Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 13
Clinical Characteristics and Outcome of Pediatric COVID-19 Patients in Ethiopia During the Early COVID-19 Pandemic: A Prospective Cohort Study
Authors Weldetsadik AY , Abayneh M, Abraha M, Sirgu S, Bekele D
Received 21 January 2022
Accepted for publication 28 April 2022
Published 5 May 2022 Volume 2022:13 Pages 165—174
DOI https://doi.org/10.2147/PHMT.S359333
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Abate Yeshidinber Weldetsadik,1 Mahlet Abayneh,1 Mebratu Abraha,2 Sisay Sirgu,3 Delayehu Bekele4
1Department of Pediatrics and Child Health, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia; 2Research Directorate, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia; 3Department of Internal Medicine, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia; 4Department of Obstetrics and Gynecology St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia
Correspondence: Abate Yeshidinber Weldetsadik, Department of Pediatrics and Child Health, St Paul’s Hospital Millennium Medical College, PO. Box 1271, Addis Ababa, Ethiopia, Tel +251 911993975, Email [email protected]
Introduction: Most previous pediatric COVID-19 studies reported milder disease in children. However, there are limited pediatric data from low-income settings. We aimed to assess the characteristics and outcomes of pediatric COVID-19 in Ethiopia.
Setting: St. Paul’s COVID-19 treatment center; a tertiary COVID-19 center. Pediatric care was provided in a dedicated ward but with a common ICU.
Methods: St. Paul’s Hospital COVID-19 cohort (SPC-19) included inpatient COVID-19 RT-PCR confirmed cases from August 2020 to January 2021. Data were extracted from case report forms attached to patient charts and completed by the clinicians. Data were uploaded into the Redcap database and exported to SPSS 20 for analysis. Binary logistic regression and chi-square test were used in the analysis.
Results: Seventy-nine patients 0– 19 years were included from the SPC-19 cohort over 6 months. Pediatric admissions accounted for 11% of cases in the cohort. The mean age (SD) was 6.9 (± 6.36) years and 40 (50.6%) were female. The disease was asymptomatic or mild in 57 (72.2%), moderate in 15 (19%), and severe or critical in 7 (8.8%). The commonest presentations in symptomatic children were prostration (26.6%) followed by vomiting (12.7%), fever and cough (11.4% each), and dyspnea (10%). About 53 (67%) children had multimorbidity, and 14 (17.7%) children died. All deaths were in children with comorbidities with tuberculosis and malignancy being associated with 43% of deaths. Nearly 5% of children reported long-COVID symptoms highlighting the need for prolonged follow-up in those children.
Conclusion: Despite lower admissions and severity, high mortality and morbidity was documented in our pediatric cohort. The presence of comorbidity and inadequate care organization likely contributed to high mortality. COVID-19 centers of low-income settings should emphasize optimizing the care of children with COVID-19 and multimorbidity, and vaccination should be considered in those children to prevent high morbidity and mortality.
Keywords: child, inpatient, COVID-19, cohort studies, multimorbidity, Ethiopia
Introduction
Just in 2 years of the COVID-19 pandemic, more than 326 million people were infected, and more than 5.5 million died worldwide as of January 17, 2022.1 Despite the tremendous effect, COVID-19 has brought on our lives, the direct health effect of COVID −19 in children has initially followed a relatively benign course, with children contributing only 5% of the affected population.2,3 However, COVID-19 is expected to have more lasting and serious indirect consequences on child health.4,5
COVID-19 pandemic was documented to affect children differently from adults, most reporting milder disease in children.2,3 Reports from adult patients were mild in 80% with 14% severe and 5% critical cases2 and an overall case fatality rate of 2–3%. Mortality was much higher at 10.5% in patients with comorbidities and 49.0% in those with a critical illness.2 Contrary to this, COVID-19 caused mainly asymptomatic and mild-to-moderate disease in more than 90% and severe or critical illness in <5% children.6
COVID-19 was characterized by the occurrence of previously undocumented new symptoms and complications as the pandemic epicenter shifted from China to Europe and the US. Multi-system Inflammatory Syndrome of Childhood related to COVID-19 (MIS-C) was initially reported from Europe,7 followed by the US8 and subsequently from other countries including Ethiopia.9
