Back to Journals » International Journal of General Medicine » Volume 14
Clinical Characteristics and Prognosis of HER2 Gene Phenotype in Patients with Non-Small Cell Lung Cancer
Authors Diao WY, Ding CL, Yuan BY, Li Z, Sun N, Huang JB
Received 11 July 2021
Accepted for publication 27 September 2021
Published 1 December 2021 Volume 2021:14 Pages 9153—9161
DOI https://doi.org/10.2147/IJGM.S328908
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Wei-Ying Diao,1,* Cheng-Long Ding,1,* Bo-Yang Yuan,2 Zan Li,3 Na Sun,4 Jia-Bin Huang5
1Department of Pathology, The First Affiliated Hospital of Jiamusi University, Jimusi City, Heilongjiang Province, 154002, People’s Republic of China; 2Department of Acupuncture and Moxibustion, The First Affiliated Hospital of Jiamusi University, Jimusi City, Heilongjiang Province, 154002, People’s Republic of China; 3Department of Analytical Chemistry Teaching and Research, Jiamusi University, Jiamusi City, Heilongjiang Province, 154002, People’s Republic of China; 4Graduate Department, Jiamusi University, Jiamusi, Heilongjiang Province, 154002, People’s Republic of China; 5Department of Geratology, The First Affiliated Hospital of Jiamusi University, Jiamusi, Heilongjiang Province, 154002, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jia-Bin Huang Email [email protected]
Introduction: We aim to investigate the relationship between HER2 gene phenotype and clinical characteristics, distribution and prognosis of non-small cell lung cancer (NSCLC) patients.
Methods: A total of 249 NSCLC patients admitted to the oncology department of our hospital from January 2015 to January 2018 were retrospectively analyzed. The clinicopathological information, CT signs, clinical efficacy and long-term prognosis were collected and compared.
Results: A total of 249 NSCLC patients underwent HER2 gene testing, 21 of them (8.43%) complied with HER2 alterations [HER2 (+)], and there were significant differences in tumor stages among patients with different HER2 phenotypes (P< 0.05). Among 21 NSCLC patients with HER2 (+), HER2 gene mutation was found in 17 patients (81%), and HER2 gene amplification in 4 patients (19%). Among the HER2 mutations, 12 cases (57%) were 20 exon mutations, and 5 cases (19%) were other mutations. Analysis of CT signs showed that border lobulation/burr, necrosis sign and pleural depression were correlated with HER2 gene mutation (P< 0.05). The incidence of EGRF mutation in HER (+) patients was significantly lower than that in HER (-) patients (P< 0.05), but there was no significant difference in the incidence of ALK gene mutation among different HER phenotypes (P> 0.05). The disease control rate of HER2 (+) patients was significantly lower than that of HER2 (-) patients, and the 12-month progression-free survival rate and survival rate of HER2 (+) patients were significantly higher than those of HER2 (-) patients (P< 0.05). There was no significant difference in the incidence of ADR among HER2 patients with different phenotypes, but the incidence of ADR (adverse drug reaction) in HER2 (+) patients with Grade 3 or 4 was significantly higher than that in the control group (P< 0.05).
Discussion: The incidence of HER2 gene mutations in NSCLC patients is relatively low, but it is far commoner in patients with stage IIIB∼IV, among which exon 20 mutations are the most prevalent. In CT signs, the lesion lobulated sign/spiculated sign, necrosis signs, and pleural depression signs are related to HER2 gene mutations. In addition, HER2 gene mutations play a crucial role in the clinical prognosis and treatment safety of patients.
