Back to Journals » Journal of Asthma and Allergy » Volume 13
Clinical Characteristics and Proposed Wheat-Cofactor Challenge Protocol with a High Diagnostic Yield in Adult-Onset IgE-Mediated Wheat Allergy
Authors Thongngarm T , Wongsa C , Pacharn P , Piboonpocanun S , Sompornrattanaphan M
Received 14 July 2020
Accepted for publication 2 September 2020
Published 23 September 2020 Volume 2020:13 Pages 355—368
DOI https://doi.org/10.2147/JAA.S271429
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Torpong Thongngarm, 1 Chamard Wongsa, 1 Punchama Pacharn, 2 Surapon Piboonpocanun, 3 Mongkhon Sompornrattanaphan 1
1Division of Allergy and Clinical Immunology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; 2Division of Allergy and Clinical Immunology, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; 3Institute of Molecular Biosciences, Mahidol University, Salaya, Nakhonpathom, Thailand
Correspondence: Mongkhon Sompornrattanaphan
Division of Allergy and Clinical Immunology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Tel/Fax +66 2419 8263
Email [email protected]
Background: IgE-mediated wheat allergy in adults can be childhood or adulthood onset. Adult-onset wheat allergy has been reported, but data on clinical characteristics and practical food challenge protocols are scarce.
Objective: We aimed to describe the clinical characteristics of adult-onset wheat allergy, laboratory results, and outcomes of a modified 3-day challenge protocol using a combination of aspirin, wheat, and exercise.
Patients and Methods: Patients with histories compatible with adult-onset wheat allergy were recruited from Siriraj Hospital, Thailand. Clinical history, skin prick tests (SPTs), and specific IgE (sIgE) levels were ascertained. Patients with no food challenge contraindications were asked to volunteer for wheat challenge. A modified 3-day protocol using 300 mg of acetylsalicylic acid, 60– 75 g of wheat flour, and exercise was used for confirmatory diagnosis of conventional wheat allergy (WA) and wheat-dependent exercise-induced anaphylaxis (WDEIA).
Results: Thirty-three patients were recruited. The mean age of onset was 29.7 years (SD 10.5). SPTs yielded positivity rates of 9.1%, 84.8%, and 81.8% in commercial wheat, in-house gliadin, and in-house glutenin extracts, respectively. sIgE yielded a positivity rate of 61% and 88% in wheat and ω 5-gliadin, respectively. Eighteen patients underwent oral wheat challenges. Of these, 17 patients (94.4%) had positive challenges leading to definite diagnoses of WA (35%), and WDEIA (65%). One WDEIA patient developed hypotensive anaphylaxis in the protocol.
Conclusion: WDEIA was the most common phenotype. Our modified 3-day protocol could differentiate WA and WDEIA and yielded a high positivity rate (94.4%). It should be used cautiously as severe reactions can occur.
Keywords: anaphylaxis, food allergy, food challenge, wheat allergy, gliadin, lipid transfer protein
Introduction
Food allergy to wheat is more common in children with an estimated prevalence of 0.2–1% and likely to outgrow with a resolution rate of 65% by the age of 12 years.1,2 Therefore, wheat allergy in adults could be the persistence of child-onset or adult-onset after the age of 18 years despite previous tolerance. Adult-onset wheat allergy has been reported in many countries,3–6 but there is still a paucity of data. Wheat is an important cause of IgE-mediated food allergy in Thailand, Korea, and Japan.7 In Japan, wheat is the most common cause of new-onset food allergy in adults older than 20 years.3
Adult-onset IgE-mediated wheat allergy not only has a unique clinical pattern but also a distinct mechanism of sensitization. It can present as an outbreak in adults resulting from cutaneous sensitization to hydrolyzed wheat protein in facial soap.8 A distinct phenotype called “wheat-dependent exercise-induced anaphylaxis (WDEIA)” was reported to be common in adolescence and adults. Due to being under-recognized from physicians, WDEIA is frequently overlooked and misdiagnosed as chronic urticaria, exercise-induced anaphylaxis, and idiopathic anaphylaxis with a time lag to the diagnosis of 32–62 months.9
WDEIA could occur with the presence of the cofactors other than exercises, such as pollen exposure, concomitant ingestions of non-steroidal anti-inflammatory drugs (NSAIDs) or alcohol, the presence of menses in females, infection, and stress.9–12 Some authors used the term “wheat-dependent cofactor-augmented anaphylaxis”12 or “ω5-gliadin allergy”13 instead of WDEIA.