The serious conditions associated with COVID-19 in children are severe pneumonia with or without ARDS and sepsis mainly as acute COVID-19, and MIS-C as a post-infectious late complication.2,6–9 While comorbidities are important predictors of mortality and morbidity in adults, there is scarce data in children with inconsistent results.10,11 Lower mortality was reported in children from 29 high-income countries associated with COVID-1912 including in children with MIS-C (2–3%).13 However, mortality from COVID-19 may be higher in LMICs where care may be less well organized, the health system is easily overwhelmed, and pediatric intensive care units (PICUs) may not be available or under-functioning.14–16 There is also lesser use of available services by sick children during a pandemic.17
To date, most data on pediatric COVID-19 came from high- and middle-income countries with limited data from low- and middle-income countries (LMICs), especially from Africa.10,11,14,16 However, despite early similar pediatric results,14 clinical presentations and outcomes may differ and are not well understood in LMICs where predictors and therapeutic approaches differ from the origin of those previous reports. Children in LMICs had different environmental and multifaceted health factors with nutritional deprivation and dominantly prevalent infectious disease including TB and HIV.18–21
Ethiopia reported its first case of COVID-19 on March 13, 2020, and documented more than 450,000 infections and 7000 deaths as of January 17, 2022.22 Similar to other low-income settings, little is known about COVID-19 in children in Ethiopia. A pre-print that included 90 children from Addis Ababa showed less severe disease with no mortality, but the children were older age, less ill, and with no comorbidity that it was not representative of all COVID-19 sick children.23 High COVID-19 vulnerability and regional differences are documented in Ethiopia so that close monitoring and follow-up are required to mitigate the many effects of COVID-19.24 COVID-19 also impacts the mental and psychological health of patients, families, and health care providers tremendously.25–27 Recent studies have documented those negative health impacts from isolation and quarantine.25 Significant worsening of mental disorders including depression, anxiety, posttraumatic stress disorders, and suicide in physicians was also noted during the COVID-19 pandemic.26,27
As systematically collected data from COVID-19 treatment centers of LMICs is important in addressing the gap, our prospective cohort was designed to unravel the epidemiology, clinical pictures, outcome, and predictors of outcome in children with COVID-19 in Ethiopia.
Materials and Methods
Study Setting
St. Paul’s hospital, one of the biggest hospitals with a medical college in the country, established a COVID-19 treatment center in May 2020 in collaboration with the Federal Ministry of Health. It organized more than 350 beds, 14 intensive care and 28 high dependency unit beds with mechanical ventilators and renal replacement therapy. The pediatric unit of the COVID-19 hospital dedicated five neonatal and 22 pediatric beds but critical patients cared in the same ICU/HDU with adults based on bed availability. The center used to admit all types of patients including asymptomatic COVID-19 cases for observation.
Study Design
A prospective cohort was done over 6 months from August 2020 to January 2021.
Study Population
The study included all RT-PCR confirmed COVID-19 children and adolescents admitted to our COVID-19 treatment center during the study period.
Data Collection Method
Data collection was prospective, starting from the day of enrollment till the end date of follow-up. The end date of the follow-up (censoring) was day 90 after discharge or the day participant died if the child died while under care or follow-up. At admission, the managing physician and nurse informed the patients/and guardians about the SPC19 cohort study and obtained informed consent.
A case reporting form (CRF) was attached to the medical charts of each patient and completed by the medical providers. The research nurse then extracted these data from the CRFs into the Redcap-based electronic database on tablets. The data collectors synced their tablets daily to a central SPHMMC data server.
The study collected 1) baseline data, including, patient demographic data, vital signs, physical examination findings, diagnostic laboratory tests, and imaging findings, 2) inpatient follow-up data, including, the clinical course of illness and investigation results; 3) post-discharge follow-up data collected by phone calls at day 14, 28 and 90 days after discharge.
Data Collection Tool
The tools were adopted from the WHO acute respiratory infection clinical characterization data tool CRF and customized to the local context. Mild, moderate, and severe diseases were defined as the WHO case definition of SARS.28
Data Analysis
Data from the central database was exported into SPSS version 20 statistical packages for windows, for analysis. Descriptive statistics: Frequencies, percentages, cross tabs, and graphs were used to describe the patient’s characteristics, proportions of cases, and describe case patterns. Analytical techniques: Simple and multiple binary logistic regressions were used to see the predictors of outcome in children with COVID-19 in our cohort.
