Back to Journals » International Journal of General Medicine » Volume 15
Clinical Characteristics and Outcomes in Chronic Kidney Disease Patients with Tuberculosis in China: A Retrospective Cohort Study
Authors Xiao J, Ge J, Zhang D, Lin X, Wang X, Peng L , Chen L
Received 6 April 2022
Accepted for publication 19 July 2022
Published 19 August 2022 Volume 2022:15 Pages 6661—6669
DOI https://doi.org/10.2147/IJGM.S367090
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jing Xiao,1 Jianjian Ge,1 Dingxin Zhang,1 Xinqiang Lin,1 Xiaoshuang Wang,1 Li Peng,2 Liqun Chen1
1Department of Nephrology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, 400016, People’s Republic of China; 2Department of Respiratory, The First Affiliated Hospital of Chongqing Medical University, Chongqing, 400016, People’s Republic of China
Correspondence: Liqun Chen, Department of Nephrology, The First Affiliated Hospital of Chongqing Medical University, Youyi Road 1, Chongqing, 4000416, People’s Republic of China, Tel +86 13883557769, Email [email protected]
Background: The diverse manifestations of tuberculosis (TB) in chronic kidney disease (CKD) patients can cause difficulty in diagnosis, delayed treatment, even death. Therefore, this study investigated the clinical characteristics and the risk factors for mortality in CKD patients with TB.
Methods: This retrospective study included 167 patients diagnosed with active TB at two tertiary medical centers in Chongqing within six years. Clinical characteristics and outcomes of anti-TB treatment in patients with and without CKD were collected, and the predictive mortality values of variables were analyzed.
Results: Of the 167 patients, 66.7% (44/66) hemodialysis (HD), 41.1% (21/51) pre-HD, and 32.0% (16/50) non-CKD patients had extrapulmonary TB. The pleura and lymph node were the common sites in CKD patients. Clinical presentations of cough and hemoptysis in CKD patients were less common than those in non-CKD patients, 13.7% (16/117) of CKD patients even not having any clinical symptoms. The positive rates of tuberculin skin test, TB-polymerase chain reaction and acid-fast bacilli in sputum in HD patients were lower than those in pre-HD and non-CKD patients (p< 0.05). CKD patients were more prone to gastrointestinal and neurological side effects during anti-TB treatment. The mortality rates of non-CKD, pre-HD and HD patients was 6.1%, 31.9% and 37.3%, respectively. Multivariate Cox analysis revealed that age≥ 40 years (HR: 5.871; p=0.019), hypoalbuminemia (HR:2.879; p=0.004), CKD stage 4– 5 (HR:4.719; p=0.018) and HD (HR:6.13; p=0.005) were associated with mortality.
Discussion: CKD patients with TB have atypical clinical manifestations and high mortality. Age, hypoalbuminemia, CKD stage 4– 5, and HD were independent predictors of mortality.
Keywords: chronic kidney disease, extrapulmonary TB, pulmonary TB, anti-TB therapy, treatment outcome, hemodialysis
Introduction
Chronic kidney disease (CKD) is a significant global health issue, with an estimated rate of 8–16% worldwide.1 In China, the overall occurrence of CKD is 10.8% and appears to have increased in recent years. The mortality rate in CKD patients is about 3.2 per 1000 person-years.2,3 Tuberculosis (TB) is a leading cause of infectious morbidity and mortality worldwide. In 2019, approximately 10 million people suffered from TB, 12% of whom died as a result.4 A recent systematic review and meta-analysis found that all-cause mortality was nearly three times higher in TB patients compared with the controls.5
CKD and TB are common disorders. Patients with CKD are more susceptible to TB infection or reactivation of latent TB infection (LTBI) due to their immunosuppression. Shu et al found the risk of TB increases from CKD stage 3, and in stage 5, the risk is even higher than that of those receiving dialysis.6 Previous studies suggested that patients with CKD–especially those on hemodialysis (HD)–have higher incidence of TB than the general population.7,8 The reported overall mortality of CKD patients with TB was 35%, attributed to delayed diagnosis and anti-TB drug-related side effects.9,10
China has the second-largest incidence of TB worldwide, with an estimated 833,000 TB patients in 2019, accounting for 8.4% of global TB patients.4 Currently, China does not have complete data on the epidemiology of TB in CKD patients. The prospective single-center observation study showed that the incidence of TB in non-dialysis CKD patients was 1.6% in Taiwan.11 According to reports from some small samples in various regions, the risk of TB infection in CKD patients is about 4–30 times higher than that of the general population in China.12 In the present study, we retrospectively analyzed the clinical characteristics of TB and outcomes of anti-TB treatment in CKD patients with TB to identify risk factors for mortality.