Keywords: non-small cell lung cancer, HER2 genotype, CT signs, afatinib
As one of the most prevalent malignant tumors worldwide, lung cancer has become the top cause of cancer-related death in the urban population of China. Non-small cell lung cancer (NSCLC) includes squamous cell carcinoma, adenocarcinoma, and large cell carcinoma, in which cancer cells grow and divide more slowly, and spread and metastasize are less drastic.1,2 NSCLC accounts for about 80% of overall lung cancers, and approximately 75% of patients have progressed in the advanced stage when diagnosed, with somber 5-year survival rate.3 At present, the treatment of NSCLC mainly includes surgery, radiotherapy, chemotherapy, immunotherapy, etc. A large number of studies have shown that the occurrence and development of lung cancer are consequent to the unbalanced regulation of multiple gene expression, which is closely related to the stability of genes in organisms.4,5 Human Epithelial Factor Receptor-2 (HER2) is a member of the epithelial factor receptor family. Studies demonstrated that HER2 overexpression is an enhancer of EGFR signaling, and it plays a role in signal transduction in cancer development and progress when HER2 is dysfunctional.6 Afatinib and trezizumab have been recommended as targeted therapies for patients with HER2 mutations; yet due to the relatively low incidence of HER2 mutations and differences in genetic changes in different patients, no consensus has been achieved on the clinicopathological characteristics and efficacy of HER2 mutations in NSCLC patients.7,8 This study was conducted with a goal to explore the clinicopathological characteristics and prognosis of patients with HER2 gene mutations by analyzing patients with NSCLC treated in our hospital from January 2015 to January 2018.9
Patients and Methods
Participants
A retrospective analysis was conducted on 249 NSCLC patients admitted to the Oncology Department of our hospital from January 2015 to January 2018. Inclusion criteria: (1) age 18 to 75 years; (2) pathologically confirmed NSCLC with at least one measurable solid tumor; (3) Eastern Cooperative Oncology Group (ECOG)10 score 0~1, expected survival time ≥3 months; (3) complete medical records; (4) no serious organ dysfunction; (5) no primary tumors outside the lung; (6) all underwent genetic testing for lung cancer. Exclusion criteria: (1) have ever received HER2 inhibitor treatment; (2) have received chemotherapy, radiotherapy, surgery and targeted therapy within 4 weeks before enrollment; (3) there is active central nervous system metastasis, cancerous meningitis, spinal cord compression, etc. assessed by CT; (4) poor compliance with treatment, withdrew from treatment midway or discontinued drug without authorization. This retrospective study was approved by the Ethical Review Committee of the First Affiliated Hospital of Jiamusi University and conducted in accordance with the 1964 Declaration of Helsinki and its subsequent amendments. All patient data was maintained with confidentiality. Due to the feature of retrospective analysis, patient’s informed consent were not necessary.
Treatment Methods
All patients were treated with carboplatin pemetrexed for 6 cycles. Among them, carboplatin (Qilu Pharmaceutical Co., Ltd., National Medicine Standard H10920028), was intravenously given at 300 mg/m2, and pemetrexed (Jiangsu Aosaikang Pharmaceutical Co., Ltd., National Medicine Standard H20080624) was intravenously given at 500mg/m2. Patients with HER2 variants were treated with afatinib (Boehringer Ingelheim International GmbH, approval number H2017007) on this basis, orally 40 mg/d. All patients were treated continuously until the disease progressed or the adverse reaction became unbearable. The initial dose, reduction (or temporary interruption) and termination are determined by the attending physician. If the first-line afatinib treatment shows disease progression, the patient is allowed to carry out any follow-up treatment required, including continuing afatinib treatment, changing to other programs, or no longer undergoing any treatment.
Outcome Measures
Imaging Features
All patients underwent Light-Speed 64-slice spiral CT (GE Company, USA) after admission. The patient was in the supine position, and the scan range was from sternoclavicular joint to the diaphragm; parameter was set as follows: slice thickness 0.625 mm, slice spacing 0.625 mm, reconstruction slice thickness 5.000 m. The enhanced scan was performed by a one-time bolus injection of ioversol at a speed of 2.5 mL/s and a dose of 1.3 to 1.5 mL/kg. Scanning was started 90 s after the injection. The lung window was −550HU, the lung window was 1400HU wide, the mediastinal window was 350HU, and the mediastinal window was 35HU. When reading the film, the location, diameter (maximum diameter of the lung window cross section), traits (regular and irregular), edge, density (partial solid nodules, ground glass-like density nodules, solid nodules), necrosis symptoms and other CT signs were recorded.
Clinical Efficacy
According to the RECIST1.1 standard,11 the efficacy was classified as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). CR was considered if the tumor disappears completely and lasts for more than 4 weeks; PR was deemed if the sum of the long diameters of all measurable lesions has been reduced by more than 30%; SD was regarded if the sum of the long diameters of the lesions has reduced by less than 30% and increased by less than 20%; PD was deemed if the length of the lesion increased by more than 20%, or new lesions occurred. Objective response rate (ORR)=(CR +PR) /(CR+ PR+ SD+ PD)×100%. Disease control rate (DCR)= (CR+ PR+SD) /(CR+ PR+ SD+ PD)×100%.
