Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Clinical Characteristics and Management of Cerebral Venous Sinus Thrombosis in Patients with Essential Thrombocythemia
Authors Jiao L , Huang X , Fan C, Zhao H, Li Z, Shen H , Chen J, Duan J
Received 5 December 2020
Accepted for publication 1 April 2021
Published 22 April 2021 Volume 2021:17 Pages 1195—1206
DOI https://doi.org/10.2147/NDT.S294712
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Lidong Jiao,1 Xiaoqin Huang,1 Chunqiu Fan,1 Hong Zhao,2 Zhen Li,3 Huixin Shen,1 Jian Chen,4 Jiangang Duan5
1Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Hematology, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Department of Ophthalmology, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 4Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 5Department of Emergency, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Xiaoqin Huang
Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, 100053, People’s Republic of China
Tel +86 10-83198899– 8319
Email [email protected]
Background and Objective: Essential thrombocythemia (ET) is a rare cause of cerebral venous sinus thrombosis (CVST). Analysis of the risk factors and treatment therapies of CVST in ET has yielded controversial findings.
Subjects and Methods: We retrospectively investigated the clinical characteristics of CVST events in ET and compared baseline characteristics, causative factors, hematological effects, and treatments between ET patients with and without CVST.
Results: Overall, 91 of 115 patients who met the ET diagnosis were included in this study. Among them, 23 (25.27%) patients met the diagnostic criteria of ET with CVST for inclusion, 14 (60.87%) of whom were females, with a median age of 34 (range 25– 50). CVST diagnosis was made concomitantly to ET in 19 patients (82.61%). The most common symptom and sites of thrombosis of CVST was an acute or subacute headache and sigmoid sinuses, respectively. Compared with ET patients without CVST, ET patients with CVST were significantly younger (37.65± 14.45 vs 60.93± 13.46, P< 0.001) and had lower prevalence of hypertension (4.34 vs 32.35%, P=0.003) and coronary artery disease (0 vs 14.71%, P = 0.045). Patients with CVST presented with significant lower platelet count (510.39± 176.71 vs 750.82± 249.10, P< 0.001) and higher score of IPSET-thrombosis (P=0.017). Multivariate logistic regression analysis indicated that age (P=0.002, OR 1.096, 95% CI 1.035– 1.161), at least one CVRF (P = 0.024, OR 0.037, 95% CI 0.002– 0.649), platelet count (P=0.045, OR 0.994, 95% CI 0.989– 1.001), and lower percentage of antiplatelet therapy (P=0.035, OR 0.307, 95% CI 0.001-1.280) significantly contributed to the risk of CVST in ET.
Conclusion: Most patients (95.65%) had a favorable outcome without recurrence after standard anticoagulant and cytoreductive treatment at last follow-up. These findings indicate that CVST may be the initial presentation of ET, with its detection crucial for early diagnosis and appropriate management. Anticoagulant and cytoreductive therapies should be recommended for preventing ET-related CVST with JAK2 V617F mutation.
Keywords: cerebral venous thrombosis, JAK2 V617F, anticoagulant therapy, cytoreductive therapy, essential thrombocythemia
Introduction
Cerebral venous sinus thrombosis (CVST) is a relatively uncommon, but potentially life-threatening cerebrovascular disease, which accounts for 0.5–1% of all stroke.1,2 Any of three changes in the blood compositions, hemodynamics, or vascular endothelium may lead to CVST. The etiology of CVST is a complex and multi-factorial process, although several risk factors have been identified, including genetic or acquired factors such as prothrombotic conditions, oral contraceptives, pregnancy and puerperium, infection, and malignancy.3 In addition, hematologic disorders, such as ET, have also been associated with venous thrombosis.4 ET, characterized by proliferation of megakaryocytes and high platelet counts, is a myeloproliferative neoplasm. Generally, this condition has been associated with thrombo-hemorrhagic complications, a leading cause of ET-related morbidity and mortality. Thrombosis mainly occurs in middle-aged and older patients, with an incidence of 11% to 29%.5,6 Although thrombosis can occur in arteries or veins, venous thrombosis is less frequent. In fact, the incidence of arterial and venous events is about 1.2% and 0.6%, respectively.4,5 Venous thrombosis in ET mainly manifests as venous events including deep venous thrombosis, pulmonary embolism, portal vein thrombosis, splanchnic veins thrombosis and cerebral venous thrombosis. However, the underlying mechanisms of thrombosis are still unknown, although it seems to have a multi-factorial and more complex pathogenesis. Previous studies have indicated hypercoagulable state, peripheral blood cell proliferation, and vascular endothelium activation in pathogenesis of venous thrombosis.7,8 Additionally, age (over 60 years) and previous thrombotic events are well-known factors in risk stratification of thrombosis. However, the exact impact of other putative features such as leukocytosis, JAK2 V617F mutation, cardiovascular risk factors CVRF, splenic enlargement remains unclear. Therefore, unraveling the right combination of these additional factors may help to guide management strategies and improve prognosis of CVST events in patients with ET.
