Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 6
Clinical characteristics and outcomes in 303 HIV-infected patients with invasive fungal infections: data from the Prospective Antifungal Therapy Alliance registry, a multicenter, observational study
Authors Marukutira T, Huprikar S, Azie N, Quan S, Meier-Kriesche H, Horn D
Received 3 September 2013
Accepted for publication 26 November 2013
Published 13 March 2014 Volume 2014:6 Pages 39—47
DOI https://doi.org/10.2147/HIV.S53910
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Tafireyi Marukutira,1 Shirish Huprikar,2 Nkechi Azie,3 Shun-Ping Quan,3 Herwig-Ulf Meier-Kriesche,3 David L Horn4
1Botswana-Baylor Children’s Clinical Centre of Excellence, Gaborone, Botswana, 2Icahn School of Medicine at Mount Sinai, New York, NY, 3Astellas Scientific and Medical Affairs, Inc, Northbrook, IL, 4David Horn LLC, Doylestown, PA, USA
Abstract: This analysis aimed to characterize the epidemiology, diagnosis, treatment, and outcomes of invasive fungal infections (IFIs) in patients with human immunodeficiency virus (HIV). Data were examined for HIV patients enrolled in the Prospective Antifungal Therapy (PATH) Alliance registry, a multicenter, observational study of patients with IFIs in North America from 2004 to 2008. Patient demographics, clinical characteristics, comorbidities, antifungal therapies, and survival were assessed. In total, 320 fungal isolates were identified from 303 HIV patients with IFIs in the PATH Alliance® registry. These included Cryptococcus (50.0%), Candida (33.1%), Histoplasma (9.1%), and Aspergillus (4.4%). Candida infection occurred mainly as candidemia (86.0%); Cryptococcus as central nervous system infection (76.7%); Histoplasma as disseminated infection (74.1%); and Aspergillus as pulmonary infection (81.8%). The CD4 cell count was #200 cells/µL in 91.2% of patients with available data. The majority of patients with Cryptococcus (77.9%), Histoplasma (100.0%), and Aspergillus (71.4%) infections had CD4 cell counts ,50 cells/µL compared with 48.9% of patients with Candida infections. Patients with candidiasis were more likely to have other conditions requiring medical services compared with patients with other IFIs. Survival probability was lower in patients with Aspergillus (0.58) and Candida (0.59) infection than in patients with Histoplasma (0.84) and Cryptococcus (0.81) infection. In the highly active antiretroviral therapy era, traditional opportunistic IFIs such as cryptococcosis and histoplasmosis still mainly occur in HIV patients with CD4 counts ,50 cells/µL. Fungal infections remain a clinical challenge in HIV patients with severe immunosuppression. Our data also suggest that HIV patients with CD4 cell counts .200 cells/µL and other underlying conditions may be susceptible to invasive candidiasis.
Keywords: human immunodeficiency virus, invasive fungal infections, Prospective Antifungal Therapy Alliance registry
Introduction
Individuals with human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) are at significant risk of invasive fungal infections (IFIs). AIDS-defining fungal infections include pulmonary and esophageal candidiasis, Pneumocystis jiroveci pneumonia, disseminated or extrapulmonary coccidioidomycosis, extrapulmonary cryptococcosis, and disseminated or extrapulmonary histoplasmosis.1 Invasive aspergillosis is relatively rare2,3 and not considered an AIDS-defining illness.1
The advent of highly active antiretroviral therapy (HAART) heralded dramatic improvements in the outcomes for AIDS patients and significant reductions in the incidence of opportunistic infections in North America.4,5 In a nationwide surveillance project in the US, the incidence of almost all AIDS-defining illnesses decreased significantly between 1992 and 1998.6,7 By 2007, the average incidence of each AIDS-defining IFI was less than one case per 100 person-years.8 Despite these advances, opportunistic fungal infections remain common in some patients, such as those with low CD4 cell counts (<250 cells/μL), and those not receiving HAART.9
Effective management of IFIs depends on knowledge of current epidemiology, risk factors, rapid diagnostic tests, and optimal treatment regimens. Randomized clinical trials are limited by financial constraints and difficulty in enrolling sufficient numbers of patients with less common IFIs.10 The development and analysis of large patient databases and registries may overcome these limitations and provide current and relevant clinical information.11–17
