Back to Journals » OncoTargets and Therapy » Volume 9
Clinical benefits of combined chemotherapy with S-1, oxaliplatin, and docetaxel in advanced gastric cancer patients with palliative surgery
Authors Liu Y, Feng Y, Gao Y, Hou R
Received 2 November 2015
Accepted for publication 24 December 2015
Published 7 March 2016 Volume 2016:9 Pages 1269—1273
DOI https://doi.org/10.2147/OTT.S99684
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Daniele Santini
Yan Liu,1 Ye Feng,2 Yongjian Gao,2 Ruizhi Hou2
1Department of Ultrasonography, 2Department of Gastrointestinal Disease, China–Japan Union Hospital, Jilin University, Changchun, People’s Republic of China
Background and aim: Advanced gastric cancer accounts for a substantial portion of cancer-related mortality worldwide. Surgical intervention is the curative therapeutic approach, but patients with advanced gastric cancer are not eligible for the radical resection. The present work aimed to investigate the efficacy and safety of palliative surgery combined with S-1, oxaliplatin, and docetaxel chemotherapy in the treatment of patients with advanced gastric cancer.
Method: A total of 20 patients who underwent palliative resection of gastric cancer in China–Japan Union Hospital of Jilin University from 2010 to 2011 were evaluated. Days 20–30 post-operative, these patients started to receive chemotherapy of S-1 (40 mg/m2, oral intake twice a day) and intravenous infusion of oxaliplatin (135 mg/m2) and docetaxel (75 mg/m2). After three cycles of chemotherapy (21 days/cycle), patients were evaluated, and only those who responded toward the treatment continued to receive six to eight cycles of the treatment and were included in end point evaluation. Patients’ survival time and adverse reactions observed along the treatment were compared with those treated with FOLFOX.
Results: Out of 20 patients evaluated, there was one case of complete response, nine cases of partial response, six cases of stable disease, and four cases of progressive disease. The total efficacy (complete response + partial response) and clinical benefit rates were 50% and 80%, respectively. Of importance, the treatment achieved a significantly longer survival time compared to FOLFOX, despite the fact that both regimens shared common adverse reactions. The adverse reactions were gastrointestinal reaction, reduction in white blood cells, and peripheral neurotoxicity. All of them were mild, having no impact on the treatment.
Conclusion: Combination therapy of S-1, oxaliplatin, and docetaxel improves the survival of gastric cancer patients treated with palliative resection, with adverse reactions being tolerated. The clinical application of the chemotherapy warrants further investigation.
Keywords: gastric cancer, palliative resection, S-1, oxaliplatin, docetaxel, combination chemotherapy
Introduction
Gastric cancer is the third leading cause of cancer deaths worldwide.1,2 The disease is characterized by its heterogeneous histology and genetic background.3 Surgical resection is potentially curative in patients with early gastric cancer, but for patients with advanced cancer, the clinical outcomes are among the poorest of all solid-organ tumors because of the high rate of distinct metastasis and recurrence.4,5 A better understanding of cancer biology has allowed the development of molecular-targeted therapies, for example, bevacizumab, sunitinib, and sorafenib, that inhibit angiogenesis.6 The usefulness of these novel agents remains to be further investigated, and chemotherapy is still the first-line therapy for patients with advanced gastric cancer. Indeed, the addition of combination chemotherapy after surgery has been demonstrated to provide benefits on patients’ survivals and quality of life in different cohorts.7,8 However, the gold standard of postoperative chemotherapy in terms of the selection of doublet or triplet regimen has yet to be established. There is a controversy over the use of triplet regimen because of the potential toxicity.9
S-1, oxaliplatin, and docetaxel are chemotherapeutics that have been widely used to treat gastric cancer. The present work aimed to elevate the therapeutic efficacy of combination chemotherapy of S-1, oxaliplatin, and docetaxel in the management of 20 patients with advanced gastric cancer after palliative surgery. A control arm of FOLFOX-treated patients was included. For the first time, data have suggested that palliative resection combined with chemotherapeutics S-1, oxaliplatin, and docetaxel would benefit the postoperative prognosis of patients with advanced gastric cancer, and of importance, the adverse reactions associated with the chemotherapy were well tolerated.