Methods
Study Design and Patients
We conducted a retrospective study of CKD patients with newly diagnosed active TB at the First Affiliated Hospital of Chongqing Medical University and the Chongqing Public Health Center from January 1, 2012, to December 31, 2018. We check the clinical data of active TB patients in electronic chart record, because active TB, as an infectious disease, needs to be reported to the Chinese Center for Disease Control and Prevention(CDC). During the same period, 50 patients without CKD who were newly diagnosed with active TB were randomly selected from the medical record system as a control group. CKD was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/ 1.73m2 for more than three months according to the 2012 Kidney Disease: Improving Global Outcome guideline. Criteria for active TB diagnosis included the following: (1) positive culture of any specimen for Mycobacterium TB or positive staining with acid-fast bacilli (AFB) of the sample, (2) histopathological evidence of TB from a tissue biopsy specimen; and (3) physician-diagnosis TB in cases without bacteriological or pathological confirmation, determined by combining the patient’s clinical manifestations and imaging findings to exclude all other diseases, together with the patient’s confirmed responsiveness to anti-TB treatment after one month of follow-up. Exclusion criteria were as follows: (1) patient who has a history of pulmonary TB (PTB), (2) incomplete clinical data, (3) TB before CKD diagnosis, (4) patient infected with the human immunodeficiency virus(HIV) or chronic hepatitis.
Treatment
All patients were treated with the standard regimen of anti-TB therapy (ATT). Anti-TB drugs were administered, including rifampicin (450mg/d), isoniazid (5mg/kg/d), ethambutol (25–35mg/kg/d), and pyrazinamide (15–25mg/kg/d); however, ethambutol (25–35mg/kg) and pyrazinamide (15–25mg/kg) were administered 3 times per week to patients with CKD stage 4–5 and 5D, which was based on expert consensus for the management of adult CKD complicated with TB in China.13 The course of treatment was 6 months for PTB and one year for extrapulmonary TB (EPTB).4 In the event of serious complications–including severe drug-associated hepatotoxicity or allergic reaction involving the skin or mucosal tissue with shock–the treatment regimen was modified as appropriate by an expert treating the infectious disease. In addition to anti-TB treatment, patients were treated to control their blood pressure, blood sugar, and anemia. Dialysis patients underwent regular HD three times per week.
Study Definitions and Variable
Baseline characteristics of all patients – including age, gender, combined diseases, basic kidney information, smoking history, and history of contact TB–were collected. In addition, clinical manifestation, TB infection site, TB screening, imaging examination results, adverse effects of anti-TB drugs, and prognosis were noted. All the survivors were followed up with until June 30, 2019. The institutional ethical committee approved the study.
Statistical Methods
All statistical analyses were performed using the Statistical Package for the Social Sciences for Windows with SPSS 13.0 (Chicago, Illinois, USA). Continuous variables were expressed as mean ±standard deviation and were analyzed using variance analysis. Categorical variables were displayed as frequencies and percentages and analyzed using the Chi-square test and Fisher’s exact test. A p-value ≤0.05 was considered significant. Univariate and multivariate Cox regression analyses were performed for prognosis. Correlations were expressed as hazard ratio(HR) and 95% confidence interval (95% CI).