Wheat used for consumption includes common wheat (Triticum aestivum), the main ingredient in bread, and durum wheat (Triticum turgidum ssp. durum), preferentially used for pasta, pizza, bulgur, semolina, and couscous.14 Bread wheat (Triticum aestivum) consists of major allergens including α-amylase/trypsin inhibitor (Tri a 28 and Tri a 29.01), αβ-gliadin, ω5-gliadin (Tri a 19), low- and high-molecular-weight glutenin, α-purothionin (Tri a 37), nonspecific lipid transfer protein (nsLTP or Tri a 14), peroxidase (Tri a Bd36 kd), thioredoxin (Tri a 25), and serine proteinase inhibitor (Tri a 29).1,9 These major allergens could induce sensitization and specific IgE (sIgE) production via Th2-biased immune dysregulation. Using sIgE to ω5-gliadin increased the diagnostic yield with a sensitivity of 91% and a specificity of 92% in adult WDEIA patients.15 However, sIgE to whole wheat extract has a low diagnostic yield and is sometimes positive without clinical relevance.16,17 Skin prick test (SPTs) using commercial wheat extract generally yielded unsatisfactory results.18
Oral food challenge tests, combined with exercise, are considered the standard confirmation for the diagnosis for WDEIA. However, negative results have been reported despite the use of the combined wheat-exercise challenge.19–25 Other cofactors such as aspirin (ASA)/NSAIDs and alcohol have been reported to be equally effective as substitutes or combined with exercise in eliciting symptoms. Not only could ASA facilitate the reaction, but it also lowered the threshold of positive challenge.12,21 Adjunction with ASA might improve the diagnostic yield, especially in cases that are unable to perform an exercise challenge to reach the target intensity. Therefore, a wheat-cofactor challenge protocol using ASA and exercise would be beneficial in WDEIA patients who needed diagnostic confirmation. Few studies have described the clinical characteristics of adult-onset wheat-allergic patients with the challenge-proven diagnosis. Herein, we aimed to describe the clinical characteristics, laboratory results, and outcomes of a novel modified 3-day wheat-cofactor challenge protocol in our cohort.
Patients and Methods
Subjects
This was a prospective single-centered study of the Thai Adult Food Allergy Cohort performed at the Division of Allergy and Clinical Immunology, Siriraj Hospital, Mahidol University, Thailand. All patients who had a history compatible with adult-onset IgE-mediated wheat allergy were considered for recruitment from November 2017 to June 2019. All patients gave written informed consent to participate and publish the anonymized results. The protocols were approved by the Institutional Review Board and Ethics Committee of the Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand; approval number: 600/2560(EC3). The study was performed following the Declaration of Helsinki.
The eligibility criteria for the recruitment of adult-onset IgE-mediated wheat allergy are summarized in Table 1. We clinically classified the eligible cases into two phenotypes: 1) conventional wheat allergy (WA) and 2) WDEIA. WA was defined as an IgE-mediated reaction occurring within 3 hours after ingestion of wheat, and WDEIA was defined as anaphylaxis occurring only if the patient had exercised within 4–6 hours of ingesting wheat and anaphylaxis not occurring if the patient ingested wheat without the exercise.26 After the recruitment and patient providing informed consent, we collected their data including demographics, clinical history, symptoms during allergic reactions. SPTs and sIgE to wheat allergens were ascertained in all cases who did not have the results of those tests done within the last 12 months. Patients who had no food challenge contraindications were asked whether they would volunteer for the wheat challenge.
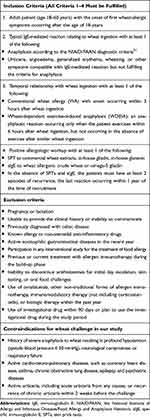 |
Table 1 Inclusion Criteria, Exclusion Criteria, and Contraindications for Wheat Challenge |
Skin Prick Test
SPT was performed on each patient’s volar surface of the forearm with a lancet using a commercial wheat extract (ALK Abello, Hørsholm, Denmark) as well as in-house-prepared gliadins, glutenins, oat, rye, barley, and millet extracts. The in-house skin test extracts were prepared by our team as described in the previous literature.27,28 Histamine phosphate (10 mg/mL) and glycerinated saline were used as the positive and negative controls, respectively. The procedure was performed according to the standard method.29 The wheal size was recorded as positive if it was ≥3 mm larger than the negative control.
Specific IgE
The sIgE for wheat, ω-5 gliadin, and lipid transfer protein (LTP) were measured using the ImmunoCAP® (Phadia, Uppsala, Sweden). A level of sIgE > 0.35 kAU/L was considered a positive result.
Wheat-Cofactors Challenge Protocol (The Modified 3-Day Protocol)
Wheat and each patient’s allergic foods were eliminated from their diets for at least 1 week before the challenge. The volunteers were admitted to the food/drug challenge unit, which was an in-hospital setting due to consideration of the safety issues according to the PRACTALL consensus report.30 Our 3-day challenge protocol was modified from previous studies19,21 as summarized in Figure 1. The details of the challenge protocol are as follows:
 |
Figure 1 Summary of modified 3-day challenge protocol. |
Day 0: A drug provocation test, using ASA with a cumulative dose of 421.5 mg with 6-hour observation, was used to exclude ASA hypersensitivity.
