative pathology were included in this study. A total of 192 patients were definitively diagnosed with IE on the basis of the following criteria: two major criteria or positive blood culture plus intraoperative exploration, or vegetations or valve lesions (found by echocardiography or during operation) plus positive pathology or valve/embolus culture. Meanwhile, 48 patients with definite IE met one major criterion plus three minor criteria.
Congenital heart disease (CHD) was coded using Q20 to Q28, and rheumatic heart disease (RHD) was coded using I05 to I09 in the International Classification of Diseases, Tenth Revision (ICD-10).
Complications of IE were defined as follows: septic shock (the presence of an acute circulatory failure in sepsis, characterized by persistent arterial hypotension—ie, systolic pressure < 90 mmHg despite adequate volume resuscitation); embolic events, including intracranial complications (eg, hemorrhage and infarction) and peripheral embolic events mainly characterized by splenomegaly or splenic embolism; functional class III–IV in accordance with the New York Heart Association (NYHA) classification; atrial fibrillation (a common arrhythmia in patients with acute conditions attributed to inflammation and hemodynamic change).6,12,13
As the main diagnostic methods for IE, transthoracic echocardiogram and blood culture were conducted on all patients. However, PCR testing of valve culture was not performed, similar to anti-Legionella, Bartonella, Coxiella burnetii, and Mycoplasma antibody tests. Indications for cardiac surgery were based on the 2015 ESC guidelines for the management of IE patients with heart failure, uncontrolled infections, embolic events, and vegetation size > 10 mm were required to undergo early surgical intervention. The time until surgery was defined as the interval between the diagnosis of IE and the date of surgery.14
Statistical Analysis
Statistical analyses were performed using SPSS version 25.0 (SPSS Inc., Chicago, United States). The data were presented as mean ± standard deviation and median for continuous variables and as frequencies and percentages for categorical variables. Comparison of baseline characteristics between groups (A: under 50 y, B: 50–65 y, and C: > 65 y) was performed using the chi-square test. In-hospital mortality was defined as death from any cause during hospitalization. Univariate analysis was performed using the chi-square or Fisher’s exact tests as appropriate for categorical variables. After univariate analysis, variables with P<0.05 were included in multivariate analysis to identify predictors for in-hospital mortality among IE patients. Odds ratios (OR) with a 95% confidence interval (CI) were calculated in logistic regression. P-value < 0.05 was considered statistically significant.
Results
Clinical Characteristics of IE Patients
The clinical features, echocardiographic data, and causative microorganisms of 240 IE patients are summarized in Table 1. The mean age of patients was 51±14 y, and 154 cases (64.2%) were male.
 |
Table 1 Clinical Features, Echocardiography and Microbiology of 240 IE Cases |
The most frequent clinical presentations were fever (78.8%) and chest tightness/asthma (43.3%). Cardiac murmur was found in 85.4% of patients during physical examination, and 136 (56.7%) patients with IE had no previous cardiac disease. Among the patients with underlying heart disease (43.3%), 51 (21.3%) patients had CHD—mainly bicuspid aortic valve (25 cases), ventricular septal defect (16 cases), and patent foramen ovale (6 cases). In addition, 21 (8.8%) cases had a history of RHD. The most prevalent comorbidities were hypertension (25.0%) and diabetes (12.5%). Cerebrovascular accident, splenomegaly, and decreased cardiac function were prominent complications observed in IE patients. Atrial fibrillation rhythm was observed in 22 patients during hospitalization.
Vegetations were detected in 212 (88.3%) patients by echocardiography. Native valve involvement was detected in 215 (89.6%) patients, followed by prosthetic valve-related IE (8.8%) and cardiac device-related IE (1.7%). The distribution of valve involvement was as follows: mitral valve (51.7%), aortic valve (48.3%), tricuspid valve (6.7%), pulmonary valve (3.3%), and multiple valves (20.4%). No vegetations were found by echocardiography in 11.7% (28/240) of patients; however, vegetations, valve lesions (such as valve perforation, perivalvular abscesses, or rupture of chordae tendineae), or pseudoaneurysms were found intraoperatively in 20 of the patients. Positive blood cultures were detected in 8 patients.