Results
Socio-Demographic Characteristics of Pediatric COVID-19 Patients
Among 700 COVID-19 patients in the SPC-19 cohort, 79 (11.3%) were children and adolescents 0–19 years of age. The mean age (SD) was 6.9 (±6.36) years and 40 (50.6%) were female. Most, 38 (48%), children were under-five with 18 (22.8%) infants (4 of them were neonates), 20 (25%) were 1–5 years, 24 (30.4%) were 6–12 years, and the remaining 17 (22%) were adolescents from 13 to 19 years. Most were from Addis Ababa (33%) and Oromia (28%) regions.
Clinical Presentation and Disease Severity
While most were asymptomatic or only mildly symptomatic, the commonest presentation in the symptomatic children was prostration (26.6%) followed by vomiting (12.7%), fever and cough (11.4% each), and dyspnea (10%) (Tables 1 and 2). Physical exam revealed delayed capillary refill time (CRT > 2 seconds) in 9 (11.4%) and Spo2 < 92 in 7 (8.8%) children. Systemic hypertension defined by either the systolic or diastolic BP was found in 10 (12.6%) children throughout the courses of their illness (Table 3).
 |
Table 1 Clinical Symptoms Related Characteristics of COVID-19 Patients Admitted at SPHMMC COVID-19 Treatment Center, 2021 |
 |
Table 2 WHO Severity Classification: Cohort of Pediatric COVID-19, SPHMMC |
 |
Table 3 Summary of Selected Laboratory Abnormalities in Children with COVID-19 Infection Admitted to SPHMMC, Aug 2020-Jan 2021 |
The presentation was asymptomatic or mild in 57 (72.2%), moderate in 15 (19%), and severe and critical in 7 (8.8%) (Table 2). About 54 (68.4%) children had comorbid disease unrelated to COVID-19, and a total of 14 (17.7%) children died during the study period.
Laboratory Abnormalities
Only sick children (40 (50.6%)) had investigations based on clinical indications at the discretion of the treating physician. Hematologic abnormalities were the most common with lymphopenia documented in more than half of those children. Neutrophilia and anemia were also commonly found with a rate of almost 2 in 5 children (Table 4). However, there was no imaging studies in our cohort of children despite the presence of indications.
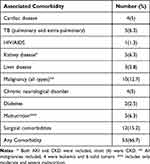 |
Table 4 Clinical Comorbid Illness-Related Characteristics of COVID-19 Patients Admitted at SPHMMC COVID-19 Treatment Center, 2021 |
Comorbidities
About 53 (67%) children had comorbid disease unrelated to COVID-19. Surgical comorbidities, Malignancy, malnutrition, tuberculosis, and renal disease were the commonest multimorbidities in those children admitted with COVID-19 (Table 5).
 |
Table 5 Factors Affecting Outcome of COVID-19 Children from Multiple Regression, SPHMMC |
Treatment and Outcome
Most children did not receive specific treatment other than observation and supportive care. Antibiotics,21 antipyretics,7 and steroids6 were the commonly prescribed medications. Antifungal1 and anticoagulation1 were rarely used, and no antivirals were used at all. Specific medications were provided based on the underlying diagnosis including anti-TB and cardiovascular drugs for tuberculosis and cardiac patients, respectively (Table 5). Only six children were admitted to the ICU because of limited bed availability, although all the severe and critical patients were eligible for admission, based on the center’s practice. Among those admissions to the ICU, Mechanical Ventilation (MV),1 Non-Invasive Ventilation (NIV),1 prone position,2 and renal replacement therapy1 were provided in addition to the continuation of care started on the floor. Inotropes (adrenaline or dopamine) use was not documented in any of the patients.
A total of 9 (11.4%) in-hospital deaths and 5 post-discharge deaths occurred in the first 2 weeks after hospital discharge with a total of 14 (17.7%) deaths in the cohort. Although it is difficult to ascertain the exact causes of death after discharge, COVID-19 and the underlying disease may have contributed to mortality. Three post-discharge deaths had acute leukemia with complications likely playing the main role for mortality, while the other two had malnutrition and pancytopenia of an undetermined cause whose mortality is possibly driven by COVID-19. A total of 18 (22.8%) serious complications occurred in the cohort. The complications were bacterial pneumonia (in 7, 1 microbiologically confirmed), severe ARDS,3 pleural effusion,1 seizure,2 stroke,1 gastrointestinal bleeding,2 pancreatitis,1 and hypoglycemia.1 Advanced post-discharge care (including oxygen supplementation and dialysis) was required in 14 (17.7%) of the discharged children. Among the survivors in the cohort, almost 5% complain of persistent symptoms including fatigue, fever, cough, joint pain, headache and other non-specific symptoms (long COVID) till the end of the 3 months follow-up.