Day 1: An exercise challenge test was performed in an ambient temperature regulated within a range of 27–30°C monitored by a thermometer. The patient performed aerobic treadmill exercise, adjusting the speed and the slope of the treadmill to achieve a target heart rate (HR) of >80% of maximum HR by age within 5 minutes, and then this target HR was maintained for 15 minutes. The exercise challenge was terminated if there were any positive reactions. If no reactions were detected, the exercise was gradually tapered down by the speed and the slope. Exercise challenge test on day 1 helped to determine the submaximal exercise intensity for each individual on day 3.
Day 2: A wheat challenge test was performed by using bread wheat since it is the most common source of wheat consumed in Thailand as opposed to pasta, pizza, bulgur, and couscous. Sliced Farmhouse® bread (President Bakery Public Company Limited, Thailand) was used. One slice of bread, weighing 24 g and containing 62% of wheat flour, contained 15 g of wheat. We started with one-eighth of a sliced bread followed by an incremental dose until a cumulative dose of 5 slices was achieved.30 We closely observed the patient for a total of 6 hours before declaring a negative wheat challenge.
Day 3: A combined wheat-exercise-ASA challenge test was performed. A 300 mg of ASA was administered, followed by the ingestion of 4-sliced bread 30 minutes later. Exercise was initiated 30 minutes after bread ingestion and pursued as day 1 protocol.
A patient who had a negative exercise challenge on day 1 proceeded to the open wheat challenge on day 2, and a patient with a negative open wheat challenge on day 2 proceeded to the combined wheat-cofactor challenge on day 3.
The challenges were terminated at the index clinical reactivity with objective signs, following PRACTALL consensus.30 Our protocol allowed for exercise-induced anaphylaxis (EIA), WA, and WDEIA to be diagnosed if a positive result occurred on days 1, 2, and 3, respectively. The contraindication of wheat challenges in our study included 1) having a history of severe anaphylaxis to wheat resulting in profound hypotension, neurological compromise, or respiratory failure and 2) having active cardio-neuro-pulmonary diseases, such as coronary heart disease, asthma, chronic obstructive lung disease, epilepsy, or psychiatric diseases.
Statistical Analysis
All analyses were performed using PASW Statistics version 18.0 (SPSS, Inc., Chicago, IL). Demographic and clinical data are summarized using descriptive statistics. Categorical data are presented as frequencies and percentages. Continuous data are presented as median with range, and mean ± standard deviation as appropriate. Mann Whitney U-test was used for comparison of medians between the two groups. The Chi-square test was used for comparison of categorical data, and the Kruskal–Wallis test was employed for continuous data with non-normal distribution. All p-values less than 0.05 were considered statistically significant.
Results
Figure 2 summarizes the flow diagram of patients’ recruitment. Forty-three patients were assessed for eligibility. Of these, 33 patients were recruited in our study. Ten patients were excluded: 8 due to child-onset wheat allergy, and 2 due to having a history of NSAIDs hypersensitivity. Eighteen patients agreed to be a volunteer for oral wheat ± exercise challenge, whereas 15 patients did not undergo challenge: 13 due to challenge refusal, and 2 due to having contraindication for the food challenge.
 |
Figure 2 Flow diagram of patient recruitment. |
Patient Characteristics
Their clinical characteristics and laboratory results of 33 patients are summarized in Table 2. There were similar demographic data, atopic background, comorbidities, onset, and duration of wheat allergy between patients who underwent challenge and who did not. Overall, WDEIA was a more common phenotype than WA (23/33, 69.7% versus 10/33, 30.3%). The mean age at the time of recruitment was 32.6 years (SD 11.3). The mean age of onset was 29.7 years (SD 10.5). The most common atopic comorbidity was allergic rhinitis (51.5%), followed by asthma (6%), and atopic dermatitis (6%). Fifty-one percent of patients had a concomitant food allergy other than wheat, including shellfish, fruits, peanut, and cow’s milk.
 |
Table 2 Clinical Characteristics of 33 Patients in Adult-Onset Wheat Allergy Cohort |
Clinical History Before Diagnosis Was Made
The majority of the patients (31/33, 94%) had experienced more than one anaphylactic episode, which ranged from 1 to 20 times (median = 4 times) before the diagnosis of WA was made. The median lag time from onset to the diagnosis was 17 months (range, 1 week to 5 years). Most patients had sought medical attention for such episodes from different primary physicians with a median number of visits of 2 (range, 1 to 5 visits). The most common diagnosis before WA/WDEIA diagnosis was made included chronic urticaria (45.5%), idiopathic anaphylaxis (21.2%), exercise-induced anaphylaxis (12.1%), and acute urticaria with systemic symptoms (9%).