Long-Term Prognosis
The long-term prognosis includes overall survival (OS) and progression-free survival (PFS). Follow up was conducted every 2 months after treatment. PFS refers to the time interval from the first day of chemotherapy to the first recorded disease progression or death; OS refers to the interval from the first day of chemotherapy to the occurrence of death. All patients were followed up for 12 months without loss of follow-up.
Statistical Analysis
All data were analyzed by SPSS 22.0, and GraphPad Prism 8.0 software was used to plot graphics. The relationship between HER2 gene phenotype and clinical characteristics was analyzed by chi-square test, and the survival curve was analyzed by Kaplan-Meier method, both of which were tested by two-sided tests. A P-value of <0.05 was claimed as statistical significance.
Results
The Clinical Characteristics and Variation Type Distribution of HER2 Gene Phenotype in NSCLC Patients
A total of 249 NSCLC patients underwent HER2 genetic testing, and 21 were HER (+) patients, accounting for 8.43%. There were 8 males and 13 females among HER (+) patients; the average age was 65.35±9.23 years old. There were 14 cases of smoking history and 7 cases of no smoking history; 14 cases of adenocarcinoma and 7 cases of squamous carcinoma were classified by histology; in the tumor staging, there were 8 patients with stage I~IIIA and 13 patients with stage IIIB~IV. The clinical information of HER2 gene mutation and non-mutation patients is shown in Table 1, and there was a significant difference in tumor staging between the two groups (P=0.019). Analysis of 21 patients with HER2 gene mutations showed that 17 cases were HER2 gene mutations, accounting for 81%, 4 cases were HER2 gene amplification, accounting for 19%; Among HER2 gene mutations, 12 cases were exon 20 mutations, accounting for 57%, 5 cases were other mutations, accounting for 19%. The distribution of HER2 gene variant types in NSCLC patients is shown in Figure 1.
 |
Table 1 Clinical Information of Patients with Different HER2 Genotypes |
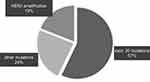 |
Figure 1 Distribution of HER2 gene variant types in NSCLC patients. |
CT Signs of the HER2 Gene in NSCLC Patients
Table 2 shows that there was a correlation between the lesion boundary lobulated sign/spiculated sign, necrosis sign, pleural depression sign and HER2 gene mutation (all P<0.05).
 |
Table 2 CT Feature Distribution of HER2 Gene Phenotype in NSCLC Patients |
The Relationship Between HER2 Gene Mutations and EGFR and ALK Gene Mutations in NSCLC Patients
Among 21 HER (+) patients, 1 had EGFR gene mutation and 20 had no mutation, 2 had ALK gene mutation and 19 had no mutation; of 228 HER (-) patients, 112 had EGFR gene mutation and 116 had no mutation, 22 cases had ALK gene mutation and 206 had no mutation. As shown in Table 3, the incidence of EGRF mutation in HER (+) patients was significantly lower than that in HER (-) patients (P<0.001). The incidence of ALK gene mutation was significantly lower than that of the control group, but there was no statistically significant difference in patients with different phenotypes of HER (P>0.05).
 |
Table 3 Relationship Between HER2 Gene Mutations and EGFR and ALK Gene Mutations in NSCLC Patients |
The Clinical Efficacy of HER2 NSCLC Patients with Different Phenotypes
Comparing the clinical efficacy of HER2 (+) and HER2 (-) patients, it was found that among HER2 (+) patients, there were 6 patients with PR, 8 patients with SD, and 7 patients with PD, ORR and DCR were 28.57% and 66.67%, respectively; Among HER2 (-) patients, there were 106 patients with PR, 90 patients with SD, and 32 patients with PD. The ORR and DCR were 46.19% and 85.96%, respectively. The clinical efficacy of HER2 NSCLC patients with different phenotypes is shown in Table 4. There was no evident difference in ORR between HER2 (+) and HER2 (-) patients. The DCR of HER2 (+) patients was strikingly lower than that of HER2 (-) patients (P=0.020).