Predictors of Outcome
Bivariate analysis showed significant association of ICU admission (p- 0.03, COR, 8.25, 95% C.I 1.25, 55.56), disease severity (p 0.002, COR-17.5, 95% C.I: 2.94, 104), and presence of comorbidity (p 0.00038, X2=12.6) with mortality. However, multivariate logistic regression of socio-demographic, clinical, and laboratory variables revealed only disease severity (AOR 8.48, 95% CI 1.27, 56.53) was an independent predictor of survival in our cohort of COVID-19 children and adolescents (Table 5).
Discussion
Pediatric COVID-19 contributed to more than 10% of COVID-19 hospital admissions in our cohort. We also documented slightly higher cases of severe and critical cases (9%) compared to previous studies.2,3 Additionally, there was a very high comorbidity (67%) and mortality (18%) rates unlike data from most previous studies.2,3,11,12,23 These differences, however, may also be affected by the limited availability of pediatric COVID-19 centers, and sick and high-risk patients are likely to be admitted to a tertiary center and are not representative of the general pediatric population. Similar to ours, a recently published multi-country sub-Saharan study on MIS-C and a case serious from Algeria documented very high mortality in children with multimorbidity and malignancy and COVID-19.16,29
Clinical Presentation and Treatment
Most of our patients were asymptomatic or with mild symptoms similar to previous pediatric data.6 The main presenting symptoms of symptomatic children are similar to other studies, with prostration, fever, cough, vomiting, and shortness of breath being the main manifestations.11,23,30 Only a few children had laboratory studies determined, and the commonest abnormalities were lymphopenia and anemia, similar to previous studies in children and adults.31 However, CRP, PCT, and other markers were not available regularly in the center and we could not document them. Additionally, imaging studies are not done for all patients and thus we could not include data on imaging of those children.
Treatment was mainly supportive with medication use only in moderate and severe illnesses. ICU admission was not possible for all with indication because of bed unavailability and as a result, the use of ICU care modalities including MV, NIV, and inotropes remain low despite the very high documented mortality. There was low steroid utilization (7.5%) in our cohort possibly as it was an early COVID-19 study before the RECOVERY trial result was available32 and likely absence of indications in most others. Antibiotics were given to all severe and critically sick and a few moderately sick children, but the bacterial infection was rarely confirmed. There was no use of hydroxychloroquine, antivirals, or other drugs with compassionate indication, and monoclonal antibodies and new antivirals were not available.
Outcome
High child mortality is documented in LMICs from any childhood diseases, and COVID-19 is not an exception as shown here in our study.33 Clinical care in LMICs is usually not well organized and unaffordable, the health system is easily overwhelmed, and PICUs may not be available or under-functioning. Mortality is high from any disease in LICs and COVID-19 mortality also is expected to be higher as a result,14–16,30 and lesser use of available services by sick children was also documented during the pandemic.17 The few but critically sick COVID-19 children with high multimorbidity likely contributed to the high mortality. As a result, COVID-19 centers in LICs must emphasize optimization of the care of children with COVID-19 and multimorbidity. Although all the deaths were in children with comorbidities, the multivariable analysis did not show comorbidity as a significant factor. While this could be related to the increased severity in those with comorbidity in our children, a similar Indian study also did not show an association between pediatric comorbidities with severity and mortality.10 Contrary to this, a large Brazilian study documented increased mortality in children with comorbidity.11 Although the differences may originate from unraveled factors, the small sample size in our and Indian studies should be noted while interpreting the result, and our data also trends towards significance in the bivariate analysis.
A striking finding of our cohort is the very high post-discharge mortality (35% of overall deaths) after discharge; most in the first 2 weeks and all by 4 weeks’ post-discharge with no death from 1 to 3 months after hospital discharge. This highlights the need to optimize post-discharge clinical care with continuation of health care, and to follow more stringent discharge criteria to avoid early and immediate post-discharge deaths, especially in the presence of multimorbidity.