Previous Wheat-Allergic Reactions
The most common symptom was urticaria, which was reported in all patients (Table 2). Syncope was reported in 12/33 (36.4%) of patients whereas documented hypotension was demonstrated in only 7/33 (21.2%). Severe anaphylaxis resulting in cardiac arrest (1/33) and intubation (1/33) were also reported. When using the clinical criteria from the National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium (NIAID/FAAN),31 anaphylaxis was diagnosed in 31 patients (94%). The severity of the reaction varied with a median of grade 2 using Ring & Messmer severity grading,32 grade 4 using Sampson severity grading,33 and moderate severity using EAACI Taskforce on Anaphylaxis.34
Skin Prick Test and Specific IgE
In Table 2, only 3 patients (9.1%) showed positive SPT to commercial wheat extract whereas 28 patients (84.8%) and 27 patients (81.8%) showed positive SPT to in-house gliadins and glutenins extracts, respectively. When using >0.35 kAU/L as the positive cut-off point, 20 patients (60.6%) and 29 patients (87.9%) had positive results for sIgE to wheat and ω5-gliadin, respectively. The median level of sIgE to wheat and ω5-gliadins were 0.44 (range, 0.05–36.8) and 3.99 kAU/L (range, 0.01–50.1), respectively. Only 1 patient (patient number 3) had a positive result for sIgE to LTP (Table 3).
Grass and Other Grains Sensitization
Overall, 7 patients (21.2%) had grass sensitization, which was indicated by SPT to common grass in Thailand (Johnson, Bermuda). The most common grass to which our cohort was sensitized was Bermuda (24.2%), followed by Johnson (9.1%). Among 33 patients, only 5 patients (15.2%) sensitized to other grains, indicated by SPT to in-house extracts of oat, rye, barley, and millet (Table 2).
Symptoms Observed During Positive Oral Wheat ± Exercise Challenge
Among the 18 patients in the challenge-accepted group, all patients had negative results from both ASA provocation test on day 0 and exercise challenge on day 1; therefore, both ASA hypersensitivity and EIA could reasonably be excluded. Six patients had a positive oral wheat challenge without exercise on day 2 so that WA was diagnosed. The remaining 12 patients underwent combined ASA, wheat, and exercise challenge on day 3. Eleven patients (11/12) had positive challenge, and WDEIA was diagnosed (Figure 3). One patient had a negative challenge result from our protocol. We summarize the clinical characteristics and laboratory results of the 18 patients in the challenge-accepted group in Table 3.
 |
Table 3 Clinical Characteristics of Patients Who Underwent Challenge Procedure |
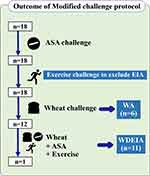 |
Figure 3 Outcomes of modified 3-day challenge protocol. |
Urticaria was the most common objective sign during the challenge (16/17, 94%). Isolated facial urticaria (7/16, 44%) was the most common site of rash observed at the point of the positive challenge, followed by generalized urticaria (5/16, 31%), isolated distal-extremities urticaria (3/16, 19%), and isolated truncal urticaria (1/16, 1%). Gastrointestinal symptoms were observed in 3/17 (17.6%) patients with positive challenge results which included emesis and abdominal pain. Only 1 patient had two episodes of emesis, which were interpreted as a positive challenge according to the PRACTALL consensus report.30
Discussion
We report the clinical characteristics of 33 adult-onset wheat-allergic patients from Thailand and performed oral wheat challenges in the majority of cases (54.5%). Overall, WDEIA was the most common phenotype. A wide range of ages of onset (18–48 years) and female-predominance were observed. Fifty-one percent of the patients had concomitant plant and food allergies, which was similar to a recent Italian adult-onset wheat allergy cohort.5 The diagnosis was often delayed despite most of our patients presenting with severe anaphylaxis, similar to the largest cohort from the UK.13 In the present study, the median time from the first symptom to WA/WDEIA diagnosis was 17 months. These delays led to more than one episode before the diagnosis was made. The reasons for these delays are unclear, but a lack of awareness might have been a contributing factor.