Due to the variability and nonspecificity of clinical manifestations, the diagnosis of CVST often remains overlooked. In order to reduce recurrent risk, morbidity and mortality of CVST in ET, accurate diagnosis and development of appropriate management therapies are imperative. Furthermore, it is important to assess patients with ET at a high risk of thrombotic complications, in order to guide proper treatment approaches. To date, available approaches for managing ET have primarily focused on dealing with thrombotic complications. In fact, only a handful of case reports are available, owing to the rarity of ET patients with CVST. Many aspects of ET-related CVST need to be clarified. For instance, some case reports have reported that various pharmacologic interventions in ET patients with CVST were effective. However, the current therapeutic options vary widely, owing to limited evidence from relevant randomized trials. Thus, the diagnosis and therapy of ET-related CVST remains challenge.
Additionally, knowledge of ET patients with CVST in Chinese populations is limited. In the present study, we sought to provide clinical information by analyzing related data at our center in the past ten years. Specifically, we aimed to describe clinical features, treatment strategies and risk factors of CVST diagnosed with ET in this cohort, and attempted to provide a better understanding and guidance for appropriate management strategies against the disorder.
Subjects and Methods
Study Subjects
Consecutive ET patients, over eighteen years old, from the departments of neurology or hematology at Xuanwu Hospital, Capital Medical University between January 2010 and September 2019, were included. These included ET patients with CVST, and ET patients without CVST as control group. ET diagnosis was defined according to the ICD-10 coding system (D47.302-Essential thrombocythemia) and the World Health Organization (WHO) diagnostic criteria. Patients collected before 2016 were based on the 2008 WHO diagnostic criteria,9 which request the diagnosis of ET requires meeting all 4 major criteria or the first 3 major criteria and the minor criterion. Major criteria: 1. Platelet count ≥ 450 ×109/L; 2. BM biopsy showing proliferation mainly of the megakaryocyte lineage with increased numbers of enlarged, mature megakaryocytes with hyperlobulated nuclei. No significant increase or left shift in neutrophil granulopoiesis or erythropoiesis and very rarely minor (grade 1) increase in reticulin fibers; 3. Not meeting WHO criteria for BCR-ABL1+ CML, PV, PMF, myelodysplastic syndromes, or other myeloid neoplasms; 4. Presence of JAK2, CALR, or MPL mutation. Minor criterion: Presence of a clonal marker or absence of evidence for reactive thrombocytosis. Patients collected after 2017 were based on 2016 WHO diagnostic criteria,10 which request as follows: 1. Platelet count ≥ 450 × 109/L; 2. Megakaryocyte proliferation with large and mature morphology. No or little granulocyte or erythroid proliferation. 3. Not meeting WHO criteria for CML, PV, PMF, MDS or other myeloid neoplasm. 4. Demonstration of JAK2 V617F or other clonal marker or no evidence of reactive thrombocytosis. CVST diagnosis was defined according to international guidelines,1 then confirmed by digital subtraction angiography (DSA), magnetic resonance imaging (MRI)/magnetic resonance venography (MRV) or cranial computed tomography venography (CTV). CVST patients were divided into acute group (<48 hours from symptoms onset to admission), subacute group (48 hours to 4 weeks from symptoms onset to admission), or chronic group (more than four weeks from symptoms onset to admission) according to course of disease as previously described.1 Follow-up started six months after initial CVST diagnosis, and continued via clinical visits or telephone follow-up, after discharge. Functional outcomes were evaluated using the modified Rankin scale (mRS), then categorized as favorable and unfavorable outcomes with mRS of 0–2 and >2, respectively. We adopted the International Prognostic Score for Thrombosis in ET (IPSET-thrombosis score)11 to stratify ET patients into the following three risk groups: low risk (0–1 points), intermediate risk (2 points), and high risk (≥3 points). Generally, the IPSET-thrombosis evaluates the following variables: age > 60 (1 point), history of thrombosis (2 points), CVRF (1 point), and JAK2 V617F mutation (2 points).