Determinants of outcome were disease severity, age <12 months, ICU admission, and presence of comorbidity on bivariate analysis, but only disease severity persisted to be significant on multivariate analysis. This is consistent with the Brazilian and other studies including the recent sub-Saharan multi-country study where age, severity, and comorbidity were the determinants of mortality.11,16,19,29
It has been predicted earlier that the indirect effects of COVID-19 in child health may be worse than its direct effect, especially in LMICs, as efforts are shifted to handling the pandemic in the already constrained health system with limited capacity.4,34 However, our result and others also inform the significant direct health effect with high morbidity and mortality in children of LMICs, especially in those with comorbidities.29 It is thus important not to underestimate the effect of COVID-19 in children of LMICs.5,16 In this scenario, it also is critical not to lose the gained success in neonatal and child mortality because of the COVID-19 pandemic.14,21,35 As children in LMICs are among the most vulnerable population, more far-reaching susceptibility and risk factors should be taken into consideration and addressed properly.24,34,35 The high rate of malnutrition and chronic infectious diseases like TB, and sub-optimal care of children with chronic illness including malignancies are imminent challenges for the health system of LMICs already functioning at its limit.20 Maintaining and improving the quality of care will also be challenged even more under these circumstances, and require special emphasis and strengthening of the health care system.20,33,35
Long-COVID
We documented symptoms of long-COVID in nearly 5% of children. Long COVID was reported to be a rare condition with a low burden in UK children despite a potential underestimation.36 This, however, is contrary to reports from adults where long COVID is highly prevalent. A recent large cohort reported 57% of COVID-19 adult patients to have one or more symptoms with 37% persisting up to 3–6 months.37
Limitations and Strength
Our study is limited by the small number of patients in the cohort, and may not be representative of all groups of children as most were with multimorbidities. Additionally, the characterization of symptomatic children was further limited by a lack of chest imaging. However, we were able to collect detailed data from patients, and the prospective cohort enabled us to see the relatively longer period outcome and symptom persistence and resolution of long-COVID.
Conclusion
Despite a relatively low rate of pediatric admissions and severity, high in-hospital and post-discharge mortality was documented in our cohort of COVID-19 children. The presence of comorbidity, and inadequate care organization for the few but critically sick children likely contributed to the high mortality. COVID-19 centers in LMICs should emphasize optimizing the care of children with COVID-19 and multimorbidity, and provision of HDU/ICU care separately or in adult ICUs as appropriate for their setting should be strongly considered. Long COVID, on the other hand, is a relatively rare occurrence. As less attention is given to this age group concerning vaccination, because of the widespread belief that they are not significantly affected by COVID-19, the high mortality and morbidity trend in children from LMICs should make us prioritize the prevention of COVID-19 in children with vaccination. We thus emphasize that pediatric COVID-19 is not as benign as we have thought, as demonstrated also from a recent multi-country sub-Saharan Africa.29
Abbreviations
ARDS, acute respiratory distress syndrome; BP, blood pressure; COVID-19, corona virus disease caused by the SARS-CoV-2 virus-2019; CRF, case report form; CRP, C-reactive protein; HDU/ICU, high dependency unit/intensive care unit; LMICs, low- and middle-income countries; MIS-C, multi-system inflammatory syndrome of childhood related to COVID-19; MV, mechanical ventilation; NIV, non-invasive ventilation; RR, respiratory rate; RT-PCR, reverse transcriptase polymerase chain reaction; PCT, procalcitonin; PICU, pediatric intensive care unit; PR, pulse rate; SD, standard deviation; SPC-19, St. Paul’s Hospital COVID-19 prospective cohort; SPHMMC, St. Paul’s Hospital Millennium Medical College; SPO2, saturation of oxygen measured by pulse-oximetry; WHO, World Health Organization.
Data Sharing Statement
Data is available with a reasonable request from the corresponding author.
Ethics Approval and Informed Consent
The study was approved by SPHMMC IRB. Informed consent was taken from parents or legal guardians of each participants and the study was done per the approved protocol and based on the principles of the Helsinki declaration.
Acknowledgments
We thank SPHMMC for funding the research and all participating patients and research team who worked in those difficult times tirelessly both in the clinical care as well as this research.