Blood cultures were positive in 104 (43.3%) patients, and 108 strains were isolated (Figure 2). Streptococcus was the most common microorganism, found in 55 (22.9%) patients and comprising 52.9% of all positive blood cultures. S. aureus and coagulase-negative Staphylococcus (CoNS) constituted 7.5% and 4.6% of the microorganisms, respectively. S. aureus was detected by blood culture in 83.3% of patients with septic shock, 28.6% of whom had an intracranial infection. Meanwhile, 5 methicillin-resistant S. aureus (MRSA) and 6 methicillin-resistant CoNS (MRCNS) strains were isolated from blood culture by antimicrobial susceptibility testing. The resistance rates of S. aureus and CoNS to penicillin were 88.9% and 90.9%, respectively. No Streptococcus and Staphylococcus resistance to vancomycin and linezolid were isolated. In addition, 1 strain of E. faecalis exhibited resistance to linezolid, but no Enterococcus showed resistance to vancomycin. The gram-negative bacilli were composed of 4 strains of Acinetobacter, 4 strains of Haemophilus, 1 strain each of Escherichia coli, Stenotrophomonas maltophilia, Brucella, Aggregatibacter actinomycetemcomitans, and Brevundimonas diminuta.
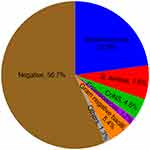 |
Figure 2 Results of blood culture in 240 IE patients. |
Among the IE patients, 56.7% had negative blood culture, and 75.0% (102/136) had previous antibiotic therapy. Cardiac surgery was conducted on 72.1% (98/136) of the patients with negative blood cultures. Meanwhile, 60 patients underwent valve cultures, 16 of which were positive (including 10 strains of Streptococcus, 3 strains of CoNS, 2 strains of S. aureus, and 1 strain of Pseudomonas schisterii). Overall, the in-hospital mortality of the IE patients was 6.3%, of which 19 patients (7.9%) died during the one-year follow-up. The mean time between diagnosis and surgery was 14 ± 13 d (median=10 d).
Comparison Between Three Groups Classified by Age
The 240 IE patients were divided into three groups by age (listed in Table 2): 104 patients in Group A, 103 patients in Group B, and 33 patients in Group C. No statistically significant difference in the clinical presentations (fever and cardiac murmur) of IE was found between the age groups. Group A had a significantly higher percentage of cases with CHD (29.8%), splenomegaly (29.8%), multivalvular infection (26.9%), and paravalvular abscess (15.4%) than other age groups. Chest tightness/asthma symptoms (50.5%), diabetes comorbidity (18.4%), and vegetation size ≥ 10 mm (60.2%) more frequently occurred in Group B than in Group A. However, the frequencies of occurrence of hypertension (63.6%), atrial fibrillation (10.7%), and enterococcal bloodstream infection (9.1%) were significantly higher in Group C than in other groups. Previous heart valve surgery, renal or liver disease, prosthetic valve infection, septic shock, and gram-negative bacilli infection were more common in Group C than in other age groups; however, no statistical difference was indicated. The proportions of surgical treatment were 72.1% (Group A), 76.9% (Group B), and 51.5% (Group C), respectively. The in-hospital mortality of the IE patients in Group C (18.2%) was significantly higher than those in Groups A (4.9%) and B (3.9%).
 |
Table 2 Characteristics of 240 Patients with IE in the Three Age Groups |
Risk Factors for in-Hospital Mortality
The risk factors associated with in-hospital mortality were analyzed. The results are listed in Table 3. Univariate analysis showed that the following factors were associated with the in-hospital mortality: age over 65 y, CHD, previous heart valve surgery, intracranial infection, septic shock, splenic embolization, cerebral hemorrhage, atrial fibrillation, NYHA class III–IV, prosthetic valve infection, gram-negative bacilli infection, and surgical treatment.