The Prospective Antifungal Therapy (PATH) Alliance registry is a multicenter, observational registry that collected data on the epidemiologic characteristics, diagnoses, treatment, and outcomes of IFIs in North America.18 Since its launch in July 2004, data from 6,845 patients have been evaluated, and numerous abstracts, articles, and presentations have been published on the trends and insights gained with regard to these aspects of IFIs in North America.12,14,18–26 In the present analysis, we explored the clinical characteristics and outcomes of IFIs in the subset of patients with HIV enrolled in the PATH Alliance® registry. Data related to some patients included in this analysis may have been previously reported.14,19,23,24
Materials and methods
Data collection
This analysis was based on prospectively collected data from HIV-infected patients with IFIs enrolled from July 1, 2004 to December 31, 2008 in 25 North American centers (23 in the US, two in Canada) participating in the PATH Alliance® registry. This database has been described in detail previously.21 Briefly, patients with proven or probable IFIs were enrolled and followed prospectively for 12 weeks, or until they died or were lost to follow-up. Inclusion criteria for each specific IFI were adapted from the 2002 guidelines of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group.27 Patient data were accrued using a real-time web-based electronic case report form.
Data for each IFI were collected, including patient demographics and clinical characteristics, comorbidities, existing medications and antifungal therapies, immunologic risk, and concomitant bacterial and viral infections. Patient characteristics included HIV-specific information such as CD4 and CD8 cell counts, and viral load. Detailed information about the IFI was collected and included the specific fungal pathogen and species, infection site, diagnostic tests, antifungal therapy, and outcomes. Incidences of Pneumocystis pneumonia were excluded from this analysis since it is not traditionally reported with other IFIs and was therefore likely underreported in the PATH Alliance registry.
Statistical analyses
The day of diagnosis of an IFI was designated as day 1. Descriptive analyses were used for overall patient characteristics and subgroup analyses (eg, pathogen, treatment cohorts). Descriptive survival analyses were used for overall patient survival and subgroup analyses by pathogen. The survival distribution function was estimated using the Kaplan–Meier method.28 Patients lost to follow-up prior to the week 12 assessment were censored on the day of their last activity documented in the database. Since patient data recorded in the PATH Alliance® registry are not publicly accessible, access to data for statistical analyses was provided to the study investigators by the program sponsor, Astellas Pharma US (Northbrook, IL, USA). Statistical analyses were performed using SAS 9.2/Enterprise Guide version 4.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Overall cohort characteristics
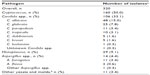
Of the 6,845 patients enrolled in the PATH Alliance® database, 303 (4.4%) were HIV-positive (Table 1). The mean age of the patients was 42.1±0.6 (range 19.0–69.0) years. Most HIV-infected patients in the cohort were adults aged 19–64 years (n=297, 98.0%), male (n=237, 78.2%), and of African-American origin (n=195, 64.4%).
A total of 320 fungal isolates were identified in 303 patients with an HIV infection (Table 2). The most common were Cryptococcus (n=160, 50.0% of all infections), Candida (n=106, 33.1%), Histoplasma (n=29, 9.1%), and Aspergillus (n=14, 4.4%). Fifteen patients were documented as having coinfection with two fungal species, ie, Aspergillus flavus + Histoplasma, Aspergillus fumigatus + Candida glabrata, A. fumigatus + Histoplasma, Candida albicans + C. glabrata (n=3), C. albicans + Candida krusei, C. albicans + Candida tropicalis (n=2), C. krusei + C. tropicalis, Candida lusitaniae + Candida parapsilosis, C. glabrata + C. parapsilosis, C. parapsilosis + C. tropicalis, C. albicans + Cryptococcus, and C. krusei + Mucor. In addition, one patient had coinfection with Fusarium + Paecilomyces + Mucor. The main sites of infection for all fungal pathogens were blood (n=188, 62.0% of patients with positive blood cultures), the central nervous system (n=128, 42.2%), and lung (n=30, 9.9%).
