Back to Journals » International Journal of Women's Health » Volume 9
Clinical behavior of a cohort of adult women with facial acne treated with combined oral contraceptive: ethinylestradiol 20 µg/dienogest 2 mg
Authors Palacio-Cardona J , Caicedo Borrero DM
Received 10 April 2017
Accepted for publication 5 September 2017
Published 16 November 2017 Volume 2017:9 Pages 835—842
DOI https://doi.org/10.2147/IJWH.S139289
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
John Palacio-Cardona,1 Diana María Caicedo Borrero2
1Student Welfare Service – Health Area, Santiago de Cali University, 2Support for Research, Academic, Scientific and Technological Services (SEACIT), Cali, Colombia
Abstract: Acne vulgaris is the most common skin disease. It affects the young adult female population and generates great impact on physical and mental health. One of the treatments with good results for affected women is combined oral contraceptive pills (COCPs). The aim of this study was to determine the clinical effect of facial acne management with ethinylestradiol 20 µg/dienogest 2 mg in a cohort of Colombian adult women. A cohort of 120 female university students was followed for 12 months. These participants were enrolled in the Sexual and Reproductive Health Program of the Santiago de Cali University. This cohort admitted women between 18 and 30 years old who had chosen to start birth control with ethinylestradiol 20 µg/dienogest 2 mg COCPs, did not have contraindications to the use of COCPs, and had been diagnosed with acne. Monthly monitoring of facial acne lesion count was performed. Relative changes in facial lesion count were identified. At the end of follow-up, the percentage of reduction of lesions was 94% and 23% of women had a 100% reduction in acne lesions. In conclusion, the continued use of the ethinylestradiol 20 µg/dienogest 2 mg COCPs reduced inflammatory and non-inflammatory acne lesions in reproductive-age women between 18 and 30 years of age with no severe acne.
Keywords: skin diseases, acne vulgaris, reproductive control agents, contraceptive agents, female contraceptive agents, oral, hormonal
Plain language summary
The study sought to measure the benefit of combined oral contraceptive therapy in acne, at concentrations which had not been studied in real-life conditions. Since acne is a disease of high prevalence and with a strong impact on the mental health of young people, this non-contraceptive benefit can be used as a treatment strategy for acne. University students who started contraceptive therapy with ethinylestradiol 20 μg/dienogest 2 mg and had mild-to-moderate acne were invited to participate in a follow-up study for 12 months. Each month, the contraceptive use was checked and the number of acne facial lesions was checked. At follow-up, the percentage of lesions reduction was 94% and 23% of women had a 100% reduction in acne lesions. It has been demonstrated in real-life conditions that for women of childbearing age with mild/moderate acne, in whom the use of combined oral contraceptive pills is not contraindicated and who wish to use them, continued use of ethinylestradiol 20 μg/2 mg dienogest is a beneficial alternative in the treatment of acne.
Introduction
Acne vulgaris is the most common chronic inflammatory skin disease in adolescents and young adults. Prevalence reports of this condition range between 12% and 59%.1,2 Approximately 80% of adult women have persistent acne, and approximately 20% have acne de novo, which appears between the ages of 21 and 25 years. Acne in adult women occurs mainly with facial lesions in mild and moderate forms of acne2 and can have both physical and emotional consequences at medium and long term, such as the presence of scars, low self-esteem, depression, and anxiety – affecting women’s quality of life.1
Acne is a disease of multifactorial origin, including factors such as follicular hyper-keratinization, Propionibacterium acnes colonization, increased sebum production, and mechanisms of innate or acquired inflammation.1,3 From the hormonal point of view, androgens have a central role in the pathogenesis of the disease;4 it is suggested that there is an increased sensitivity of hormone receptors expressed by sebocytes and keratinocytes as well as increased local androgen metabolism by enzymes.2,4 In particular, women have different endogenous sources of androgens such as their ovaries, adrenal gland, and skin,5 which permanently exposes them to the effects of androgens, which in turn may lead to persistent and very symptomatic typical acne lesions.
Understanding the pathophysiologic mechanism that operates in cases of female acne has led to proposing the use of combined oral contraceptive pills (COCPs), which have been used mainly as adjuvants,5–10 in cases of papulopustular, nodular, and conglobate acne, in women diagnosed with hyperandrogenism, with menstrual cycle disorder.4 These drugs have direct effects on androgen production and thus positive effects on the skin of women.4 Several studies have shown the effect of COCPs in acne, even compared to antibiotics.6,11 Specifically, progestogens have potent anti-androgenic activity and act by blocking androgen receptors in target organs, improving skin conditions mediated by androgen, such as skin spots and acne vulgaris action.