 |
Table 3 Risk Factors of In-Hospital Outcome in Patients with IE (Univariate Analysis) |
Multifactorial analysis was conducted on clinical variables that were determined as statistically significant by univariate analysis. The following were identified as independent predictors of in-hospital mortality (Table 4): age over 65 y (P=0.005, OR=13.357, 95% CI 2.220–80.345), intracranial infection (P=0.004, OR=19.397, 95% CI 2.586–145.500), splenic embolization (P=0.015, OR=26.493, 95% CI 1.906–368.186), cerebral hemorrhage (P=0.008, OR=12.851, 95% CI 1.959–84.326), NYHA class III–IV (P=0.016, OR=7.636, 95% CI 1.471–39.644), and prosthetic valve infection (P=0.045, OR=6.633, 95% CI 1.042–42.205).
 |
Table 4 Risk Factors of In-Hospital Mortality in Patients with IE (Multivariate Analysis) |
Discussion
The present study provided valuable insights into the clinical characteristics and prognostic risk factors of IE patients in recent years on the basis of data collected from a tertiary hospital in China.
This study identified fever and heart murmur as the most common manifestations in IE patients, followed by chest tightness/asthma. Although these findings could occur in various diseases and were nonspecific, IE should be highly suspected when they crossed or coexisted. Consistent with other reports, CHD replaced RHD as the most common underlying heart disease in IE patients, comprising 20.1%–36.7%15–18 of the heart diseases. This percentage might be attributed to the increasing trend of CHD patients in recent years; the late diagnosis for CHD contributed to the restrictions of local medical and economic conditions and a greater number of patients with CHD surviving to adulthood.15,19 The main types of IE in patients with CHD were bicuspid aortic valve and ventricular septal defect. Studies further have suggested that the proportion of IE in patients without underlying heart disease is rising, which may be related to the increase of invasive medical measures, hemodialysis, intravenous drug users, and the wide application of antibacterial drugs and immunosuppressants.20 IE has occurred predominantly in males, with a male-to-female ratio of 1.2–2.7:1,21 and has been associated with an increased proportion of valvular disease (such as CHD, particularly the bicuspid aortic valve).22,23 In the present study, the most frequent complications observed were cerebrovascular accident, splenomegaly, and decreased cardiac function. In the ESC-EORP EURO-ENDO (European infective endocarditis) registry, the most common complications are embolic events (particularly in the brain) in up to 40% of IE patients.24
Therapeutic schedules mainly rely on blood culture, the major criterion for IE, followed by identification and susceptibility testing of the isolate.25 Consistent with previous reports in China,26,27 Streptococcus was the primary causative organism in IE, followed by S. aureus, CoNS, and Enterococcus. Owing to population aging, a decrease in RHD burden, and advanced device management, the prevalence of S. aureus has increased, eventually becoming the dominant pathogen in IE in developed countries.21,28 In the current study, S. aureus had a detection rate of only 7.5% but nonetheless was the main cause of septic shock and intracranial infection related to the prognosis of IE patients. Enterococcus IE is the third leading cause of IE, ranging in frequency from 7% to 18% in developed countries.29–31 However, Enterococcus was identified in only 4 patients in this study, and the proportion was similar in another report from East China.20
In the present study, blood cultures were negative in 56.7% of cases, similar to the data in some Asiatic populations (30–61%)32–34 but surpassing the number in other developed countries (7–20%).33,35,36 The high prevalence of negative blood cultures was usually associated with the antibiotic treatment given before the blood culture, the insufficient number of blood cultures submitted, highly fastidious bacterial or nonbacterial infections, and inadequate microbiological techniques.37 In the present study, 75.0% (102/136) of the patients with negative blood cultures had previous antibiotic therapy. Multiple studies have indicated that previous administration of antibiotics can decrease the recovery rate of bacteria by 35% to 40%,38 particularly against the common bacteria causing IE, such as Streptococcus, and less commonly Staphylococcus or Enterococcus.39 Further, 24 other patients were not tested for blood cultures in our hospital because other medical centers reported negative results, or fewer than two sets of blood cultures were submitted. In addition, serological assessment for special pathogens (eg, Bartonella, C. burnetii, or Mycoplasma) and molecular biological methods (eg, PCR) that had been directly used for clinical microbiology and detection of samples (blood or resected cardiac valves) could be helpful for the diagnosis of IE.25 However, these tests were not conducted in the current study, which was the limitation of this study.