Author Contributions
All authors made a significant contribution to the study including in the conception, study design, execution, acquisition of data, analysis and interpretation; revising and critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was funded by SPHMMC. The funding institution has no role in data collection or writing of the research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. WHO coronavirus (COVID-19) dashboard [Internet]. Geneva: World Health Organization; 2022. Available from: https://covid19.who.int/.
2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi:10.1001/jama.2020.2648
3. Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–1095. PMID: 32202343; PMCID: PMC7228328. doi:10.1111/apa.15270
4. Roberton T, Carter ED, Chou VB, et al. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8(7):e901–e908. PMID: 32405459; PMCID: PMC7217645. doi:10.1016/S2214-109X(20)30229-1
5. Simba J, Sinha I, Mburugu P, et al. Is the effect of COVID-19 on children underestimated in low- and middle- income countries? Acta Paediatr. 2020;109(10):1930–1931. PMID: 32557761; PMCID: PMC7323043. doi:10.1111/apa.15419
6. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. doi:10.1542/peds.2020-0702
7. Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children: a systematic review. E Clin Med. 2020;26:100527. doi:10.1016/j.eclinm.2020.100527
8. Son MBF, Murray N, Friedman K, et al. Multisystem inflammatory syndrome in children - initial therapy and outcomes. N Engl J Med. 2021;385(1):23–34. doi:10.1056/NEJMoa2102605
9. Alemayehu T, Karibian A, Mekonnen D. A first case of Pediatric Inflammatory Multisystem Syndrome Temporally associated with SARS CoV 2 (PIMS-TS) from Ethiopia: case report and a review of literature. Ethiop Med J. 2021;59(2):177–180.
10. Kapoor D, Kumar V, Pemde H, Singh P. Impact of comorbidities on outcome in children with COVID-19 at a tertiary care pediatric hospital. Indian Pediatr. 2021;58(6):572–575. doi:10.1007/s13312-021-2244-0
11. Oliveira EA, Colosimo EA, Simões E, et al. Clinical characteristics and risk factors for death among hospitalised children and adolescents with COVID-19 in Brazil: an analysis of a nationwide database. Lancet Child Adolesc Health. 2021;5(8):559–568. doi:10.1016/S2352-4642(21)00134-6
12. Islam N, Shkolnikov VM, Acosta RJ, et al. Excess deaths associated with covid-19 pandemic in 2020: age and sex disaggregated time series analysis in 29 high income countries. BMJ. 2021;373:n1137. doi:10.1136/bmj.n1137
13. Shioji N, Aoyama K, Englesakis M, Annich G, Maynes JT. Multisystem inflammatory syndrome in children during the coronavirus disease pandemic of 2019: a review of clinical features and acute phase management. J Anesth. 2021;35(4):563–570. doi:10.1007/s00540-021-02952-6
14. Chaziya J, Freyne B, Lissauer S, et al. COVID-19 in Malawi: lessons in pandemic preparedness from a tertiary children’s hospital. Arch Dis Child. 2021;106(3):238–240. doi:10.1136/archdischild-2020-319980
15. Klingenberg C, Tembulkar SK, Lavizzari A, et al. COVID-19 preparedness-a survey among neonatal care providers in low- and middle-income countries. J Perinatol. 2021;41(5):988–997. doi:10.1038/s41372-021-01019-4
16. Arous R, Djillali IS, Rouis NO, et al. High mortality of COVID-19 in children with cancer in a single center in Algiers, Algeria. Pediatr Blood Cancer. 2021;68(6):e28898. doi:10.1002/pbc.28898
17. Abebe W, Worku A, Moges T, et al. Trends of follow-up clinic visits and admissions three-months before and during COVID-19 pandemic at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia: an interrupted time series analysis. BMC Health Serv Res. 2021;21(1):731. doi:10.1186/s12913-021-06730-8
18. Van der Zalm MM, Lishman J, Verhagen LM, et al. Clinical experience with severe acute respiratory syndrome coronavirus 2-related illness in children: hospital experience in Cape Town, South Africa. Clin Infect Dis. 2021;72(12):e938–e944. doi:10.1093/cid/ciaa1666
19. Ghisolfi S, Almås I, Sandefur JC, von Carnap T, Heitner J, Bold T. Predicted COVID-19 fatality rates based on age, sex, comorbidities and health system capacity. BMJ Glob Health. 2020;5(9):e003094. doi:10.1136/bmjgh-2020-003094
20. Zar HJ, Dawa J, Fischer GB, Castro-Rodriguez JA. Challenges of COVID-19 in children in low- and middle-income countries. Paediatr Respir Rev. 2020;35:70–74. doi:10.1016/j.prrv.2020.06.016
21. UNICEF Ethiopia. The impact of COVID-19 on Children in Addis Ababa, Ethiopia. Ethiopia:UNICEF [Internet]; 2022. Available from: https://www.unicef.org/ethiopia/documents/impact-covid-19-children-addis-ababa-ethiopia.