Results
Baseline Clinical Characteristics
During the six years, 167 patients with TB participated in this study, including 66 patients who received HD (HD group), 51 patients who did not receive HD (pre-HD group), and 50 patients without CKD (non-CKD group). Among the pre-HD group, 41.2% (21/51) had CKD stage 1–3, and 58.8% (30/51) had CKD stage 4–5. As shown in Table 1, 41.2% (21/51) had hypoalbuminemia (serum albumin<3.0mg/dl) in the pre-HD group, which was higher than the frequency in the HD (10.6%) and non-CKD (14.0%) group (p˂0.001). There was no significant difference in age, gender, underlying disease, smoking and contact TB history among those groups.
 |
Table 1 Patients Demographics and Sites of Tuberculosis(TB) |
Site of TB
Incidence rates of EPTB were higher in the HD and pre-HD groups (66.6% and 41.1%, respectively) compared with the non-CKD group (32.0%; p=0.002). The pleura, lymph node, and bone were the common sites of TB in CKD patients, while almost all were pleura TB in the non-CKD group (Table 1).
Clinical Presentations
There were no significant differences in fever or pyrexia of unknown origin and TB poisoning symptoms (anorexia, night sweats, and weight loss) among the groups. However respiratory symptoms were significantly lower in the pre-HD (58.8%) and HD (50.0%) groups compared with the non-CKD (p<0.001), while gastrointestinal symptoms and lymphadenitis were significantly higher in the HD group compared the pre-HD and non-CKD groups (p=0.012 and p=0.018, respectively). Additionally, 12.1% of patients in the HD group and 15.7% of patients in the pre-HD group did not have any clinical symptoms (Table 2).
 |
Table 2 Clinical Characteristics of the Study Patients |
TB Screening Test
As shown in Table 3, not all patients completed the TB screening test. The strong positive rate of TB antibody (TB-Ab) and the positive rates of tuberculin skin test (TST)and T-Spot in the HD group were very low (10.9%, 20.0%, and 69.2%, respectively), but there were no significant differences among the three groups. The positive rate of TB-polymerase chain reaction (TB-PCR) in sputum was 91.6% in the non-CKD group, which was higher than those in the pre-HD (50.0%) and HD (40.0%) groups (p=0.032). The positive rates of AFB-sputum smear in patients with and without HD were 11.1% and 39.5%, respectively, lower than that in non-CKD patients (51.2%, p=0.01).
 |
Table 3 Laboratory and Imaging Characteristics of the Study Patients |
Pulmonary computed tomography scans were done in all patients, A total of 56.1% (37/66) of HD patients and 74.5% (38/51) of pre-HD patients had TB-related radiologic changes, and TB mainly invaded the multiple lung lobes. Forty percent (31/75) of CKD patients and 32.6% (14/43) of non-CKD patients had pleural effusion; there were no significant differences in TB-related lung lesions among those patients.
Side Effects of Anti-TB Drugs
As shown in Table 4, 26 patients in the pre-HD group, 35 in the HD group, and 15 in the non-CKD group developed side effects during anti-TB treatment. Only one patient had acute liver failure due to severe drug-related hepatotoxicity and died (HD group). Side effects of gastrointestinal, central, and peripheral nerves disorders were more severe in the pre-HD and HD group (p=0.017, p=0.026, respectively) than in the non-CKD group (p=0.012), which included the optic nerves disorders in the HD group (15.3%). In the pre-HD group, 13 patients with CKD stage 4–5 rapidly developed high serum creatinine and uric acid levels and required dialysis during anti-TB treatment.
 |
Table 4 Side Effects and outcomes of Anti-Tuberculosis Therapy in Patients |
Outcomes
Four patients in the pre-HD group, 7 in the HD group, and 1 in the non-CKD group were lost to follow-up and did not complete the study. During the treatment period, the overall mortality rate was 34.9% (37/106). The mortality rates of non-CKD, pre-HD and HD patients was 6.1%(3/50), 31.9%(15/47), and 37.3%(22/59), respectively (p<0.001, Table 4).