Echocardiography is vital in the diagnosis and evaluation of IE. It detects vegetations in 88.3% of IE patients, with the left heart valves predominantly involved. In this study, the mitral and aortic valves are almost equally affected, with 51.7% and 48.3%, respectively. Multiple valves are involved in 20.4% of cases. These proportions are higher in another report.40 In addition, this study found that mitral valve (61.9%) was most commonly involved in RHD patients, while aortic valve (68.6%) was significantly more affected in patients with CHD. In our cohort, the incidence of prosthetic valve endocarditis was only 8.8% (21/240), which is lower than the 26–30% of the cases reported in other studies.24,40,41 However, further data indicated that 23 patients had previously undergone cardiac valve replacement or plasty, of which 21 cases developed prosthetic valve infection. Therefore, the prevention of IE in patients undergoing heart valve surgery is the most important.
In the present study, echocardiography failed to detect vegetations in 28 IE patients, but valve vegetations or lesions were found in 20 of them during operation. Transthoracic echocardiography has been shown to exhibit 70% and 50% sensitivity in detecting native valve vegetation and prosthetic valve vegetation, respectively.11 Transoesophageal echocardiography has demonstrated sensitivity and specificity exceeding 90% for vegetations and thus can be performed to confirm the diagnosis of IE in cases of high clinical suspicion, particularly in prosthetic or device-related IE. Additional imaging techniques such as 18F-FDG PET/CT or radiolabeled leucocyte single-photon emission computed tomography-computed tomography (SPECT-CT) can determine the presence of prosthetic valve infection or perivalvular lesions (abscess and aneurysm or pseudoaneurysm formation).42 In the present study, 2 patients with prosthetic valve-related IE were diagnosed by 18F-FDG PET/CT.
In our cohort, the mean age of IE patients was 51 y—that is, older than the mean age reported in a study conducted in China from 2013 to 2018.26 Meanwhile, research from developed countries has reported an increasing proportion of elderly patients with IE, which increase stems from multiple factors: more extensive investigations in elderly cases, increased survival in patients with multiple comorbidities, widespread use of invasive therapeutic interventions (such as pacemakers and defibrillators), and hemodialysis.19 The overall in-hospital mortality rate in our study was 6.3%, but a significantly high mortality rate (18.2%) was observed in the elderly (over 65 y old).
The clinical characteristics of IE patients in different age groups were compared. We found that 30% of IE patients aged less than 50 y had a history of CHD, mainly characterized by splenomegaly, multivalve infection, and paravalvular abscess. By contrast, elderly cases with IE showed lower frequencies of embolic events (particularly splenomegaly) and valvular lesions, as observed in another study.7 These patients showed higher occurrences of comorbidities and atrial fibrillation and had poorer prognosis than those of younger patients. Nonspecific features impeded diagnosis in elderly patients. In addition to the common Streptococcus, gram-negative bacteria and Enterococcus had higher detection rates in elderly patients. Therefore, clinicians should pay attention to the coverage of these pathogens during empiric anti-infective therapy. Early surgery is an effective treatment for patients with IE. However, surgical therapy in elderly patients is less frequently performed because of severe complications and the fear of surgery by patients and their families. Recent studies have demonstrated that many frailty scores can assess the physical condition of elderly patients and the mortality independently of age before cardiac surgery.43,44 Treatment success in elderly IE may be improved by comprehensive assessment of patients and cooperation between multiple specialists.
In the present study, the factors associated with increased risk of in-hospital mortality were CHD, previous heart valve surgery, septic shock, atrial fibrillation, infection with gram-negative bacilli, and nonsurgical treatment. Meanwhile, we identified the following as independent risk factors for in-hospital mortality: age over 65 y, intracranial infection, splenic embolization, cerebral hemorrhage, NYHA class III–IV, and prosthetic valve infection. Early identification of patients with high-risk features may contribute to improved outcome through early anti-infective and surgical treatment.45 A meta-analysis found that early surgery was associated with reduced in-hospital and long-term mortality, compared with non-early surgery.46
This study presents several limitations. First, our study was a retrospective single-center design. The information was limited to medical records and inevitably biased. Second, most patients were transferred to our hospital from other medical centers at the middle or late stage of the disease, resulting in atypical clinical features and negative blood culture results. Moreover, geographic and economic differences existed. With these limitations considered, the subsequent step was to conduct a multicenter prospective cohort study in the region.