Our modified 3-day challenge protocol achieved a high rate of positivity in the challenge-accepted group (94%). We began the protocol with the exercise-only challenge instead of wheat challenge on day 1 for two reasons: 1) to exclude exercise-induced anaphylaxis and 2) to avoid a possible false-interpretation from delayed wheat absorption by performing exercise-only challenge on the first day because WDEIA may have a delayed onset up to 24 hours after wheat ingestion.35 We used 300 mg of ASA adjunct with exercise to augment the clinical reactions by lowering the wheat threshold.12 ASA possibly facilitates allergen absorption from the gastrointestinal tract.36 To exclude ASA hypersensitivity, ASA challenge was performed on day 0 since there is a possibility that some patients who have not taken ASA or NSAIDs for a long period may develop hypersensitivity at some time points without relevant histories. The NSAID hypersensitivity was reported by 2.66% of women and 1.34% of men and was the third most commonly reported drug hypersensitivity in the United States, after penicillins and opiates.37 Since no patients in our cohort reacted to ASA challenge on day 0, it is optional to omit this step in patients who have not recently had relevant histories of ASA or NSAID hypersensitivities. We summarize and compare our protocol with the previous studies12,21 in Table 4. The study from Christensen et al12 used up to 80 g of gluten and demonstrated that the reaction of wheat allergy could be elicited at rest at a high dose gliadin challenge. Since Brockow et al21 reported that 10 g of gluten was equivalent to 125 g of wheat. Therefore, 80 g of gluten was equivalent to 67 slices of the Farmhouse® bread product that we used in the present study, and 67 slices would be a much higher portion size than those that are typical in adults.30 In countries where purified gluten flour might not be commercially available, using sliced wheat bread and adding cofactor could be practical and reproducible. On day 2, we used 5 slices of Farmhouse® bread, equivalent to 6 g of gluten, which is considered enough to declare a negative wheat challenge and exclude conventional wheat allergy as this amount is 2.5-fold of portion sizes for adult.38 Our proposed modified 3-day protocol aims not only to yield the diagnosis but also to differentiate between EIA, WA, and WDEIA within a single admission. The amount of wheat used in our protocol on day 3 (fixed at 4 slices of bread, equivalent to 4.8 g of gluten) was agreeably close to the median threshold of gluten dose (4.3 g) reported in Christensen’s protocol of combined gluten, ASA (1000 mg), and exercise.12 We used a lower dose of ASA (300 mg), taken 30 minutes before wheat ingestion to avoid GI side effects, which possibly occurred in some patients. The half-life of ASA is 2–3 hours, of which its effect is covered during our challenge time.
 |
Table 4 Comparison of Wheat-Cofactor Challenge Protocols in Patients with Wheat Allergy |
We did not use diluted 95% alcohol as a cofactor because a proportion of the Asian population might elicit alcohol-related syndrome, including flushing symptoms39,40 mimicking the immediate-type hypersensitivity reaction and misleading the interpretation. Therefore, taking 300 mg of ASA and wheat only once before exercise made the protocol practical and concise, and it can be easily followed by in the appropriate population in a time- and resource-limited setting.
The relatively high positivity rate from the wheat-cofactor challenge in our protocol could be due to several reasons. First, we concurrently combined both cofactors of ASA and exercise on day 3, which possibly augmented the reaction and lowered the wheat dose threshold. Second, we regulated the room temperature to range between 27°C and 30°C. There was a reported case of a temperature effect in eliciting symptoms during the food-exercise challenge in a walnut-allergic patient, in which the reactions occurred only in a warm but not in a cold environment.41 Hot and humid weather covering the entire period of the year is common in Thailand. Our clinical experience is that some Thai WDEIA patients report reactions with wheat ingestion together with non-strenuous daily life activity. Lack of awareness of cofactors, such as mild exercise, warm temperature, infection, or NSAIDs/ASA might be involved in these events. We agree with the term “wheat-dependent, cofactor-augmented anaphylaxis” proposed by Christensen et al since this term precisely covered all of the cofactors other than exercise that could result in the reactions.12 Other unknown cofactors have to be further investigated due to the unpredictable magnitude of their effects and possible interactions between cofactors.
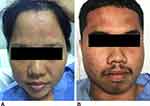
Urticaria was the most common manifestation in both clinical histories and objective symptoms during the challenges in our cohort. Interestingly, face is the most commonly affected part of the body, and this distribution was found in isolation during a challenge (Figure 4). The exact pathophysiologic mechanisms underlying this finding are unknown. Nevertheless, the skin may serve as a site for sensitization to allergens even when allergic skin inflammation is absent.42,43 This finding suggests facial skin might be the primary route of sensitization. A similar observation was reported by Yagami et al,8 in which facial rash was initially observed in patients who used wheat-containing facial soap, followed by a newly-developed wheat allergy. A case–control study from Japan also confirmed the positive correlation between the use of wheat-containing facial soap and wheat allergy development.44 The mechanistic link is supported by a murine model in which allergen sensitization occurred in barrier-impaired skin, mediated by keratinocyte thymic stromal lymphopoietin.45
SPTs using commercial wheat extract generally yielded unsatisfactory results, similar to other studies,18,46 whereas SPTs using in-house glutenin and gliadin extracts yielded a higher (>80%) positivity rate, similar to Thai wheat-allergic children patients.27 Although wheat extracts for skin testing are commercially available, the preparation, especially as an aqueous solution, may not be sensitive for the diagnosis because some major wheat allergens are alcohol-soluble.47 sIgE to wheat and ω5-gliadin were positive 60.6% and 87.9% of the patients in our cohort, respectively, while the positivity rate of ω5-gliadin is slightly lower than the large multicenter cohort from the UK.13 Besides, LTP has rarely been sensitized in our cohort whereas it was one of the major allergens reported in European wheat-allergic patients (40.9%).48 Geographic variation in sensitized allergens might explain these differences among wheat-allergic patients.