Cox Regression Analysis
Univariate Cox regression analysis showed that age≥40 years, hypoalbuminemia, CKD, and HD were associated with mortality. Multivariate Cox analysis revealed that age≥40 years (HR: 5.871; p=0.019), hypoalbuminemia (HR:2.879; p=0.004), CKD stage 4–5 (HR:4.719; p=0.018). And HD (HR:6.130; p=0.005) were independent risk factors (Table 5).
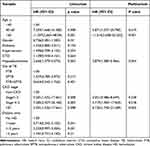 |
Table 5 Univariate and Multivariate Cox Regression Analyses for Patient Mortality |
Discussion
This study analyzed the clinical characteristics and risk factors for mortality in CKD patients with TB. The state of cell-mediated immune response impairment persists in CKD patients, which makes them susceptible to infections, including the high occurrence of TB infection.14,15 Cho et al reported that TB risk increased by 5.1% with every 10 mL/min/1.73 m2 decrease in eGFR, and there was a positive correlation between CKD stage and TB in CKD patients.16 We found that most (81.1%) of TB patients were CKD stage 4–5 and on dialysis, and 45.5% (30/66) of HD patients were infected with TB within the first year in our study. Similarly, Vikrant S found that TB occurs between 12 and 15 months on dialysis.9 These findings suggest that special attention should be paid to CKD patients due to their increased risk of TB infection, especially within the first year of dialysis.
Unlike patients with TB alone, CKD patients with TB often have atypical clinical manifestations. First, we found that the respiratory symptoms—such as cough and hemoptysis—were less common in CKD patients, even 13.7% (16/117) of patients not having any clinical symptoms, which active TB cases were diagnosed by the clinical presentation or image by the experts. Second, CKD patients were more likely to develop EPTB. Due to the accumulation of uremic toxins or abnormal vitamin D metabolism with decreased renal function leads to immune dysfunction, and active TB may develop or reactivate from LTBI.17 In our study, 41.1% (21/51) of pre-HD and 66.7% (44/66) of HD patients had EPTB, and the most common sites were the pleura, lymph node, and bone. In 2015, the overall incidence of EPTB in South Korea accounted for 13%.18 Li C-H found that 50% patients had EPTB in 114 non-dialysis CKD stage 1–5 patients.11 Previous studies have demonstrated that the incidence rate of EPTB on dialysis patients was approximately 40%.10 While India has the highest burden of TB in the world, Vikrant reported that three-fourths of dialysis (peritoneal dialysis or hemodialysis) patients had EPTB, and 40.6% dialysis patients had unilateral pleural effusion in India.9 However, our data showed that bilateral pleural effusion was more predominant in CKD patients (63.3%; 19/30), which may be related to uremia and hypoalbuminemia in addition to TB infection.
Furthermore, some CKD patients with TB can present with nonspecific symptoms–such as fever, anorexia, and weight loss–which mimic uremia and lead to difficulties in diagnosis, delay treatment, even death. Therefore, when it is highly suspected that CKD patients have TB infection, Physicians should screen for TB infection early. In our study, the positive rate of AFB sputum smear in CKD patients was very low because of the increasing frequency of EPTB. The positive rates of TST, TB-Ab, and TB-PCR sputum in CKD patients were lower than those in non-CKD patients, especially the positive rates of TST and TB-Ab in HD patients were only 10.9% and 20.0%, respectively. It is well known that TST and TB-Ab lack sensitivity with negative rates of up to 50%.9 Impaired cellular immunity in CKD patients could explain the high rate of negative and false-negative results of TST and TB-Ab, suggesting that these are unreliable tests in CKD patients. The newer interferon-gamma release assays (IGRAs), including T-Spot and Quanti FERON-TB, have a sensitivity of 100% and a specificity of 62% in detecting active TB infection, superior to TST.19 In our study, the positive rate of T-Spot was very high in both CKD and non-CKD patients (82.6% and 100%, respectively), but the sample size was relatively small, the large number of prospective studies are needed for further confirm.