Conclusion
This study demonstrates that patients without previous cardiac disease have an increased risk of IE. Attention should be paid to the early diagnosis and treatment of CHD, and the prevention of IE after cardiac valve surgery. Streptococcus remains as the primary causative pathogen of IE in developing countries. However, the presence of S. aureus, gram-negative bacteria as well as Enterococci with a higher prevalence in elderly patients, requires additional consideration by clinicians. Previous antibiotic therapy may be the main cause of a negative blood culture, which is not conducive to the clinical criteria for the diagnosis of IE. Compared with younger patients, elderly patients have more comorbidities and complications but lower rates of surgery, leading to higher in-hospital mortality. On the basis of this finding, the benefits and risks of surgery for elderly patients need to be evaluated via multidisciplinary cooperation. Intracranial infection, splenic embolization, cerebral hemorrhage, NYHA class III–IV, and prosthetic valve infection were identified as independent risk factors for in-hospital mortality in IE patients.
Ethical Statement
Study participants had provided informed consent prior to the start of the study. Written informed consent was provided by the patients’ relatives to allow the case details to be published. This study was designed following the Declaration of Helsinki and approved by the ethics committee of Jiangsu Province Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vandersluis YR, Suri S. Infective endocarditis and orthodontic implications in children: a review of the literature. Am J Orthod Dentofacial Orthop. 2020;157(1):19–28. doi:10.1016/j.ajodo.2019.03.027
2. Damasco PV, Correal JCD, Cruz-Campos ACD, et al. Epidemiological and clinical profile of infective endocarditis at a Brazilian tertiary care center: an eight-year prospective study. Rev Soc Bras Med Trop. 2019;52:e2018375. doi:10.1590/0037-8682-0375-2018
3. Yuan XC, Liu M, Hu J, Zeng X, Zhou AY, Chen L. Diagnosis of infective endocarditis using echocardiography. Medicine. 2019;98(38):e17141. doi:10.1097/MD.0000000000017141
4. Williams ML, Doyle MP, McNamara N, Tardo D, Mathew M, Robinson B. Epidemiology of infective endocarditis before versus after change of international guidelines: a systematic review. Ther Adv Cardiovasc Dis. 2021;15:17539447211002687. doi:10.1177/17539447211002687
5. Kreitmann L, Montaigne D, Launay D, et al. Clinical characteristics and outcome of patients with infective endocarditis diagnosed in a department of internal medicine. J Clin Med. 2020;9:864. doi:10.3390/jcm9030864
6. Blanchard V, Pagis B, Richaud R, et al. Infective endocarditis in French Polynesia: epidemiology, treatments and outcomes. Arch Cardiovasc Dis. 2020;113(4):252–262. doi:10.1016/j.acvd.2019.12.007
7. Arminanzas C, Farinas-Alvarez C, Zarauza J, et al. Role of age and comorbidities in mortality of patients with infective endocarditis. Eur J Intern Med. 2019;64:63–71. doi:10.1016/j.ejim.2019.03.006
8. Chirillo F. It is not how old you are, it is how you are old: need for changes in the management of infective endocarditis in the elderly. Heart. 2017;103(20):1562–1564. doi:10.1136/heartjnl-2017-311411
9. Ostergaard L, Smerup MH, Iversen K, et al. Differences in mortality in patients undergoing surgery for infective endocarditis according to age and valvular surgery. BMC Infect Dis. 2020;20(1):705. doi:10.1186/s12879-020-05422-8
10. Olmos C, Vilacosta I, Habib G, et al. Risk score for cardiac surgery in active left-sided infective endocarditis. Heart. 2017;103(18):1435–1442. doi:10.1136/heartjnl-2016-311093
11. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075–3128. doi:10.1093/eurheartj/ehv319
12. Olmos C, Vilacosta I, Fernandez C, et al. Contemporary epidemiology and prognosis of septic shock in infective endocarditis. Eur Heart J. 2013;34(26):1999–2006. doi:10.1093/eurheartj/ehs336
13. Wei XB, Huang JL, Liu YH, et al. Incidence, risk factors and subsequent prognostic impact of new-onset atrial fibrillation in infective endocarditis. Circulation J. 2020;84(2):262–268. doi:10.1253/circj.CJ-19-0854
14. Suardi LR, de Alarcon A, Garcia MV, et al. Blood culture-negative infective endocarditis: a worse outcome? Results from a large multicentre retrospective Spanish cohort study. Infect Dis. 2021;53(10):755–763. doi:10.1080/23744235.2021.1925342
15. Wu Z, Chen Y, Xiao T, Niu T, Shi Q, Xiao Y. Epidemiology and risk factors of infective endocarditis in a tertiary hospital in China from 2007 to 2016. BMC Infect Dis. 2020;20(1):428. doi:10.1186/s12879-020-05153-w
16. Wei XB, Liu YH, Huang JL, et al. Prediabetes and diabetes are both risk factors for adverse outcomes in infective endocarditis. Diabet med. 2018;35(11):1499–1507. doi:10.1111/dme.13761
17. Zhu W, Zhang Q, Zhang J. The changing epidemiology and clinical features of infective endocarditis: a retrospective study of 196 episodes in a teaching hospital in China. BMC Cardiovasc Disord. 2017;17(1):113. doi:10.1186/s12872-017-0548-8
18. Yang F, Zhang B, Yu J, et al. Epidemiology and the prognosis of healthcare-associated infective endocarditis in China: the significance of non-nosocomial acquisition. Emerg Microbes Infect. 2015;4(7):e38. doi:10.1038/emi.2015.38
19. Shah ASV, McAllister DA, Gallacher P, et al. Incidence, microbiology and outcomes in patient hospitalized with infective endocarditis. Circulation. 2020;141:2067–2077.
20. Xu H, Cai S, Dai H. Characteristics of infective endocarditis in a tertiary hospital in East China. PLoS One. 2016;11(11):e0166764. doi:10.1371/journal.pone.0166764
21. Tagliari AP, Steckert GV, da Silveira LMV, Kochi AN, Wender OCB. Infective endocarditis profile, prognostic factors and in-hospital mortality: 6-year trends from a tertiary university center in South America. J Card Surg. 2020;35(8):1905–1911. doi:10.1111/jocs.14787
22. Yew HS, Murdoch DR. Global trends in infective endocarditis epidemiology. Curr Infect Dis Rep. 2012;14(4):367–372. doi:10.1007/s11908-012-0265-5
23. Oliver L, Lavoute C, Giorgi R, et al. Infective endocarditis in octogenarians. Heart. 2017;103(20):1602–1609. doi:10.1136/heartjnl-2016-310853
24. Habib G, Erba PA, Iung B, et al. Clinical presentation, aetiology and outcome of infective endocarditis. results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J. 2019;40(39):3222–3232. doi:10.1093/eurheartj/ehz620
25. Cheng J, Hu H, Fang W, et al. Detection of pathogens from resected heart valves of patients with infective endocarditis by next-generation sequencing. Int J Infect Dis. 2019;83:148–153. doi:10.1016/j.ijid.2019.03.007
26. Ma L, Ge Y, Ma H, Zhu B, Miao Q. Infective endocarditis at a tertiary-care hospital in China. J Cardiothorac Surg. 2020;15(1):135. doi:10.1186/s13019-020-01183-2
27. Xu N, Fu Y, Wang S, Li S, Cai D. High level of D-dimer predicts ischemic stroke in patients with infective endocarditis. J Clin Lab Anal. 2020;34:e23206. doi:10.1002/jcla.23206
28. Toyoda N, Chikwe J, Itagaki S, Gelijns AC, Adams DH, Egorova NN. Trends in infective endocarditis in California and New York State, 1998–2013. JAMA. 2017;317(16):1652–1660. doi:10.1001/jama.2017.4287