Data Collection
The following relevant information was retrospectively collected from medical records of all enrolled patients: demographic variables including gender, age, pregnancy history, potential risk factors including medical history of birth control pills and malignant tumor, clinical symptoms and signs, laboratory investigations including evaluation of the other causes of coagulopathy, detection of JAK2 V617F mutation, imaging material (such as CTV, MRI, MRV, DSA), treatment modalities, as well as clinical outcomes.
Statistical Analysis
All statistical analyses were performed in SPSS version 19.0 (SPSS Inc., Chicago, USA). Continuous variables, for normal distribution, were presented as means ± standard deviations (SD) of the mean, then compared by Student’s t-test. Additionally, median and interquartile range (IQR) or range were displayed for those of abnormal distribution and analyzed with Mann‐Whitney U-test. On the other hand, categorical variables were reported as counts and percentages, then analyzed with Pearson chi-square or Fisher’s exact tests for frequency less than five. Moreover, multivariate logistic regression analysis was performed to identify variables associated with CVST in ET. To adjust for confounding factors, with P < 0.01 and the factors that clinically considered to be closely related to CVST. Data followed by P <0.05 were considered statistically significant.
Results
Demographic Characteristics
A total of 115 patients, with medical records and diagnosed with ET were retrospectively reviewed. Among them, 24 ET patients did not have complete information or secondary reversible risk factors, were therefore excluded from analysis. A total of 91 patients (79.13%) at the median age of 60 (Range 17–89) were included in the study. We identified 23 ET patients with CVST available for analysis, including 9 (39.13%) males and 14 (60.87%) females, which accounted for 25.27% of 91 patients. The median age, at the diagnosis of CVST, was 34 (Range 25–50), whereas the median duration of presenting CVST was 182 days (Range 20–365). The diagnosis of CVST was made concomitantly to ET in 19 (82.61%) patients, and after the diagnosis of ET in 4(17.39%) patients. A summary of demographic and clinical characteristics of CVST patients is outlined in Table 1. Bone marrow smears of case 7 are presented in Figure 1.
 |
Table 1 The Baseline Features of the 23 ET Patients with CVST |
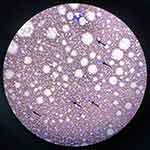 |
Figure 1 Bone marrow smear in case 7. Bone marrow cytomorphology showed hypercellularity and markedly increased megakaryocytes (black arrows) at low magnification (100×). |
Neurological Features of CVST in ET
A summary of clinical features of CVST is presented in Tables 1 and 2. The number of Et patients with CVST presenting with acute, subacute and chronic episodes was 12 (52.17%), 4 (17.39%), and 7 (30.43%), respectively. Headache was the first and most commonly reported complaint in all patients. Headache usually presents as a refractory and diffuse occurrence, and is distributed to the anterior/posterior bilateral head. In addition, we also recorded sensory disturbance, impaired cognition, mental abnormalities, as well as loss of hearing and visual symptoms (such as blurred vision, visual field defect, amaurosis fugax and metamorphopsia).
 |
Table 2 Clinical Manifestations of ET Patients with CVST |
Analysis of Cerebral Spinal Fluid
All patients underwent lumbar puncture, with severe elevated (≥330 mm H2O) and mild to moderate elevated (180–330mm H2O) ICP recorded in 11 (47.83%) and 9 (39.13%) cases, respectively. Normal pressure was recorded in 3 cases (13.04%). On the other hand, the patients exhibited normal leukocyte number, protein, glucose, and chloride levels of cerebrospinal fluid (CSF).