Our wheat-cofactor challenge, using a combination of ASA, wheat, and exercise, yielded a high positive rate (94.4%), but severe systemic reaction occurred in one patient. In Table 3, patient number 17 developed hypotensive syncope and generalized urticaria, resulting in the use of epinephrine administration despite the absence of previous cardiopulmonary diseases. A negative challenge in patient number 18 could be explained by the limited yield of challenge protocol itself12,21 and her strict avoidance of wheat ingestion. She had only two episodes of reactions occurring within the last 5 years after wheat allergy onset, the last of which occurred 11 months before we performed the challenges. The possible natural resolution was suspected as previously described in patients who avoid wheat intake.5 However, she still avoids wheat ingestion and no anaphylaxis occurs ever since.
Our study had some limitations. First, we did not perform a challenge in all cases. However, the additional criteria of repeated reaction and evidence of IgE sensitization would firmly increase the likelihood of wheat-allergic symptoms of patients in our cohort. Second, our sample size was limited due to recruitment from a single-center, which might not reflect the entire population. Further studies should focus on the identification of major allergens in this adult-onset WA population. We hypothesize that the allergen sensitized in this population might be different from the childhood-onset wheat allergy as there are many routes of sensitization reported in adults.8,49 The concomitant food allergy in our cohort was defined as a compatible history of IgE-mediated reaction with positive food sensitization, demonstrated by either skin tests or food-specific IgE. Some of the reactions described by the patients could occur with a varied amount of food intake and various cofactors. Therefore, it is difficult to state that it was exercise-induced as we did not perform other food-exercise challenges in the same way as wheat.
In conclusion, adult-onset wheat allergy should be suspected in adult patients with anaphylaxis-like reactions with unidentifiable causes. A combination of relevant history and positive sIgE test to ω5-gliadin is crucial in leading to the diagnosis whereas SPT with commercial wheat extract is less helpful. Although our challenge protocol could be positive in those who had negative ω5-gliadin-specific IgE, it should be considered cautiously as it could lead to a severe systemic reaction. Further study should focus on the identification of major allergens in this adult-onset wheat allergy population.
Abbreviations
GI tract, gastrointestinal tract; IgE, Immunoglobulin E; NSAIDs, non-steroidal anti-inflammatory drugs; SPT, skin prick test; sIgE, specific immunoglobulin E; WA, wheat allergy; WDEIA, wheat-dependent exercise-induced anaphylaxis; ω5-gliadin, omega5-gliadin.
Ethical Approval
The study and protocol were approved by the Institutional Review Board and Ethics Committee of the Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand; approval number: 600/2560(EC3). The study was performed following the Declaration of Helsinki. Written informed consent was obtained from the patient to participate.
Consent for Publication
Written informed consent for publication was obtained from the patient. The patient was informed that de-identified data would be used in scientific research and publications.
Acknowledgments
We acknowledge the contributions of Ms. Orathai Theankeaw and Ms. Aree Jameekornrak Taweechue for research assistance and Ms. Khemajira Karaketklang for statistical analyses. We would like to thank Dr. Anthony Tan for editing the English language.
Authorship
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This study was funded by Siriraj Research Development Fund from Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand.
Disclosure
All authors declare no personal or professional conflicts of interest for this work.
References
1. Cianferoni A. Wheat allergy: diagnosis and management. J Asthma Allergy. 2016;9:13–25. doi:10.2147/JAA.S81550
2. Keet CA, Matsui EC, Dhillon G, Lenehan P, Paterakis M, Wood RA. The natural history of wheat allergy. Ann Allergy Asthma Immunol. 2009;102(5):410–415. doi:10.1016/S1081-1206(10)60513-3
3. Ebisawa M, Ito K, Fujisawa T. Committee for Japanese Pediatric Guideline for Food Allergy TJSoPA, Clinical Immunology TJSoA. Japanese guidelines for food allergy 2017. Allergol Int. 2017;66(2):248–264. doi:10.1016/j.alit.2017.02.001
4. Kivity S. Adult-onset food allergy. Isr Med Assoc J. 2012;14(1):70–72.
5. Scibilia J, Rossi Carlo M, Losappio Laura M, et al. Favorable Prognosis of Wheat Allergy in Adults. Journal of Investigational Allergology and Clinical Immunology. 2019;29(2):118–123. doi:10.18176/jiaci.0296
6. Hamada Y, Chinuki Y, Fukutomi Y, et al. Long-term dynamics of omega-5 gliadin-specific IgE levels in patients with adult-onset wheat allergy. J Allergy Clin Immunol Pract. 2020;8(3):1149–51 e3.
7. Lee AJ, Thalayasingam M, Lee BW. Food allergy in Asia: how does it compare? Asia Pac Allergy. 2013;3(1):3–14.
8. Yagami A, Aihara M, Ikezawa Z, et al. Outbreak of immediate-type hydrolyzed wheat protein allergy due to a facial soap in Japan. J Allergy Clin Immunol. 2017;140(3):879–81 e7.