Patients and methods
Cohort characteristics
The clinical data of gastric cancer patients receiving palliative resection from 2010 to 2011 in China–Japan Union Hospital at Jilin University were retrieved. This study was approved by the Institutional Review Board of Ethics Committee of China-Japan Union Hospital. Analysis was made on the following two different postoperative chemoregimens: Group 1 (n=20) treated with combination therapy of S-1, oxaliplatin, and docetaxel and Group 2 (n=24) received FOLFOX. In Group 1, there were 12 males and eight females (age 38–72, mean age 59.2±10.4), while Group 2 consisted of 13 males and eleven females (age 48–70, mean age 60.5±6.9). Written informed consents were obtained from all patients, and only cases who fulfilled the following criteria were eligible for this study: 1) histopathological diagnosis is available; 2) Karnofsky score ≥60 with expected survival of ≥3 months; 3) normal bone marrow reserve and liver/kidney function; 4) lesion size can be clearly detected by computed tomography or magnetic resonance imaging to give measurable outcome; 5) no second primary tumor; and 6) no history of chemotherapy treatment. Patients’ characteristics are summarized in Table 1.
  | Table 1 Characteristics of patients in Groups 1 and 2 |
Chemotherapy
During treatment, Group 1 received a combination therapy of S-1, oxaliplatin, and docetaxel, of which, one course of the treatment consisted of 21 days. On day 1, patients were intravenously infused 75 mg/m2 docetaxel (TXT) for 1 hour and 135 mg/m2 oxaliplatin (LOHP) supplemented with 500 mL of 5% glucose for 3 hours. Oral S-1 capsules (Gio capsules) were administered at a dose level of 40 mg/m2 twice daily after meals for 14 days consecutively, followed by a 7-day rest. Patients were treated with three courses of chemotherapy before they were subjected to an efficacy assessment, in which responders were then given six to eight courses of chemotherapy. During the treatment, patients were administered prophylactic antiemetic and hepatoprotective therapeutics and, whenever necessary, granulocyte colony-stimulating factor. Dependent on the patient’s condition, dexamethasone may have been given before and after the intravenous infusion of docetaxel. At the end of the treatment, safety and efficacy were evaluated again.
During treatment, Group 2 received FOLFOX regimen. At the start of the treatment on day 1, patients were intravenously infused 85 mg/m2 oxaliplatin and 400 mg leucovorin over 2 hours, followed by an intravenous bolus of fluorouracil (5-FU) at a dose level of 400 mg/m2. Subsequently, a continuous intravenous infusion of 5-FU (2.4 g/m2) was applied for 48 hours. Antiemetic prophylaxis and hepatoprotective agents were given along with the chemotherapy. Granulocyte colony-stimulating factor was given, if necessary.
Evaluation of treatment response and adverse events
Treatment response of each patient was evaluated following Response Evaluation Criteria In Solid Tumors; imaging was taken every 3 weeks and was classified as complete response (CR), partial response (PR), stable disease (D), and progressive disease (PD). Response rate was calculated as the sum of CR and PR, and clinical benefit rate as the sum of CR, PR, and D. Adverse reactions observed were assessed following World Health Organization guidelines on the evaluation of acute and subacute adverse reactions of anticancer drugs into 0–IV severity degree. Patients were followed up after chemotherapy until their death or unless the follow-up was terminated. Time to progression (TTP) was defined as the period from the start of chemotherapy to the appearance of signs of disease progression. Median survival time (MST) represented how long the studied patients remained alive from the beginning of chemotherapy.
Statistical analysis
Survivals and adverse reactions of the two treatment groups were compared using log-rank test and chi-square test, respectively. Difference with P-value <0.05 was regarded as statistically significant.
Results
Clinical outcomes of combination chemotherapy of S-1, oxaliplatin, and docetaxel
After 3-week treatment, each of the 20 patients was clinically evaluated for the outcomes and any adverse events associated with the treatment. The total efficacy was 50%, with one patient with CR and six patients presented PR. In addition to these patients, nine and four patients demonstrated D and PD, respectively, making the clinical benefit rate 80%. Up to the last follow-up in June 2013, the median TTP was 7.6 months, while MST was 10 months. For the patients treated with FOLFOX, the median TTP and MST were 5.5 months and 9 months, respectively. The differences in MST and TTP between the two treatment groups were statistically significant (P<0.05).