Neurological Imaging Findings
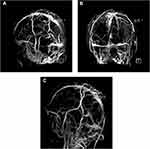
Tables 1 and 2 show the details of the lesion sites of brain parenchyma and the involved venous sinuses. MRI revealed parenchymal lesions including cerebral infarction, cerebral venous infarction, hemorrhagic cerebral venous infarction and cerebral hemorrhage. Meanwhile, MRV or DSA showed that all cases suffered from more than one sinus thrombosis. The most common sites of CVST were the sigmoid sinus and transverse sinus, followed by the superior sagittal sinus, jugular vein and straight sinus. Additionally, the cortical venous, confluence of sinuses and inferior sagittal sinus could also be involved occasionally. MRV appearances of case 7 see Figure 2.
Risk Factors Associated with CVST Patients with ET
Table 3 shows the comparison of clinic-hematological characteristics between groups of ET patients with and without CVST. Summarily, 68 ET patients without CVST were included as control group. Compared with ET patients without CVST, ET patients with CVST were significantly younger, had lower prevalence of hypertension and coronary artery disease, significantly lower platelet counts and higher IPSET-thrombosis scores (p<0.001 or p<0.05). Conversely, there was no significant difference in presence of JAK2 V617F mutation, white blood cell count, red blood cell count, hemoglobin values and hematocrit between two groups (p>0.05). Table 4 shows the results of Multivariate logistic regression analysis of risk factors associated with CVST in ET patients, which indicated that age, at least one CVR, platelet count and lower percentage of antiplatelet therapy were the significant risk of CVST (p<0.05). Additionally, there are no significant differences between the groups with regards to age (over 60 years), stroke history, and risk stratification, which have previously been recognized as classical risk factors (p > 0.05). Meanwhile, evaluation of the other causes of coagulopathy, including antithrombin III, protein C and protein S, antiphospholipid antibodies, homocysteine, antinuclear, revealed normal level. Inquired histories of pregnancy, birth control pills, infection, trauma, surgery and malignant tumor were all negative.
 |
Table 3 Comparison of ET Patients with CVST and without CVST |
 |
Table 4 Risk Factors Associated with CVST in ET Patients in Multivariate Logistic Regression |
Treatment and Outcomes
After CVST diagnosis, all patients received anticoagulation treatment. Most of CVST patients were treated with subcutaneous injection of enoxaparin, followed by warfarin and dabigatran as long-term systemic anticoagulation. Meanwhile, most of patients received antiplatelet therapy. Additionally, ET patients were treated with hydroxyurea, IFN-α and immunosuppressant. At the same time, individual patients also received endovascular treatment including intravenous sinus thrombolysis in 5 (21.74%) cases, endovascular thrombectomy and stents in 4 (17.39%) cases due to cerebral hernia. Some of patients received symptomatic treatment such as 20% mannitol dehydration for intracranial hypertension and antiepileptic drugs for epilepsy, based on clinical situation. A majority of patients (20/23, 86.97%) showed clinical improvement, with only one patient discontinuing the treatment because of whose family members asking the doctor to give up treatment after a rapid deterioration of cerebral hernia. During the follow-up period, 22 (95.65%) patients exhibited clinically improved outcomes (mRS>2) without recurrence of CVST and ET, with no occurrence of bleeding complications (see Tables 1 and 2).
Discussion
CVST represents as an unusual type of vascular event in ET. In the present study, we retrospectively analyzed clinical features, management strategies, and risk factors of 23 CVST patients, who simultaneously met the diagnostic criteria of ET. Summarily, our findings indicated that headache was the first and most common CVST presentation, multiple sinus thrombosis was a frequent occurrence, ET patients with CVST had favorable outcomes, and anticoagulation as well as cytoreductive treatment had a synergistic effect in preventing recurrence. In addition, young age, at least one CVRF, abnormal platelet count, and a lower percentage of antiplatelet therapy significantly contributed to the risk of CVST.