22. World Health Organization. WHO Coronavirus (COVID-19) Dashboard [Internet]. Geneva: World Health Organization; 2022. Available from: https://covid19.who.int/region/afro/country/et.
23. Leulseged TW, Hassen IS, Maru EH, et al. COVID-19 in hospitalized Ethiopian children: characteristics and outcome profile (pre-print). medRxiv. 2020;41. doi:10.1101/2020.10.30.20223115
24. Alene KA, Gelaw YA, Fetene DM, et al. COVID-19 in Ethiopia: a geospatial analysis of vulnerability to infection, case severity and death. BMJ Open. 2021;11:e044606. doi:10.1136/bmjopen-2020-044606
25. Jain A, Bodicherla KP, Raza Q, Sahu KK. Impact on mental health by “Living in Isolation and Quarantine” during COVID-19 pandemic. J Fam Med Prim Care. 2020;9(10):5415. doi:10.4103/jfmpc.jfmpc_1572_20
26. Jolly TS, Pandian GSDB, Batchelder E, Jain A. Posttraumatic stress disorder exacerbation as a result of public masking in times of COVID-19. Prim Care Companion CNS Disord. 2020;22(6):27191. doi:10.4088/PCC.20l02828
27. Laboe CW, Jain A, Bodicherla KP, Pathak M. Physician suicide in the era of the COVID-19 pandemic. Cureus. 2021;13(11):e19313.
28. World Health Organization. WHO case report form. [Internet]. Geneva: World Health Organization; 2022. Available from: https://www.who.int/publications/i/item/global-covid-19-clinical-platform-case-report-form-(crf)-for-post-covid-conditions-(post-covid-19-crf-).
29. Nachega JB, Sam-Agudu NA, Machekano RN, et al. Assessment of clinical outcomes among children and adolescents hospitalized with COVID-19 in 6 Sub-Saharan African Countries. JAMA Pediatr. 2022;176:e216436. doi:10.1001/jamapediatrics.2021.6436
30. Irfan O, Muttalib F, Tang K, et al. Clinical characteristics, treatment and outcomes of paediatric COVID-19: a systematic review and meta-analysis. Arch Dis Child. 2021;106:440–448. doi:10.1136/archdischild-2020-321385
31. Bergamaschi G, Borrelli de Andreis F, Aronico N, et al. Anemia in patients with covid-19: pathogenesis and clinical significance [published correction appears in Clin Exp Med. 2021. Clin Exp Med. 2021;21(2):239–246. doi:10.1007/s10238-020-00679-4
32. Horby P, Lim WS, Emberson JR, et al.; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693–704. doi:10.1056/NEJMoa2021436
33. GBD 2015 Child Mortality Collaborators. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortalities, 1980–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1725–1774. doi:10.1016/S0140-6736(16)31575-6
34. Gashaw T, Hagos B, Sisay M. Expected impacts of COVID-19: considering resource-limited countries and vulnerable population. Front Public Health. 2021;9:614789. doi:10.3389/fpubh.2021.614789
35. Estifanos AS, Kazmi K, Morris SK. Could COVID-19 reverse the modest gains made in newborn health in Ethiopia? Matern Child Health J. 2021;25(6):849–854. PMID: 33942230; PMCID: PMC8091985. doi:10.1007/s10995-021-03175-7
36. Molteni E, Sudre CH, Canas LS, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2 [published correction appears in Lancet Child Adolesc Health. 2021 Aug 31;]. Lancet Child Adolesc Health. 2021;5(10):708–718. doi:10.1016/S2352-4642(21)00198-X
37. Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9):e1003773. doi:10.1371/journal.pmed.1003773
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