9. Scherf KA, Brockow K, Biedermann T, Koehler P, Wieser H. Wheat-dependent exercise-induced anaphylaxis. Clin Exp Allergy. 2016;46(1):10–20.
10. Asero R, Piantanida M, Pinter E, Pravettoni V. The clinical relevance of lipid transfer protein. Clin Exp Allergy. 2018;48(1):6–12. doi:10.1111/cea.13053
11. Romano A, Scala E, Rumi G, et al. Lipid transfer proteins: the most frequent sensitizer in Italian subjects with food-dependent exercise-induced anaphylaxis. Clin Exp Allergy. 2012;42(11):1643–1653. doi:10.1111/cea.12011
12. Christensen MJ, Eller E, Mortz CG, Brockow K, Bindslev-Jensen C. Wheat-Dependent Cofactor-Augmented Anaphylaxis: A Prospective Study of Exercise, Aspirin, and Alcohol Efficacy as Cofactors. J Allergy Clin Immunol Pract. 2019;7(1):114–121. doi:10.1016/j.jaip.2018.06.018
13. Kennard L, Thomas I, Rutkowski K, et al. A Multicenter Evaluation of Diagnosis and Management of Omega-5 Gliadin Allergy (Also Known as Wheat-Dependent Exercise-Induced Anaphylaxis) in 132 Adults. J Allergy Clin Immunol Pract. 2018;6(6):1892–1897. doi:10.1016/j.jaip.2018.02.013
14. Battais F, Richard C, Jacquenet S, Denery-Papini S, Moneret-Vautrin DA. Wheat grain allergies: an update on wheat allergens. Eur Ann Allergy Clin Immunol. 2008;40(3):67–76.
15. Le TA, Al Kindi M, Tan JA, et al. The clinical spectrum of omega-5-gliadin allergy. Intern Med J. 2016;46(6):710–716. doi:10.1111/imj.13091
16. Venter C, Maslin K, Arshad SH, et al. Very low prevalence of IgE mediated wheat allergy and high levels of cross-sensitisation between grass and wheat in a UK birth cohort. Clin Transl Allergy. 2016;6(1):22. doi:10.1186/s13601-016-0111-1
17. Nilsson N, Nilsson C, Ekoff H, et al. Grass-Allergic Children Frequently Show Asymptomatic Low-Level IgE Co-Sensitization and Cross-Reactivity to Wheat. Int Arch Allergy Immunol. 2018;177(2):135–144. doi:10.1159/000489610
18. Soares-Weiser K, Takwoingi Y, Panesar SS, et al. The diagnosis of food allergy: a systematic review and meta-analysis. Allergy. 2014;69(1):76–86. doi:10.1111/all.12333
19. Pacharn P, Jirapongsananuruk O, Daengsuwan T, Vichyanond P, Visitsunthorn N. Wheat-dependent, exercise-induced anaphylaxis in Thai children: a report of 5 cases. Asian Pac J Allergy Immunol. 2009;27(2–3):115–120.
20. Hanakawa Y, Tohyama M, Shirakata Y, Murakami S, Hashimoto K. Food-dependent exercise-induced anaphylaxis: a case related to the amount of food allergen ingested. Br J Dermatol. 1998;138(5):898–900. doi:10.1046/j.1365-2133.1998.02254.x
21. Brockow K, Kneissl D, Valentini L, et al. Using a gluten oral food challenge protocol to improve diagnosis of wheat-dependent exercise-induced anaphylaxis. J Allergy Clin Immunol. 2015;135(4):977–84 e4. doi:10.1016/j.jaci.2014.08.024
22. Romano A, Di Fonso M, Giuffreda F, et al. Diagnostic work-up for food-dependent, exercise-induced anaphylaxis. Allergy. 1995;50(10):817–824. doi:10.1111/j.1398-9995.1995.tb05055.x
23. Dohi M, Suko M, Sugiyama H, et al. Food-dependent, exercise-induced anaphylaxis: a study on 11 Japanese cases. J Allergy Clin Immunol. 1991;87(1 Pt 1):34–40. doi:10.1016/0091-6749(91)90210-F
24. Okazaki M, Kitani H, Mifune T, et al. Food-dependent exercise-induced anaphylaxis. Intern Med. 1992;31(8):1052–1055. doi:10.2169/internalmedicine.31.1052
25. Kohno K, Matsuo H, Takahashi H, et al. Serum gliadin monitoring extracts patients with false negative results in challenge tests for the diagnosis of wheat-dependent exercise-induced anaphylaxis. Allergol Int. 2013;62(2):229–238. doi:10.2332/allergolint.12-OA-0495
26. Panel NI-SE, Boyce JA, Assa’ad A, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1–S58.
27. Phisitbuntoon T, Jirapongsananuruk O, Pacharn P, et al. A potential role of gliadin extract skin prick test in IgE-mediated wheat allergy. Asian Pac J Allergy Immunol. 2020;125.