However, Xu YZ found that IGRA may detect more LTBI in immunocompromised patients.20 IGRA cannot distinguish between active TB and LTBI because it cannot measure the infection itself, which have important limitations and poor predictive value for active TB in CKD patients. Therefore, selecting a test with better performance may help early detection and better treatment of TB in patients with a high risk of developing active TB.
CKD patients with TB, especially those on hemodialysis, have a higher mortality rate. In our cohort, the overall mortality rate was 34.9% (37/106). Among CKD patients with and without dialysis, the mortality rate was 37.3% (22/59) and 31.9% (15/47), respectively. However, Vikrant reported that among 107 CKD patients with TB infection in India, the overall mortality rate was 7%; the mortality rate was 34.8% for dialysis patients.9 Reis-Santos et al reported that the overall mortality rate was about 34.4% in 1077 CKD patients with TB.10 There are several possible reasons for the poor prognosis of CKD patients with TB in our study. First, we found that the CKD patients tended to develop TB drug-related side effects compared to non-CKD patients (57.5% vs 30.6%), even though renal function-based dosage adjustment was performed, moreover, the CKD patients, especially HD patients, were more prone to gastrointestinal and neurological disturbances during anti-TB treatment. A retrospective analysis in Japan found that the adverse events increased with the severity of CKD (20.4–44.4%) in 241 CKD patients with TB (including 11 dialysis patients).21 Some previous studies have reported that the rate of adverse effects of anti-TB drugs ranges from 24.9% to 46.34% in dialysis patients.9,21 Ezer et al reported that the incidence of optic neuropathy associated with ethambutol was 2.3%;22 however, in our cohort, 15.3% of patients on dialysis had optic neuropathy, which was higher than pre-HD (6.4%) and non-CKD (6.1%) patients. This difference may be related to the decreased clearance and metabolism of anti-TB drugs due to concomitant renal function decline in patients with CKD. Therefore, the higher drug-related side effects may result incomplete adherence to anti-TB drugs or therapy was discontinued, and lead to poor patient compliance, even death. Second, we found that age ≥40 years, hypoalbuminemia, CKD stage 4–5, and dialysis risk factors for mortality using Cox regression analysis. The mean age of CKD patients was higher than non-CKD patients, which could mean older CKD patients are more susceptible to TB. Furthermore, 13 out of 51 patients with CKD stage 4–5 received dialysis due to deterioration of renal function during anti-TB treatment in our study. Similarly, Chang et al reported that 7.1% of patients had kidney injury during anti-TB treatment, and 27% of those patients did not recover within six months.23 Anti-TB drugs could induce nephrotoxicity, and mycobacterium tuberculosis may cause interstitial nephritis and amyloidosis, which can aggravate the progression of kidney disease. In our study, 41.2% of pre-HD patients and 10.6% of HD patients had hypoalbuminemia at enrollment resulting from gastrointestinal symptoms, such as nausea and vomiting, due to uremic toxins or side effects of anti-TB drugs, or proteinuria, and chronic inflammation (TB infection). Severe hypoalbuminemia possibly making them prone to secondary serious infections or death. Furthermore, we also found that 37 of 106 CKD patients with TB died during the follow-up, and ten patients died of heart disease and 15 patients died of infection, including five patients with PTB infection complicated with respiratory failure. A systematic review and meta-analysis found that patients diagnosed with TB have a higher risk of cardiovascular diseases (CVD) morbidity and mortality compared with those not diagnosed with TB.24 However, in our study, the primary cause of death among CKD patients with TB was an infection, followed by heart disease. To our knowledge, this is the first time the cause of death for CKD patients with TB has been reported. In addition, we found that some CKD patients with underlying diseases, such as diabetes (26.5%) or autoimmune diseases (15.8%), are prone to infections due to weakened immunity in our study. On the other hand, in China, approximately 71% of patients with TB live in rural areas and are not treated on time because of their poor economic conditions.25,26
Our study has several limitations. The first was its retrospective design. The second limitation was that one patient with non-CKD and 11 patients with CKD were lost to follow-up during treatment because they returned to their hometowns. Third, the single-center design and small sample size may limit the ability to generalize our findings to the overall population.