Adverse events associated with the combination therapy have also been assessed. The events could be categorized into blood toxicity, gastrointestinal reaction, and peripheral neurotoxicity. Myelosuppression was seen, in which 85% of patients showed leukopenia. Other adverse events included thrombocytopenia (65%), nausea and vomiting (50%), diarrhea (40%), peripheral neurotoxicity characterized by reversible limb parenthesis that was aggravated by low temperature (50%), and alopecia (60%; Table 2). Of importance, all adverse events were within Grades I–III, having no significant impact on treatment efficacy. Besides, no treatment-related mortality was seen during the study.
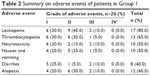  | Table 2 Summary on adverse events of patients in Group 1 |
Combination chemotherapy of S-1, oxaliplatin, and docetaxel prolonged patients’ survivals after palliative resection
The survival times of patients receiving different regimens were followed, and the results showed that those treated with combination therapy of S-1, oxaliplatin, and docetaxel survived longer than their counterparts who received FOLFOX. As determined by log-rank test, the difference between the two groups was statistically significant (P=0.03; Figure 1). Of note, both treatment regimens shared common adverse reactions, including gastrointestinal reaction, blood toxicity reaction, neurotoxicity, and alopecia (Table 3). None of these reactions showed significant difference in incidence between the two treatment groups as determined by chi-square test (Table 3).
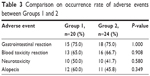  | Table 3 Comparison on occurrence rate of adverse events between Groups 1 and 2 |
Discussion
Gastric cancer is highly prevalent, accounting for a substantial portion of cancer-related mortality globally. Surgical intervention is potentially curative for patients with early gastric cancer. However, unfortunately, many patients have locally advanced tumors or metastasis and are diagnosed incurable at the time of presentation. Management of patients with advanced gastric cancer is challenging, and thus far, whether the patients should receive palliative resection has remained controversial. Experiences accumulated from our clinical practices suggest that palliative resection is beneficial to patients’ outcomes because the surgery is able to significantly mitigate cancer-related bleeding to ameliorate bowel obstruction, and of importance, to enhance the efficacy of chemotherapy by reducing tumor burden. In fact, in accordance with our clinical experiences, studies from different medical centers have collectively suggested that palliative gastrectomy has positive impact on patients’ survivals and quality of life.10
Adjuvant chemotherapy is administered to patients treated with palliative resection with an aim to eradicate both cancer cells being sheared off during the surgery and residual foci after tumor resection. The gold standard of such chemotherapy has yet to be established. In this context, the present work demonstrated for the first time that comparing with FOLFOX, the combination chemotherapy of S-1, oxaliplatin, and docetaxel achieved a better postoperative prognosis in patients with advanced gastric cancer. Notably, the severity of adverse reactions associated with the chemotherapy did not compromise treatment efficacy despite the debates over the use of triplet chemotherapy in the treatment of cancer patients because of the toxicity observed.