Generally, CVST is a multi-factorial and uncommon cerebrovascular disorder that occurs in young patients. Previous studies have shown that ET, a chronic clonal myeloproliferative neoplasm characterized by thrombocytosis,1 is a common hematological disease, and one of the underlying etiologies of CVST. Arterial or venous thrombosis is a significant complication in ET, whose incidence has been found to be as high as 11–25%.5,6 Even so, CVST is not a frequent thrombosis complication of ET, as evidenced by an estimated prevalence of 0.3–1.3% and severe thrombotic events (8–31%).5,12 The clinical manifestations of CVST are highly variable, while the clinical course of ET is often indolent, which often leads to missing or delaying diagnosis. In the present study, we found a 20% CVST incidence in ET, higher than previously reported. This incidence was also more common (60.87%) in females.13,14 That may be related to the more severe cases referred to our hospital as the national cerebrovascular disease center.
The clinical presentations of CVST vary depending on the location of cerebral venous thrombosis. Most (82.61%) patients suffered from CVST before ET diagnosis, with more than half of the newly diagnosed cases found to be asymptomatic. Therefore, it is recommended to follow up clinical course of CVST patients, to ensure continued ET detection. In the present study, the most frequent CVST presentations were those associated with intracranial hypertension, including headache, nausea/vomiting, and papilledema. This was consistent with previous studies, which have reported that CVST mainly manifests as acute onset, with the most frequent and first symptom being headache, a primary reason for visiting the doctors. The most common locations of venous sinus affected in ET with CVST were the sigmoid, transverse, and superior sagittal sinuses. Previous studies also have shown that non-infectious CVST is more common in the superior sagittal sinus and transverse sinus.15,16 We think the sites of the CVST are commonly associated with the predisposing causes, pathogens easily invade cavernous sinus, mastoid process and middle ear, inducing thrombosis in cavernous sinus and sigmoid sinus. While venous sinus thrombosis caused by non-inflammatory diseases, such as ET, is more likely to occurs in the transverse and superior sagittal sinuses. Sigmoid sinus thrombosis is often accompanied by the thrombosis of superior sagittal sinus thrombosis or transverse sinus, which may be caused by the spread of the latter two.
To date, the underlying mechanism of CVST in ET patients remains unclear, although several path-mechanisms have been proposed. For example, previous studies have suggested that hypercoagulable state and vascular endothelium activation might be the critical pathological mechanisms of venous thrombosis.7,8 In addition, several risk factors, including age over 60 years, CVRF, history of thrombosis, leukocytosis, and presence of JAK2 V617F mutations, have been considered significant predictors of CVST in general ET.17,18 In the present study, however, we found that CVST occurred in patients at a much younger age. CVST incidence and CVRF in young patients were significantly different from those in the general ET population, indicating that the IPSET-thrombosis score might not be suitable for young ET patients.19,20
To date, the effect of platelet number on the risk of thrombosis remains controversial. In the present study, despite platelet count correlating with CVST in ET patients, CVST cases had lower platelet counts than non-CVST counterparts. Several studies have reported a lack of correlation between platelet counts and thromboembolic complications.21 In addition, increased platelet, leukocyte, and endothelial activation have been consistently reported in ET patients, suggesting a pathogenetic link between activated platelets and thrombosis.22 Conversely, other studies have demonstrated the role of enhanced platelets at diagnosis as protective factors for thrombosis in patients with ET.23 Our data indicated that platelet count is not the sole risk factor of CVST. Moreover, thrombocytopenia in CVST patients may be related to platelet depletion, anticoagulant therapy, cytoreductive therapy.
JAK2 V617F mutation has been frequently reported (around 50–60%) in ET patients, necessitating a change in the diagnostic methods. In fact, this has been associated with increased risk of thrombosis in ET.24,25 In the present study, we found no significant differences in the positive rate of JAK2 V617F mutations between ET patients with and without CVST. This may be attributed to the weight of age and the JAK2 allele. However, due to technical limitations, we were unable to assay the JAK2 V617F allele burden, which is more valuable in influencing disease phenotype and thrombosis risk.24 Future therapeutic strategies should target standardizing management and thrombotic reduction risk since thrombosis is the primary cause of morbidity in ET. For acute CVST, heparin or LMWH with early initiation before oral anticoagulation is recommended. In our study, all patients received LMWH and sequential warfarin or dabigatran, as the systemic anticoagulation therapy, soon after CVST diagnosis. Meanwhile, about 35% of patients successfully underwent endovascular treatment due to clinical deterioration or no improvement despite anticoagulation, or with severe neurological deficits, which improved their outcomes with no recurrence during follow-up. These results suggested that endovascular treatment might be an alternative for CVST patients with ET, in failed anticoagulant therapy or severe cases. In fact, this therapy has been extensively adopted to treat refractory CVST.26 According to the international guidelines,27,28 ET patients’ management strategy was based on risk stratification using the IPSET-thrombosis score:29 low risk, intermediate-risk, and high-risk group. While the IPSET score is used to evaluate the overall probability of thrombosis, the IPSET score does not include some well-known causes of venous thrombosis, and so its application in this study inevitably has some limitations.