29. Cresti A, Chiavarelli M, Scalese M, et al. Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc Diagn Ther. 2017;7(1):27–35. doi:10.21037/cdt.2016.08.09
30. Keller K, von Bardeleben RS, Ostad MA, et al. Temporal trends in the prevalence of infective endocarditis in Germany between 2005 and 2014. Am J Cardiol. 2017;119(2):317–322. doi:10.1016/j.amjcard.2016.09.035
31. Fernandes E, Olive C, Inamo J, Roques F, Cabie A, Hochedez P. Infective endocarditis in French West Indies: a 13-year observational study. Am J Trop Med Hyg. 2017;97(1):77–83. doi:10.4269/ajtmh.16-0514
32. Ren Z, Mo X, Chen H, Peng J. A changing profile of infective endocarditis at a tertiary hospital in China: a retrospective study from 2001 to 2018. BMC Infect Dis. 2019;19(1):945. doi:10.1186/s12879-019-4609-8
33. Watt G, Lacroix A, Pachirat O, et al. Prospective comparison of infective endocarditis in Khon Kaen, Thailand and Rennes, France. Am J Trop Med Hyg. 2015;92(4):871–874. doi:10.4269/ajtmh.14-0689
34. Mirabel M, Rattanavong S, Frichitthavong K, et al. Infective endocarditis in the Lao PDR: clinical characteristics and outcomes in a developing country. Int J Cardiol. 2015;180:270–273. doi:10.1016/j.ijcard.2014.11.184
35. Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the international collaboration on endocarditis-prospective cohort study. Arch Intern Med. 2009;169(5):463–473. doi:10.1001/archinternmed.2008.603
36. Nakatani S, Mitsutake K, Ohara T, et al. Recent picture of infective endocarditis in Japan–lessons from cardiac disease registration (CADRE-IE). Circulation J. 2013;77(6):1558–1564. doi:10.1253/circj.CJ-12-1101
37. Santa-Ana-Tellez Y, Mantel-Teeuwisse AK, Dreser A, Leufkens HG, Wirtz VJ. Impact of over-the-counter restrictions on antibiotic consumption in Brazil and Mexico. PLoS One. 2013;8(10):e75550. doi:10.1371/journal.pone.0075550
38. Zamorano J, Sanz J, Moreno R, et al. Comparison of outcome in patients with culture-negative versus culture-positive active infective endocarditis. Am J Cardiol. 2001;87(12):1423–1425. doi:10.1016/S0002-9149(01)01570-3
39. Pecoraro AJK, Pienaar C, Herbst PG, et al. Causes of infective endocarditis in the Western Cape, South Africa: a prospective cohort study using a set protocol for organism detection and central decision making by an endocarditis team. BMJ Open. 2021;11(12):e053169. doi:10.1136/bmjopen-2021-053169
40. Zaqout A, Mohammed S, Thapur M, et al. Clinical characteristics, microbiology, and outcomes of infective endocarditis in Qatar. Qatar Med J. 2020;2020(2):24. doi:10.5339/qmj.2020.24
41. Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart. 2005;91(5):571–575. doi:10.1136/hrt.2003.032128
42. Rajani R, Klein JL. Infective endocarditis: a contemporary update. Clin Med. 2020;20(1):31–35. doi:10.7861/clinmed.cme.20.1.1
43. Sundermann SH, Dademasch A, Seifert B, et al. Frailty is a predictor of short- and mid-term mortality after elective cardiac surgery independently of age. Interact Cardiovasc Thorac Surg. 2014;18(5):580–585. doi:10.1093/icvts/ivu006
44. Wu Z, Chen Y, Xiao T, Niu T, Shi Q, Xiao Y. The clinical features and prognosis of infective endocarditis in the elderly from 2007 to 2016 in a tertiary hospital in China. BMC Infect Dis. 2019;19(1):937. doi:10.1186/s12879-019-4546-6
45. Marques A, Cruz I, Caldeira D, et al. Risk factors for in-hospital mortality in infective endocarditis. Arq Bras Cardiol. 2020;114(1):1–8. doi:10.36660/abc.20180194
46. Liang F, Song B, Liu R, Yang L, Tang H, Li Y. Optimal timing for early surgery in infective endocarditis: a meta-analysis. Interact Cardiovasc Thorac Surg. 2016;22(3):336–345. doi:10.1093/icvts/ivv368
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