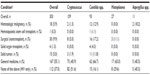
CD4 cell counts were available for 146 patients (48.2%; Table 3). The majority of these patients (n=101, 69.2%) had an absolute CD4 count <50 cells/μL. Very few patients (n=13, 8.9%) had an absolute CD4 count >200 cells/μL. The median CD4 cell count was 24 cells/μL (range 0–1,468). The median CD4:CD8 ratio was 0.1 (0.0–1.9). Median CD4 cell counts and CD4:CD8 ratios were highest in patients with Candida infections (Table 3).
  | Table 3 Immunologic and viral load status in different fungal infections |
HIV viral load counts were available for 116 patients (38.3%; Table 3). Most of these patients (n=114, 98.2%) had HIV viral loads of ≥50 copies/mL. Median HIV viral load was 92,100 copies/mL (range 0–4,306,000). Median viral loads were highest in patients with Cryptococcus and Histoplasma infections (Table 3).
Some patients presented with other underlying conditions in addition to HIV as summarized in Table 4. Overall, 30 patients underwent nontransplant surgery, 18 presented with a hematologic malignancy, 15 presented with a solid tumor, four required a solid organ transplant, and one received hematologic stem cell therapy. Of the remaining patients, 167 had conditions classified under general medicine services, and predisposing or comorbid conditions were not reported in 112 patients.
  | Table 4 Other underlying conditions |
Of all patients with HIV, those with candidiasis presented with the highest incidence of other conditions (Table 4). Sixteen patients with this IFI required surgery, 12 presented with a hematologic malignancy, 11 presented with a solid tumor, and five required either solid organ (n=4) or hematologic stem cell (n=1) transplantation. Only 15 patients with candidiasis presented with no underlying condition other than HIV.
Specific pathogens
Cryptococcosis
Cryptococcosis was the most frequently reported IFI (n=159, 52.5% of all patients). Median CD4 cell count and HIV viral load in patients with Cryptococcus infection were 17 cells/μL (range 0–391) and 117,000 copies/mL (0–4,306,000), respectively (Table 3). The CD4 count was <50 cells/μL in 77.9%, and ≤200 cells/μL in 97.4% of the patients with cryptococcosis. The main sites of infection were not mutually exclusive and included central nervous system (n=122), blood (n=83), and lung (n=11). Cryptococcus infection was diagnosed in the majority of patients by culture (n=119), antigen test (n=108), and/or histopathologic examination (n=9). On day 3, 69 patients (43.4%) with cryptococcosis had received monotherapy with either lipid-based amphotericin B (n=37), fluconazole (n=18), amphotericin B deoxycholate (n=12), or 5-fluorocytosine (n=3). An additional 67 patients (42.1%) received combination therapy with lipid-based amphotericin B and 5-fluorocytosine (n=31), amphotericin B deoxycholate and 5-fluorocytosine (n=24), or other combinations (n=12). By day 3, three patients had died, and treatment records were incomplete for the remaining 19 patients due to missing data and loss to follow-up.
Candidiasis
Candidiasis was the second most frequently reported IFI (n=93, 30.7% of all patients). The most common Candida species was C. albicans, which accounted for 45.3% of all Candida species. Median CD4 cell count and median viral load in these patients were 50 cells/μL (0–1,468) and 16,600 copies/mL (50–750,000), respectively. The CD4 count was <50 cells/μL in 48.9% and ≤200 cells/μL in 78.7% of the patients with candidiasis. The majority of cases presented as candidemia (n=80, 86.0%). Other infection sites were not mutually exclusive and included skin (n=4), abdomen (n=3), and lung (n=2). Candida infection was diagnosed by culture or tissue biopsy in all patients. On day 3, monotherapy with echinocandins (n=28) or fluconazole (n=22) were commonly used for the treatment of candidemia, but amphotericin B (lipid-based; n=5) and combination therapy (n=5) were rarely used. By day 3, nine patients had died, and treatment records were incomplete for the remaining 24 patients due to missing data and loss to follow-up.