28. Srisuwatchari W, Piboonpocanun S, Wangthan U, Jirapongsananuruk O, Visitsunthorn N, Pacharn P. Clinical and in vitro cross-reactivity of cereal grains in children with IgE-mediated wheat allergy. Allergol Immunopathol (Madr). 2020. doi:10.1016/j.aller.2019.11.008
29. Heinzerling L, Mari A, Bergmann KC, et al. The skin prick test - European standards. Clin Transl Allergy. 2013;3(1):3. doi:10.1186/2045-7022-3-3
30. Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, et al. Standardizing double-blind, placebo-controlled oral food challenges: american Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012;130(6):1260–1274. doi:10.1016/j.jaci.2012.10.017
31. Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391–397. doi:10.1016/j.jaci.2005.12.1303
32. Ring J, Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;309(8009):466–469. doi:10.1016/S0140-6736(77)91953-5
33. Sampson HA. Anaphylaxis and emergency treatment. Pediatrics. 2003;111(6 Pt 3):1601–1608.
34. Muraro A, Roberts G, Clark A, et al. The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology. Allergy. 2007;62(8):857–871. doi:10.1111/j.1398-9995.2007.01421.x
35. Rongfei Z, Wenjing L, Nan H, Guanghui L. Wheat - Dependent Exercise-Induced Anaphylaxis Occurred With a Delayed Onset of 10 to 24 hours After Wheat Ingestion: A Case Report. Allergy Asthma Immunol Res. 2014;6(4):370–372. doi:10.4168/aair.2014.6.4.370
36. Matsuo H, Morimoto K, Akaki T, et al. Exercise and aspirin increase levels of circulating gliadin peptides in patients with wheat-dependent exercise-induced anaphylaxis. Clin Exp Allergy. 2005;35(4):461–466. doi:10.1111/j.1365-2222.2005.02213.x
37. Giavina-Bianchi P, Jares E, Aun MV, Thong B. Drug hypersensitivity reactions in the Americas: similarities and differences. Ann Allergy Asthma Immunol. 2019;122(5):447–448. doi:10.1016/j.anai.2019.02.007
38. Bird JA, Leonard S, Groetch M, et al. Conducting an Oral Food Challenge: an Update to the 2009 Adverse Reactions to Foods Committee Work Group Report. J Allergy Clin Immunol Pract. 2020;8(1):75–90 e17.
39. Luft FC. Asian flushing presents opportunities for disease prevention. J Mol Med (Berl). 2016;94(11):1195–1197. doi:10.1007/s00109-016-1470-8
40. Andrici J, Hu SXH, Eslick GD. Facial flushing response to alcohol and the risk of esophageal squamous cell carcinoma: A comprehensive systematic review and meta-analysis. Cancer Epidemiol. 2016;40:31–38. doi:10.1016/j.canep.2015.10.011
41. Jo E-J, Yang M-S, Kim Y-J, et al. Food-dependent exercise-induced anaphylaxis occurred only in a warm but not in a cold environment. Asia Pac Allergy. 2012;2(2):161–164. doi:10.5415/apallergy.2012.2.2.161
42. Lack G, Fox D, Northstone K, Golding J; Avon Longitudinal Study of Parents and Children Study Team. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348(11):977–985. doi:10.1056/NEJMoa013536
43. Han H, Roan F, Ziegler SF. The atopic march: current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol Rev. 2017;278(1):116–130.
44. Fukutomi Y, Taniguchi M, Nakamura H, Akiyama K. Epidemiological link between wheat allergy and exposure to hydrolyzed wheat protein in facial soap. Allergy. 2014;69(10):1405–1411. doi:10.1111/all.12481
45. Leyva-Castillo JM, Hener P, Jiang H, Li M. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J Invest Dermatol. 2013;133(1):154–163. doi:10.1038/jid.2012.239
46. Li PH, Thomas I, Wong JC-Y, Rutkowski K, Lau C-S. Differences in omega-5-gliadin allergy: East versus West. Asia Pac Allergy. 2020;10(1):e5. doi:10.5415/apallergy.2020.10.e5
47. Pacharn P, Kumjim S, Tattiyapong P, Jirapongsananuruk O, Piboonpocanun S. Identification of wheat sensitization using an in-house wheat extract in Coca-10% alcohol solution in children with wheat anaphylaxis. Asian Pac J Allergy Immunol. 2016;34(2):153–158.
48. Pastorello EA, Farioli L, Conti A, et al. Wheat IgE-Mediated Food Allergy in European Patients: α-Amylase Inhibitors, Lipid Transfer Proteins and Low-Molecular-Weight Glutenins. Int Arch Allergy Immunol. 2007;144(1):10–22. doi:10.1159/000102609
49. Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, et al. EAACI Molecular Allergology User’s Guide. Pediatr Allergy Immunol. 2016;27(Suppl 23):1–250.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.