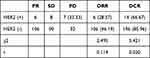 |
Table 4 Clinical Efficacy of NSCLC Patients with Different Phenotypes of HER2 |
Comparison of 12-Month PFS Rate and OS Rate of NSCLC Patients with Different Phenotypes of HER2
Analysis of the 12-month PFS rate and OS rate of NSCLC patients with different phenotypes of HER2 found that there were 0 patients with 12-month PFS in HER2 (+) patients, 8 patients with 12-month OS, with the rate of 0 and 38.95%, respectively; there were 82 patients with 12-month PFS in HER2 (-) and 178 patients with 12-month OS, with the rate of 35.96% and 78.07%, respectively. As shown in Table 5, the 12-month PFS rate and OS rate of HER2 (+) patients were drastically higher than those of HER2 (-) patients (all P<0.05).
 |
Table 5 Comparison of 12-Month PFS Rate and OS Rate of NSCLC Patients with Different Phenotypes of HER2 |
PFS and OS Curve of HER2 (+) and HER2 (-) Patients
Among 21 HER2 (+) patients, 8 patients survived and 13 patients died at a follow-up of 12 months; all patients had disease progression. The PFS and OS curve of HER2 (+) patients are shown in Figure 2. Among 228 HER2 (+) patients, 178 patients survived and 40 patients died at a follow-up of 12 months; 146 patients had disease progression. The PFS and OS curve of HER2 (-) patients are shown in Figure 3. The PFS and OS of HER2 (-) were better than the HER2 (+) patients.
 |
Figure 2 PFS curve and OS curve of HER2 (+) patients. |
 |
Figure 3 PFS curve and OS curve of HER2 (-) patients. |
Adverse Reactions of NSCLC Patients with Different Phenotypes of HER2 During Treatment
Adverse reactions occurred in 19 cases HER2 (+), and 14 cases were Grade 3 or 4; Adverse reactions occurred in 185 cases of HER2 (-), and 82 cases were Grade 3 or 4. There was no significant difference in the incidence of adverse reactions in patients with different phenotypes of HER2, whereas the incidence of Grade 3 or 4 adverse reactions in patients with HER2 (+) was markedly higher than that of the control group (P<0.05, Table 6).
 |
Table 6 Adverse Reactions of NSCLC Patients with Different Phenotypes of HER2 During Treatment |
Discussion
NSCLC is the leading cause of cancer-related deaths globally. It is characterized by rapid tumor proliferation, high metastasis, strong invasiveness and drug resistance. Despite the considerable improvements and achievements in surgical procedures and chemotherapy regimens in recent years, its 5-year survival rate remains unsatisfying.12 Currently, platinum-based combination chemotherapy is considered the standard treatment for NSCLC, but only 30–40% of patients obtained an excellent curative effect, and the drug resistance mechanism is still elusive. HER2 is a member of the epithelial factor receptor family and can enhance a series of signal transduction pathways involved in cell proliferation, differentiation and migration.13 HER2 gene mutation and gene amplification have been found in a variety of cancers such as breast cancer and gastric cancer.14 The positive expression of HER2 is closely related to the prognosis of HER2 targeted therapy, also the positive expression of HER2 indicates a poor prognosis of tumor diseases. Studies have shown that the tumorigenic mechanisms of HER2 oncogene include inhibiting apoptosis and promoting proliferation; increasing the invasiveness of tumor cells; promoting tumor angiogenesis and lymphangiogenesis.15
Most studies have shown that HER2 targeted drug therapy can benefit patients, but HER2 histological biopsy will cause adverse events such as pneumothorax and lesion damage and bleeding. Also, the puncture sampling is unrepresentative and cannot reflect all the information.16,17 This study analyzed the CT signs of NSCLC patients, and the results showed that the boundary lobulated sign/spiculated sign, necrosis sign, pleural depression sign and HER2 gene mutations are related, indicating that the lesion boundary lobulated sign/spiculated sign, necrosis sign, pleural depression sign are more likely to have HER2 gene mutations, which provides a reference for the selection of clinical treatment options. For those who are at higher risk for puncture such as elderly population, with emphysema, poor puncture sites, severe bleeding tendency, coronary heart disease, etc., CT detection can help predict the mutation of HER2 gene.