Histoplasmosis
Histoplasmosis was the third most frequently reported IFI (n=27, 8.9% of all patients). Median CD4 cell count and median viral load in these patients were 13 cells/μL (1–38) and 110,000 copies/mL (1,580–750,000), respectively. The CD4 count was <50 cells/μL in all patients with histoplasmosis. The sites of infection were not mutually exclusive and included blood (n=20), lung (n=4), abdomen (n=3), central nervous system (n=2), and skin (n=1). The majority of cases of infection with Histoplasma were diagnosed through culture (n=24). The other three infections were diagnosed by antigen test (n=1), histopathologic examination (n=1), or both (n=1). The most common antifungal therapy used for histoplasmosis on day 3 was monotherapy with either lipid-based amphotericin B (n=12) or an azole (itraconazole, fluconazole, or voriconazole; n=6). Combination therapy was not used to treat Histoplasma infection. By day 3, two patients had died, and records were incomplete for the remaining seven patients due to missing data and loss to follow-up.
Invasive aspergillosis
Invasive aspergillosis was reported in 14 patients (4.6%), three of whom also had infections with fungal pathogens other than Aspergillus spp. Of the Aspergillus spp., the most common were A. fumigatus (78.6%) and A. flavus (18.2%). In the 11 patients with Aspergillus spp. infections only, median CD4 cell count and median viral load were 32 cells/μL (3–254) and 7,720 copies/mL (0–86,500), respectively. The CD4 count was <50 cells/μL in 71.4% and ≤200 cells/μL in 85.7% of these patients. The most common site of infection was the lung (n=9). Other sites of infection were not mutually exclusive and included central nervous system (n=2) and single cases from skin, sinus, and eye. Of the 14 Aspergillus infections, eight were proven and six were probable, and were confirmed by culture (n=11), histopathologic or cytopathologic examination (n=7), computed tomography scan (n=7), magnetic resonance imaging (n=3), and/or galactomannan antigen (n=2). On day 3, treatment records indicated that two patients received monotherapy with either lipid-based amphotericin B or voriconazole; three patients received combination therapy with voriconazole and echinocandins; or voriconazole and amphotericin B; or with amphotericin B and echinocandins. By day 3, one patient had died, and records were incomplete for the remaining five patients due to missing data and loss to follow-up.
Survival
The Kaplan–Meier plots in Figure 1 show overall survival, as well as survival for each fungal pathogen. Overall, the 30-day and 90-day survival probabilities for all patients were 0.80 and 0.72, respectively. The survival probabilities in patients with Aspergillus (0.70 at day 30 and 0.58 at day 90) and Candida (0.67 at day 30 and 0.59 at day 90) infections were lower than those observed with Histoplasma (0.84 at day 30 and 0.84 at day 90) and Cryptococcus (0.89 at day 30 and 0.81 at day 90) infections.
  | Figure 1 Ninety-day post-diagnosis survival. |
Discussion
This is the first analysis of the PATH Alliance® registry emphasizing the epidemiology and outcomes of IFIs in HIV-infected patients. Our findings highlight the occurrence of IFIs in a subset of patients with low CD4 cell counts and high viral loads despite the availability of HAART.
Most patients in the cohort were adults aged 19–64 years, male, and of African-American origin, which is consistent with the epidemiology of patients with HIV in the US.29 HIV was not well controlled in this cohort, as evidenced by the high proportions of patients with low CD4 cell counts (≤200 cells/μL) and detectable HIV viral load (≥50 copies/mL). At least 91% of patients in this cohort had AIDS by virtue of a CD4 count ≤200 cells/μL. Almost 70% had CD4 counts <50 cells/μL and were at significant risk of developing an opportunistic IFI.1,7,30 In the present analysis, the majority of patients with Cryptococcus, Histoplasma, and Aspergillus infections had extremely low CD4 counts, which is consistent with earlier reports.30–33 Cryptococcosis most often occurs with CD4 counts <50 cells/μL;30 disseminated histoplasmosis usually occurs in patients with CD4 counts <150 cells/μL;31,34 and HIV-associated aspergillosis typically occurs with CD4 counts <100 cells/μL.35 Although candidemia has also been reported in patients with HIV and very low CD4 cell counts (ranging from ~10 cells/μL36 to 125 cells/μL37), over 20% of the patients in this cohort with candidiasis had CD4 counts >200 cells/μL.