In this work, S-1, oxaliplatin, and docetaxel have been chosen with full consideration of their efficacy and safety. The clinical uses of these chemotherapeutics have been reported. S-1, which is an oral capsule, has been proved a safe and efficient anticancer therapeutic by randomized controlled clinical trials.11,12 Animal studies also collectively suggested that S-1 is superior to 5-FU because of its less cytotoxicity and enhanced therapeutic efficacy.13 The favorable pharmacologic property of S-1 is attributed to the actions of its three key components: tegafur, gimeracil, and oteracil potassium. Tegafur is metabolized by liver microsomal cytochrome P450 into 5-FU. 5-FU has profound anticancer efficacy and has been used for the treatment of gastrointestinal and breast cancers in Japan since 1957.11,14 Gimeracil inhibits dihydropyrimidine dehydrogenase (DPD) that metabolizes 5-FU. The inclusion of gimeracil in S-1 capsule prolongs the half-life of 5-FU, thus enhancing the treatment efficacy. Different individuals express differential levels of DPD. Inhibition of DPD by gimeracil also minimizes the difference of responsiveness toward S-1 between individuals. Oteracil potassium prevents gastrointestinal toxicity associated with the phosphorylation of 5-FU.15 Docetaxel, which is taxane in nature, is a chemotherapeutic widely administered for treating gastrointestinal cancers. Docetaxel inhibits cell division by interfacing microtubules and induces apoptosis of cancer cells. Clinical use of docetaxel has been well established. Monotherapy with docetaxel treated 17%–24% of advanced gastric cancer in a Phase II study,16 while combination therapy of docetaxel with other anticancer drugs could further increase the success rate to 26%–60%.17
Platinum-based anticancer drugs are nonspecific cytotoxic agents targeting DNA synthesis of cancer cells. Oxaliplatin is the third generation of such agents, having advantages over the predecessors cisplatin and carboplatin. Oxaliplatin acts in synergy with 5-FU and does not show cross drug resistance with cisplatin and carboplatin. Comparing with cisplatin, the gastrointestinal toxicity associated with oxaliplatin is much less severe. Myelosuppression commonly seen in cancer chemotherapy is also milder in oxaliplatin than carboplatin. Nephrotoxicity and ototoxicity are less observed in oxaliplatin treatment probably because of its much slower hydrolysis rate than that in cisplatin and carboplatin. However, patients treated with an elevating dose of oxaliplatin suffered from peripheral neuropathy.14
Conclusion
To conclude, the present work provided evidence indicating that palliative resection combined with triplet regimen of S-1, oxaliplatin, and docetaxel can benefit the survival of patients with advanced gastric cancer. The adverse reactions observed are well tolerated. Strikingly, the use of S-1 capsule renders the design of treatment regimen much convenience and definitely shortens the length of hospitalization. The combination therapy of S-1, oxaliplatin, and docetaxel warrants further systemic studies in larger numbers of patients with gastric cancer.
Funding
This study was supported by a grant from the Latitudinal Research Project of Jilin University (3R215B163430).
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Bray F, Ferlay J, Laversanne M, et al. Cancer incidence in five continents: inclusion criteria, highlights from volume X and the global status of cancer registration. Int J Cancer. 2015;137(9):2060–2071. | ||
McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol. 2014;11(11):664–674. | ||
D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240(5):808–816. | ||
Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87(2):236–242. | ||
Chan MM, Sjoquist KM, Zalcberg JR. Clinical utility of ramucirumab in advanced gastric cancer. Biologics. 2015;9:93–105. | ||
Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–321. | ||
Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–4393. | ||
Sudo K, Yamada Y. Advancing pharmacological treatment options for advanced gastric cancer. Expert Opin Pharmacother. 2015;16(15):2293–2305. | ||
Hanazaki K, Sodeyama H, Mochizuki Y, et al. Palliative gastrectomy for advanced gastric cancer. Hepatogastroenterology. 2001;48(37):285–289. | ||
Lee HH, Hur H, Kim SH, Park AR, Kim W, Jeon HM. Outcomes of modified FOLFOX-6 as first line treatment in patients with advanced gastric cancer in a single institution; retrospective analysis. Cancer Res Treat. 2010;42(1):18–23. | ||
Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28(9):1547–1553. | ||
Malet-Martino M, Martino R. Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): a review. Oncologist. 2002;7(4):288–323. | ||
Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358(1):36–46. | ||
Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–1820. | ||
Giuliani F, Gebbia V, De Vita F, et al. Docetaxel as salvage therapy in advanced gastric cancer: a phase II study of the Gruppo Oncologico Italia Meridionale (G.O.I.M.). Anticancer Res. 2003;23(5b):4219–4222. | ||
Murad AM, Skare NG, Vinholes J, Lago S, Pecego R. Phase II multicenter trial of docetaxel, epirubicin, and 5-fluorouracil (DEF) in the treatment of advanced gastric cancer: a novel, safe, and active regimen. Gastric Cancer. 2006;9(2):99–105. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