In this study, 21 patients with HER2 gene mutations were analyzed. Among them, 17 cases were HER2 gene mutations, accounting for 81%, and 4 cases were HER2 gene amplification, accounting for 19%. Among HER2 gene mutations, 12 cases were mutations in exon 20, accounting for 57%, 5 cases were other mutations, accounting for 19%. HER gene mutations mainly manifest gene mutations and gene amplification. Previous studies have shown that gene mutations, especially exon 20 mutations, are the most common, which is consistent with the results of this study.18 Moreover, a prior study pointed out that HER2 mutations are exclusive through EGFR, KRAS and ALK gene mutations in NSCLC.19 In this study, the rate of HER2 mutations in EGFR-negative patients was significantly higher than that in EGFR-positive patients, but HER2 mutations rate in ALK-positive and negative patients were not considerably different, which may be related to the low positive rate of ALK itself.
Afatinib is a potent and irreversible dual inhibitor of EGFR and HER2 tyrosine kinase. It can inhibit the secretion of tumor growth factors by inhibiting the angiogenesis of tyrosine kinase, thereby reducing its stimulation on vascular endothelial cells, and inhibiting angiogenesis. In this study, the DCR of HER2 (+) patients was 66.67%, which was remarkably lower than the 85.96% of HER2 (-) patients. At present, studies on the clinical efficacy of afatinib are inconsistent. In a Phase II study conducted in patients with lung adenocarcinoma, 7 patients with HER2 mutations received afatinib monotherapy with an ORR of 0;20 Another phase II study on afatinib showed that in NSCLC patients with HER2 exon 20 mutations, the ORR was 7.7% (1/13).21 The DCR of HER2 (+) patients was substantially lower than that of HER2 (-) patients, and the 12-month PFS rate and OS rate of HER2 (+) patients were significantly higher than those of HER2 (-) patients (P<0.05). Due to that this study is a retrospective study, all HER2 (+) patients were treated with afatinib, and it is impossible to determine whether it can improve the clinical efficacy. Whether HER2 mutation is a prognostic factor or predictor of NSCLC is a hot research issue. A meta-analysis stated that HER2 mutation and gene amplification are not mutually related; HER2 gene mutation is associated with poor prognosis of NSCLC; conversely, HER2 amplification has no correlation with prognosis.22 Thus, the relationship between HER2 mutations and the prognosis of NSCLC and the clinical efficacy of afatinib have yet to be confirmed by prospective studies. And certain limitations should be highlighted: 1) although it is a retrospective study, and cases were selected in strict accordance with the inclusion and exclusion criteria, bias still exist; 2) 249 patients were selected in this study, but the incidence of HER2 was low (only 21 cases). Hence, a larger sample is needed, and more genes related to lung cancer require further clinical in-depth research.
Altogether, the incidence of HER2 gene mutations in NSCLC patients is relatively low, but it is far commoner in patients with stage IIIB~IV, among which exon 20 mutations are the most prevalent. In CT signs, the lesion lobulated sign/spiculated sign, necrosis signs, and pleural depression signs are related to HER2 gene mutations. In addition, HER2 gene mutations play a crucial role in the clinical prognosis and treatment safety of patients.