Conclusion
CKD patients with TB–especially those on dialysis–had atypical clinical manifestations, a low positive rate in TB screening tests, high mortality, high incidence of gastrointestinal side effects, and neurological disturbance during anti-TB treatment. Age>40 years, hypoalbuminemia, CKD stage 4–5, and HD were independent predictors of mortality in CKD patients with TB.
Abbreviations
CKD, chronic kidney disease; TB, tuberculosis; LTBI, latent TB infection; EPTB, extrapulmonary TB; HD, hemodialysis; AFB, acid-fast bacilli; ATT, anti-tuberculosis therapy; PUO, pyrexia of unknown origin; TST, tuberculin skin test; TB-PCR, TB-polymerase chain reaction; IGRA, interferon-gamma release assays; CVD, cardiovascular diseases; CTD, Connective tissue disease.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University (IRB number: 2019-206). Informed consent was waived by the ethics committee because we retrospectively collected data without using identifying information or applying any interventions.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi:10.1016/S0140-6736(13)60687-X
2. Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. doi:10.1016/S0140-6736(12)60033-6
3. Wang J, Wang F, Liu S, Zhou M, Zhang L, Zhao M. Reduced kidney function, albuminuria, and risks for all-cause and cardiovascular mortality in China: a population-based cohort study. BMC Nephrol. 2017;18:188–197. doi:10.1186/s12882-017-0603-9
4. World Health Organization. Global tuberculosis report 2020; 2020. Available from: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131.
5. Romanowski K, Baumann B, Basham CA, Ahmad Khan F, Fox GJ, Johnston JC. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2019;19:1129–1137. doi:10.1016/S1473-3099(19)30309-3
6. Shu -C-C, Yu-Feng Wei Y-F, Yeh Y-C, et al. The impact on incident tuberculosis by kidney function impairment status: analysis of severity relationship. Respir Res. 2020;21:51–59. doi:10.1186/s12931-020-1294-5
7. Bardenheier BH, Pavkov ME, Winston CA, et al. Prevalence of tuberculosis disease among adult us-bound refugees with chronic kidney disease. J Immigr Minor Health. 2019;21:1275–1281. doi:10.1007/s10903-018-00852-8
8. Cheng KC, Liao KF, Lin CL, Liu CS, Lai SW. Chronic kidney disease correlates with increased risk of pulmonary tuberculosis before initiating renal replacement therapy: a cohort study in Taiwan. Medicine. 2018;97:39–45.
9. Vikrant S. Tuberculosis in dialysis: clinical spectrum and outcome from an endemic region. Hemodialysis Int. 2019;23:88–92. doi:10.1111/hdi.12693
10. Reis-Santos B, Gomes T, Horta BL, Maciel EL. The outcome of tuberculosis treatment in subjects with chronic kidney disease in Brazil: a multinomial analysis. J Bras Pneumol. 2013;39(5):585–594. doi:10.1590/S1806-37132013000500009
11. Li C-H, Chen H-J, Chen W-C, et al. The risk of tuberculosis infection in non-dialysis chronic kidney disease patients. Front Med. 2021;13(8). doi:10.3389/fmed.2021.715010
12. Yuan FH, Guang LX, Zhao SJ. Clinical comparisons of 1498 chronic renal failure patients with and without tuberculosis. Ren Fail. 2005;27:149–153. doi:10.1081/JDI-48243
13. Li H. Expert consensus on the management of adult chronic kidney disease and tuberculosis in China. Chin J Blood Purif. 2016;15(11):577–586. In Chinese.