HAART has emerged as the standard of care among patients with CD4 cell counts <500 cells/μL and is associated with marked reductions in the incidence of IFIs in patients with HIV.7 Results from this query of the PATH Alliance registry suggest that the majority of HIV patients with an IFI were either not receiving or were nonadherent to HAART. However, information on antiretroviral therapy was not collected to confirm this hypothesis.
Patients with invasive candidiasis were more likely to have another condition requiring clinical services such as malignancy, nontransplant surgery, hematologic stem cell therapy, or solid organ transplantation. The presence of central venous catheters is a significant independent risk factor for candidemia in patients with HIV36–38 and it is possible that these patients were more likely to have central venous catheters due to the additional condition. However, information on the use of central venous catheter was not collected.
Culture and antigen tests were the most frequently used diagnostic tests for IFIs in this patient group. All cases of candidemia were diagnosed with traditional culture methods,39 similar to previous reports of candidemia in patients with HIV.36,37 However, cases of Candida infection in the lung or skin reported here may not be classified as IFIs as per revised 2008 guidelines.40 Diagnosis of cryptococcosis is most often made by latex agglutination test for capsular polysaccharide, and/or by blood or cerebrospinal fluid culture.41 The majority of Cryptococcus infections in this study were detected by one or both of these methods.
In general, treatment strategies adhered to current guideline recommendations. Lipid-based amphotericin B is the recommended first-line treatment for disseminated histoplasmosis30 and was administered most frequently in this cohort. Based on the results of a number of randomized clinical trials,42–45 echinocandins or fluconazole are the preferred treatments for invasive candidiasis46 and were administered most frequently in this cohort. Voriconazole is recommended for invasive aspergillosis,47 and was received by most treated patients in this cohort. Notably, voriconazole was also prescribed in combination with either amphotericin B or echinocandins in some patients. By contrast, induction therapy with amphotericin B and 5-fluorocytosine is recommended for Cryptococcus infection, but was used in fewer than half of patients with cryptococcosis.
Survival in this cohort was highest in patients with cryptococcosis and histoplasmosis. Survival in patients with HIV and cryptococcosis generally ranges from 75% to 90%,48,49 which is similar to survival reported in the present analysis. Although survival in patients with disseminated histoplasmosis is typically poor (50%–65% for Histoplasma infection),50,51 survival rates in the PATH Alliance® registry were higher. Survival in patients with invasive aspergillosis was lower compared with patients with cryptococcosis and histoplasmosis, but was better than existing data that report long-term survival rates <30%.2,3 In contrast, survival in patients with invasive candidiasis was poor and similar to previous reports of 40%–60% survival in patients with HIV.36,37
We acknowledge that our analysis has significant limitations. Although the database was designed to capture CD4 cell count and HIV viral load data, these parameters were available for <50% of patients. Furthermore, the database did not capture HIV therapy, so we were unable to analyze the presence or absence of antiretroviral therapy. However, we believe that most patients in this cohort were not adherent to antiretroviral therapy. Although the initial sample size was reasonable, analysis regarding treatment and outcomes was limited by incomplete treatment information and loss of follow-up in a significant number of patients. In addition, the 2002 guidelines of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group were used for this analysis, but when the guidelines were revised in 2008, the new guidelines could not be adopted in totality. All skin and lung Candida infections in the PATH Alliance® registry are listed in this manuscript as IFIs; however, not all cases may have histologic evidence of invasive disease, and this is also a limitation. Finally, since this was a database of patients with IFIs and not a database of HIV patients, we were unable to determine incidence and prevalence rates.
Conclusion
In the HAART era, traditional opportunistic IFIs such as cryptococcosis and histoplasmosis still occur in HIV patients with CD4 cell counts <50 cells/μL. Fungal infections remain a clinical challenge in HIV patients with severe immunosuppression. Our data also suggest that HIV patients with CD4 cell counts >200 cells/μL and other conditions may be at an increased risk for invasive candidiasis. Survival rates for HIV patients with cryptococcosis and histoplasmosis have improved compared with survival rates for invasive candidiasis and aspergillosis, which remain poor. Clinicians should remain vigilant for IFIs in HIV patients with low CD4 cell counts, even in the HAART era.