Funding
This work was supported by the basic research project of the Heilongjiang Provincial Education Department (grant number: 2020-KYYWF-0289).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Balata H, Fong KM, Hendriks LE, et al. Prevention and Early Detection for NSCLC: advances in Thoracic Oncology 2018. J Thorac Oncol. 2019;14(9):1513–1527. doi:10.1016/j.jtho.2019.06.011
2. Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc. 2019;94(8):1623–1640. doi:10.1016/j.mayocp.2019.01.013
3. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322(8):764–774. doi:10.1001/jama.2019.11058
4. Petrosyan F, Daw H, Haddad A, Spiro T, Sood R. Gene expression profiling for early-stage NSCLC. Am J Clin Oncol. 2015;38(1):103–107. doi:10.1097/COC.0b013e31828d95d8
5. Pasini A, Delmonte A, Tesei A, Calistri D, Giordano E. Targeting chromatin-mediated transcriptional control of gene expression in non-small cell lung cancer therapy: preclinical rationale and clinical results. Drugs. 2015;75(15):1757–1771. doi:10.1007/s40265-015-0461-3
6. Jebbink M, de Langen AJ, Boelens MC, Monkhorst K, Smit EF. The force of HER2 - A druggable target in NSCLC? Cancer Treat Rev. 2020;86:101996. doi:10.1016/j.ctrv.2020.101996
7. Baraibar I, Mezquita L, Gil-Bazo I, Planchard D. Novel drugs targeting EGFR and HER2 exon 20 mutations in metastatic NSCLC. Crit Rev Oncol Hematol. 2020;148:102906. doi:10.1016/j.critrevonc.2020.102906
8. Masood A, Kancha RK, Subramanian J. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in non-small cell lung cancer harboring uncommon EGFR mutations: focus on Afatinib. Semin Oncol. 2019;46(3):271–283. doi:10.1053/j.seminoncol.2019.08.004
9. Postmus PE, Kerr KM, Oudkerk M, et al. ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv1–iv21. doi:10.1093/annonc/mdx222
10. Wakelee HA, Dahlberg SE, Brahmer JR, et al.; Eastern Cooperative Oncology Group. Differential effect of age on survival in advanced NSCLC in women versus men: analysis of recent Eastern Cooperative Oncology Group (ECOG) studies, with and without bevacizumab. Lung Cancer. 2012;76(3):410–415. doi:10.1016/j.lungcan.2011.12.006
11. Grimaldi S, Terroir M, Caramella C. Advances in oncological treatment: limitations of RECIST 1.1 criteria. Q J Nucl Med Mol Imaging. 2018;62(2):129–139. doi:10.23736/S1824-4785.17.03038-2
12. Gubens MA, Davies M. NCCN guidelines updates: new immunotherapy strategies for improving outcomes in non-small cell lung cancer. J Natl Compr Canc Netw. 2019;17(5.5):574–578. doi:10.6004/jnccn.2019.5005
13. Connell CM, Doherty GJ. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open. 2017;2(5):e000279. doi:10.1136/esmoopen-2017-000279
14. Lei YY, Huang JY, Zhao QR, et al. The clinicopathological parameters and prognostic significance of HER2 expression in gastric cancer patients: a meta-analysis of literature. World J Surg Oncol. 2017;15(1):68. doi:10.1186/s12957-017-1132-5
15. Iqbal N, Iqbal N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: overexpression and Therapeutic Implications. Mol Biol Int. 2014;2014:852748. doi:10.1155/2014/852748
16. Siddiqui MR, Railkar R, Sanford T, et al. Targeting Epidermal Growth Factor Receptor (EGFR) and Human Epidermal Growth Factor Receptor 2 (HER2) Expressing Bladder Cancer Using Combination Photoimmunotherapy (PIT). Sci Rep. 2019;9(1):2084. doi:10.1038/s41598-019-38575-x
17. Palle J, Rochand A, Pernot S, Gallois C, Taïeb J, Zaanan A. Human Epidermal Growth Factor Receptor 2 (HER2) in Advanced Gastric Cancer: current Knowledge and Future Perspectives. Drugs. 2020;80(4):401–415. doi:10.1007/s40265-020-01272-5
18. Jang J, Son J, Park E, et al. Discovery of a highly potent and broadly effective epidermal growth factor receptor and HER2 exon 20 insertion mutant inhibitor. Angew Chem Int Ed Engl. 2018;57(36):11629–11633. doi:10.1002/anie.201805187
19. Arcila ME, Chaft JE, Nafa K, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res. 2012;18(18):4910–4918. doi:10.1158/1078-0432.CCR-12-0912
20. De Grève J, Moran T, Graas MP, et al. Phase II study of Afatinib, an irreversible ErbB family blocker, in demographically and genotypically defined lung adenocarcinoma. Lung Cancer. 2015;88(1):63–69. doi:10.1016/j.lungcan.2015.01.013
21. Dziadziuszko R, Smit EF, Dafni U, et al. Afatinib in NSCLC With HER2 Mutations: results of the Prospective, Open-Label Phase II NICHE Trial of European Thoracic Oncology Platform (ETOP). J Thorac Oncol. 2019;14(6):1086–1094. doi:10.1016/j.jtho.2019.02.017
22. Liu L, Shao X, Gao W, et al. The role of human epidermal growth factor receptor 2 as a prognostic factor in lung cancer: a meta-analysis of published data. J Thorac Oncol. 2010;5(12):1922–1932. doi:10.1097/JTO.0b013e3181f26266
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