14. Romanowski K, Clark EG, Levin A, Cook VJ, Johnston JC. Tuberculosis and chronic kidney disease: an emerging global syndemic. Kidney Int. 2016;90(1):34–40. doi:10.1016/j.kint.2016.01.034
15. Moran E, Baharani J, Dedicoat M, et al. Risk factors associated with the development of active tuberculosis among patients with advanced chronic kidney disease. J Infect. 2018;77(4):291–295. doi:10.1016/j.jinf.2018.06.003
16. Cho PJ, Wu CY, Johnston J, Wu MY, Shu CC, Lin HH. Progression of chronic kidney disease and the risk of tuberculosis: an observational cohort study. Int J Tuberc Lung Dis. 2019;23(5):555–562. doi:10.5588/ijtld.18.0225
17. Jean G, Souberbielle JC, Chazot C. Vitamin D in chronic kidney disease and dialysis patients. Nutrients. 2017;9(4):328–343. doi:10.3390/nu9040328
18. Kim EJ, Lee W, Jeong WY, et al. Chronic kidney disease with genitourinary tuberculosis: an old disease but ongoing complication. BMC Nephrol. 2018;19:193–201. doi:10.1186/s12882-018-0994-2
19. Al Jahdali H, Ahmed AE, Balkhy HH, et al. Predictive value of the tuberculin skin test and Quanti FERON-tuberculosis Gold In-Tube test for the development of active tuberculosis in hemodialysis patients. Ann Thorac Med. 2016;11:114–120. doi:10.4103/1817-1737.180023
20. Xu YZ, Yang QL, Zhou JG, et al. Comparison of QuantiFERON-TB Gold In-Tube and QuantiFERON-TB gold-plus in the diagnosis of mycobacterium tuberculosis infections in immunocompromised patients: a real- world study. Microbiol Spectr. 2022;3(10):1–4.
21. Saito N, Yoshi Y, Kaneko Y, et al. Impact of renal function-based anti- tuberculosis drug dosage adjustment on efficacy and safety outcomes in pulmonary tuberculosis complicated with chronic kidney disease. BMC Infect Dis. 2019;19:374, 2–8. doi:10.1186/s12879-019-4010-7
22. Ezer N, Benedetti A, Darvish-Zargar M, Menzies D. Incidence of ethambutol-related visual impairment during treatment of active tuberculosis. Int J Tubercu Lung Dis. 2013;17(4):447–455. doi:10.5588/ijtld.11.0766
23. Chang CH, Chen YF, Wu VC, et al. Acute kidney injury due to anti-tuberculosis drugs: a five-year experience in an aging population. BMC Infect Dis. 2014;14:23. doi:10.1186/1471-2334-14-23
24. Basham C-A, Smith S-J, Romanowski K, et al. Cardiovascular morbidity and mortality among persons diagnosed with tuberculosis: a systematic review and meta-analysis. PLoS One. 2020;15(7):e0235821. doi:10.1371/journal.pone.0235821
25. Gao L, Lu W, Bai L, et al. Latent tuberculosis infection in rural China: baseline results of a population-based, multi-centre, prospective cohort study. Lancet Infect Dis. 2015;15:310–319. doi:10.1016/S1473-3099(14)71085-0
26. Zhang R, Pu J, Zhou J-N, et al. Factors predicting self-report adherence (SRA) behaviours among DS-TB patients under the “Integrated model”: a survey in Southwest China. MC Infect Dis. 2022;22:201–212.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