Acknowledgments
The authors are grateful for the contribution of all the PATH investigators who provided data for the PATH Alliance® registry.
Disclosure
DH has received consultancy fees/honoraria from Astellas Pharma. TM received a scholarship from the World Health Organization for a Fellowship in Clinical Research and Drug Development undertaken at Astellas Pharma. NA, SPQ, and HUMK are employees of Astellas Pharma. SH has no conflict of interest to declare. Editorial support, funded by Astellas Pharma, was provided by Radhika Bhatia and Neil M Thomas of Envision Scientific Solutions.
References
Schneider E, Whitmore S, Glynn KM, Dominguez K, Mitsch A, McKenna MT. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years – United States, 2008. MMWR Recomm Rep. 2008;57(RR-10):1–12. | |
Holding KJ, Dworkin MS, Wan PC, et al. Aspergillosis among people infected with human immunodeficiency virus: incidence and survival. Adult and Adolescent Spectrum of HIV Disease Project. Clin Infect Dis. 2000;31(5):1253–1257. | |
Mylonakis E, Barlam TF, Flanigan T, Rich JD. Pulmonary aspergillosis and invasive disease in AIDS: review of 342 cases. Chest. 1998;114(1):251–262. | |
Hogg RS, Heath KV, Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279(6):450–454. | |
Palella FJ Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. | |
Jones J, Hanson D, Dworkin M, et al. Surveillance for AIDS-defining opportunistic illnesses, 1992–1997. MMWR Surveill Summ. 1999;48(2):1–22. | |
Kaplan JE, Hanson D, Dworkin MS, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30 Suppl 1:S5–S14. | |
Buchacz K, Baker RK, Palella FJ Jr, et al. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS. 2010;24(10):1549–1559. | |
Aberg J, Powderly W. HIV: primary and secondary prophylaxis for opportunistic infections. Clin Evid (Online). 2010;2010:0908. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3217757/. Accessed August 21, 2013. | |
Anaissie EJ. Trial design for mold-active agents: time to break the mold – aspergillosis in neutropenic adults. Clin Infect Dis. 2007;44(10):1298–1306. | |
Baddley JW, Winthrop KL, Patkar NM, et al. Geographic distribution of endemic fungal infections among older persons, United States. Emerg Infect Dis. 2011;17(9):1664–1669. | |
Horn DL, Neofytos D, Anaissie EJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the Prospective Antifungal Therapy Alliance Registry. Clin Infect Dis. 2009;48(12):1695–1703. | |
Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006:overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50(8):1091–1100. | |
Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48(3):265–273. | |
Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50(8):1101–1111. | |
Park BJ, Pappas PG, Wannemuehler KA, et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg Infect Dis. 2011;17(10):1855–1864. | |
Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005;41(9):1232–1239. | |
Azie N, Neofytos D, Pfaller M, Meier-Kriesche HU, Quan SP, Horn D. The PATH (Prospective Antifungal Therapy) Alliance® registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis. 2012;73(4):293–300. | |
Davis JA, Horn DL, Marr KA, Fishman JA. Central nervous system involvement in cryptococcal infection in individuals after solid organ transplantation or with AIDS. Transpl Infect Dis. 2009;11(5):432–437. | |
Fishman JA, Horn D, Marr KA, et al. Preliminary results from the Prospective Antifungal Therapy Alliance (PATH AllianceTM). Proceedings of the 43rd Annual Meeting of The Infectious Diseases Society of America, October 6–9, 2005; San Francisco, CA, USA. | |
Horn DL, Fishman JA, Steinbach WJ, et al. Presentation of the PATH Alliance registry for prospective data collection and analysis of the epidemiology, therapy, and outcomes of invasive fungal infections. Diagn Microbiol Infect Dis. 2007;59(4):407–414. | |
Horn DL, Neofytos D, Fishman J, et al. Use of the PATH Alliance database to measure adherence to IDSA guidelines for the therapy of candidemia. Eur J Clin Microbiol Infect Dis. 2007;26(12):907–914. | |
Horn DL, Sae-Tia S, Neofytos D. Aspergillus osteomyelitis: review of 12 cases identified by the PATH Alliance Registry. Diagn Microbiol Infect Dis. 2009;63(4):384–387. | |