Observation and follow-up strategies are recommended for low-risk asymptomatic patients, whereas life-long oral anticoagulation is the cornerstone of CVST. High-risk ET patients under 60 years old, especially those with concurrent CVST, should also receive both cytoreductive and low-dose aspirin therapy.30 Considering the complication of CVST, mutations of JAK2 V617F allowed categorize our patients into the high-risk ET group. Consequently, all patients were subjected to systemic anticoagulation; with 73.91 and 86.96% of the patients receiving antiplatelet therapy and cytoreductive treatment (hydroxyurea or IFN-α), respectively. The benefits of antiplatelet therapy in patients with ET remain controversial. In addition, the efficacy of low-dose antiplatelet therapy, as primary prevention, is yet to be proven using randomized clinical trials. Even in young ET patients, without extremely elevated platelets, the routine low-dose aspirin may be a protective factor for thrombosis.31 In the current study, most CVST patients with ET were younger than their non-CVST counterparts, whereas the proportion of platelet therapy was lower in CVST relative to non-CVST patients. However, the relationship between this therapy with the complications of CVST in ET requires further validation. Fortunately, similar to the CVST in the general population, most CVST cases in ET have a relatively favorable prognosis. Consequently, a majority of patients improved after aggressive treatment, with no fatal outcomes and CVST relapse reported during the follow-up period.
Our study had some potential limitations, owing to its retrospective design, single-center case series, and observational results. Firstly, the research was performed in a national referral center with severe vascular disease cases. Therefore, the potential selection bias cannot fully represent the actual conditions of ET patients with CVST. Secondly, since CVST is a rare complication of ET, the relatively small sample size and incomplete evaluation index may not fully determine a causal connection. Finally, differences in genetic background may have contributed to the aforementioned inconsistencies with published studies. The practical significance of these unsolved findings needs to be verified in the future.
Conclusion
Overall, CVST may be the first manifestation of ET. Clinicians should regard a condition as ET, when patients with CVST exhibit increased platelet counts, and the JAK2 V617F mutation contributes to the diagnosis and stratification of the risk of thrombosis. Early identification and systematic anticoagulant, combined with cytoreductive treatment, should be considered for preventing ET recurrence.
Data Sharing Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
All enrolled patients provided written informed consent in accordance with the guideline of the Declaration of Helsinki of the World Medical Association Assembly. The investigation was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University.
Acknowledgments
We thank physicians, patients, and family members who provided clinical information.
Funding
The study was funded by the Fund of the Capital Medical University (17JL-02) and the National Key Research and Development Program of China (2016YFC1300600; 2016YFC0901004).
Disclosure
The authors declare no conflicts of interest concerning the research and its publication.
References
1. Saposnik G, Barinagarrementeria F, Brown RJ, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158–1192.
2. Devasagayam S, Wyatt B, Leyden J, et al. Cerebral venous sinus thrombosis incidence is higher than previously thought. Stroke. 2016;47(9):2180–2182.
3. Bousser M, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162–170.
4. Grinfeld J, Nangalia J, Baxter EJ, et al. Classification and personalized prognosis in myeloproliferative neoplasms. N Engl J Med. 2018;379(15):1416–1430.
5. Tefferi A, Elliott M. Thrombosis in myeloproliferative disorders: prevalence, prognostic factors, and the role of leukocytes and JAK2V617F. Semin Thromb Hemost. 2007;33(4):313–320.