Klevay MJ, Horn DL, Neofytos D, Pfaller MA, Diekema DJ. Initial treatment and outcome of Candida glabrata versus Candida albicans bloodstream infection. Diagn Microbiol Infect Dis. 2009;64(2):152–157. | |
Neofytos D, Horn DL, Anaissie E, et al. PATH Alliance Registry: review of invasive fungal infections in 217 stem cell recipients. Proceedings of the 45th Annual Meeting of the Infectious Diseases Society of America, October 4–7, 2007; San Diego, CA, USA. | |
Olyaei AJ, Strasfeld L, Horn DL, et al. Dosing of antifungal therapy in dialysis dependant patients. Proceedings of the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/Infectious Diseases Society of America 46th Annual Meeting, October 25–28, 2008; Washington, DC, USA. | |
Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34(1):7–14. | |
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. | |
Centers for Disease Control and Prevention. Diagnoses of HIV infection and AIDS in the United States and dependent areas. HIV Surveillance Report 2011. Available from: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/. Accessed 21 August, 2013. | |
Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009; 58(RR-4):1–207. | |
Gutierrez ME, Canton A, Sosa N, Puga E, Talavera L. Disseminated histoplasmosis in patients with AIDS in Panama: a review of 104 cases. Clin Infect Dis. 2005;40(8):1199–1202. | |
Huang L, Crothers K. HIV-associated opportunistic pneumonias. Respirology. 2009;14(4):474–485. | |
Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807–825. | |
Wheat LJ, Connolly-Stringfield PA, Baker RL, et al. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine (Baltimore). 1990;69(6):361–374. | |
Wallace JM, Lim R, Browdy BL, et al. Risk factors and outcomes associated with identification of aspergillus in respiratory specimens from persons with HIV disease. Pulmonary Complications of HIV Infection Study Group. Chest. 1998;114(1):131–137. | |
Launay O, Lortholary O, Bouges-Michel C, Jarrousse B, Bentata M, Guillevin L. Candidemia: a nosocomial complication in adults with late-stage AIDS. Clin Infect Dis. 1998;26(5):1134–1141. | |
Tumbarello M, Tacconelli E, de Gaetano Donati K, Morace G, Fadda G, Cauda R. Candidemia in HIV-infected subjects. Eur J Clin Microbiol Infect Dis. 1999;18(7):478–483. | |
Bertagnolio S, de Gaetano Donati K, Tacconelli E, et al. Hospital-acquired candidemia in HIV-infected patients. Incidence, risk factors and predictors of outcome. J Chemother. 2004;16(2):172–178. | |
Bodey G, Anaissie E, Edwards JJ. Definitions of candida infections. In: Bodey G, editor. Candidiasis: Pathogenesis, Diagnosis and Treatment. 2nd ed. New York, NY, USA: Raven Press; 1993. | |
De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–1821. | |
Warkentien T, Crum-Cianflone NF. An update on cryptococcus among HIV-infected patients. Int J STD AIDS. 2010;21(10):679–684. | |
Kullberg BJ, Sobel JD, Ruhnke M, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet. 2005;366(9495):1435–1442. | |
Kuse ER, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369(9572):1519–1527. | |
Pappas PG, Rotstein CM, Betts RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45(7):883–893. | |
Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356(24):2472–2482. | |
Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–535. | |
Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(3):327–360. | |
Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. AIDS. 2007;21(16):2119–2129. | |
Lortholary O, Poizat G, Zeller V, et al. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS. 2006;20(17):2183–2191. | |
Tobon AM, Agudelo CA, Rosero DS, et al. Disseminated histoplasmosis: a comparative study between patients with acquired immunodeficiency syndrome and non-human immunodeficiency virus-infected individuals. Am J Trop Med Hyg. 2005;73(3):576–582. | |
Baddley JW, Sankara IR, Rodriquez JM, Pappas PG, Many WJ Jr. Histoplasmosis in HIV-infected patients in a southern regional medical center: poor prognosis in the era of highly active antiretroviral therapy. Diagn Microbiol Infect Dis. 2008;62(2):151–156. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.