6. Dan K, Yamada T, Kimura Y, et al. Clinical features of polycythemia vera and essential thrombocythemia in Japan: retrospective analysis of a nationwide survey by the Japanese Elderly Leukemia and Lymphoma Study Group. Int J Hematol. 2006;83(5):443–449.
7. Vannucchi AM. Insights into the pathogenesis and management of thrombosis in polycythemia vera and essential thrombocythemia. Intern Emerg Med. 2010;5(3):177–184.
8. Arachchillage DR, Laffan M. Pathogenesis and management of thrombotic disease in myeloproliferative neoplasms. Semin Thromb Hemost. 2019;45(06):604–611.
9. Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22(1):14–22.
10. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405.
11. Barbui T, Finazzi G, Carobbio A, et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood. 2012;120(26):5128–5133, 5252.
12. De Stefano V, Rossi E, Za T, et al. JAK2 V617F mutational frequency in essential thrombocythemia associated with splanchnic or cerebral vein thrombosis. Am J Hematol. 2011;86(6):526–528.
13. Geyer HL, Kosiorek H, Dueck AC, et al. Associations between gender, disease features and symptom burden in patients with myeloproliferative neoplasms: an analysis by the MPN QOL International Working Group. Haematologica. 2017;102(1):85–93.
14. Tefferi A, Fonseca R, Pereira DL, et al. A long-term retrospective study of young women with essential thrombocythemia. Mayo Clin Proc. 2001;76(1):22–28.
15. Korathanakhun P, Petpichetchian W, Sathirapanya P, et al. Cerebral venous thrombosis: comparing characteristics of infective and non-infective aetiologies: a 12-year retrospective study. Postgrad Med J. 2015;91(1082):670–674.
16. Ferro JM, Bousser MG, Canhao P, et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis - endorsed by the European Academy of Neurology. Eur J Neurol. 2017;24(10):1203–1213.
17. Artoni A, Bucciarelli P, Martinelli I. Cerebral thrombosis and myeloproliferative neoplasms. Curr Neurol Neurosci Rep. 2014;14:11.
18. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857–5859.
19. Palandri F, Latagliata R, Polverelli N, et al. Mutations and long-term outcome of 217 young patients with essential thrombocythemia or early primary myelofibrosis. Leukemia. 2015;29(6):1344–1349.
20. Barbui T, Thiele J, Carobbio A, et al. Disease characteristics and clinical outcome in young adults with essential thrombocythemia versus early/prefibrotic primary myelofibrosis. Blood. 2012;120(3):569–571.
21. Campbell PJ, Maclean C, Beer PA, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood. 2012;120(7):1409–1411.
22. Patrono C, Rocca B, De Stefano V. Platelet activation and inhibition in polycythemia vera and essential thrombocythemia. Blood. 2013;121(10):1701–1711.
23. Latagliata R, Montanaro M, Cedrone M, et al. High platelet count at diagnosis is a protective factor for thrombosis in patients with essential thrombocythemia. Thromb Res. 2017;156:168–171.
24. Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790.
25. Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366(9501):1945–1953.
26. Konakondla S, Schirmer CM, Li F, et al. New developments in the pathophysiology, workup, and diagnosis of Dural Venous Sinus Thrombosis (DVST) and a systematic review of endovascular treatments. Aging Dis. 2017;8(2):136–148.
27. Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29(6):761–770.
28. Besses C, Hernandez-Boluda JC, Perez EM, et al. Current opinion and consensus statement regarding the diagnosis, prognosis, and treatment of patients with essential thrombocythemia: a survey of the Spanish Group of Ph-negative Myeloproliferative Neoplasms (GEMFIN) using the Delphi method. Ann Hematol. 2016;95(5):719–732.
29. Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2019 update on diagnosis, risk-stratification and management. Am J Hematol. 2019;94(1):133–143.
30. Chu DK, Hillis CM, Leong DP, et al. Benefits and risks of antithrombotic therapy in essential thrombocythemia: a systematic review. Ann Intern Med. 2017;167(3):170–180.
31. Masarova L, Verstovsek S. Therapeutic approach to young patients with low–risk essential thrombocythemia: primum non nocere. J Clin Oncol. 2018;O2018793497.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.