A large number of COCPs are available in Colombia, including ethinylestradiol 0.02 mg/dienogest 2 mg. Dienogest provides anti-androgenic properties useful in the treatment of symptoms of androgenization, seborrhea, hirsutism, endometriosis, and acne. In addition, low doses of estrogen, combined with different progestogens including dienogest, have a lower risk of developing adverse effects associated with this component.12–17 Several clinical trials have documented the efficacy of this combination with percentages of improvement of 80% after six cycles of treatment and total response after 12 cycles;8,11,12 however, it is important to document the effect of this treatment in a context of application in real-life conditions. Consequently, this study was developed with the aim of establishing the clinical effect of facial acne management with ethinylestradiol 20 μg/dienogest 2 mg, in a real-life cohort of Colombian adult women.
Subjects and methods
An observational study was performed, following a cohort of female students between 18 and 30 years of age with a diagnosis of mild-to-moderate acne, whose treating physician prescribed ethinylestradiol 20 μg/dienogest 2 mg as birth control method. These women attended the Sexual and Reproductive Health Program of the Santiago de Cali University (Cali, Colombia).
A sequential convenience sampling was used, excluding women with a history of using other COCPs within 2 months prior to the start of this monitoring, patients in acne treatment with oral medications, overweight women, patients with a history of atopy or severe acne conglobata, and patients with the completion of gestation history within 2 previous months at study entry. The sample was calculated considering a decreased percentage of total facial acne lesions of 15%,6 accuracy of the estimate of 95%, a 95% confidence, and a percentage of loss to follow-up of 20%. In this way, a final sample of 121 women was obtained. The protocol was approved by the ethics committee of the Santiago de Cali University, and all participants provided written informed consent. This study adhered to the principles of the Declaration of Helsinki and the National Ethical Framework: Resolution 008430 of 1993.
For inclusion of women in the cohort, the principal researcher, following the research protocol, invited to participate candidates who began their birth control with ethinylestradiol 20 μg/2 mg dienogest, after leaving birth control consultation with their treating physician. After explaining the information about the study and checking the inclusion and exclusion criteria, informed consent was obtained. Data such as demographics (age, ethnicity), personal history of hyperandrogenism, mental disorder, and family history with acne were collected. Tracking of acne lesions was made throughout a 12-month period, counting the number of inflammatory lesions (papules, pustules, and nodules) and comedones (both open and closed), in each of the face areas (forehead, chin, cheeks, and nose area). The counts were performed by a trained physician. In addition, patients were asked about the use of acne products, tobacco consumption, and side effects related to the treatment. To this end, an instrument designed by the research team, based on the relevant scientific literature,18–20 was used.
For analysis, variables of interest were described according to their scale of measurement using frequencies or measures of central tendency and dispersion. Distribution behavior of quantitative variables (weight, height, body mass index [BMI], and facial acne lesion count) was explored using the Shapiro–Wilks test.21
Reduction in acne lesions was evaluated using absolute and relative changes in facial lesion count of comedones, papules, pustules, and nodules and the sum of these at 6 and 12 months of follow-up. In addition, a bivariate analysis of the median relative difference by age group was performed by race, BMI, use of acne products, family history of acne and individual history of polycystic ovary, stress disorder or anxiety, and smoking; hypothesis test for the Wilcoxon test20 was used. p-values <0.05 were considered significant. Analyses were performed using STATA 10.
Results
A total of 120 women using ethinylestradiol 20 μg/dienogest 2 mg diagnosed with acne were recruited, and the percentage of nonparticipation was 12.4%. Follow-up median was 9 months (interquartile range [IQR]: 3–10). The percentage of loss to follow-up was 34.2% at 12 months (Figure 1). Most losses were voluntary retirements not related to either adverse events or the ethinylestradiol 20 μg/dienogest 2 mg taken, due to “not needing birth control” (two women), lack of time (two), travel (three), not interest (13), fear of taking pills (four), and family problems (one). On the other hand, four women were withdrawn having reached the improvement in acne lesions and only two due to no improvement. The median age of the study population was 21 years (IQR: 19–22). Regarding epidemiological and clinical characteristics that can affect the presence of acne lesions, it was found that 39% of participants were afro-descendants, 21% used non-prescribed topical presentations for acne, 12.5% had a history of estrogenic disease, and 22.5% had a history of smoking (Table 1). In addition, median weight was 57 kg, height 1.60 m, and BMI 22.2 kg/m2 (Table 1).
  | Figure 1 Recruitment and follow-up flow diagram. |
As for the lesions associated with acne, the most frequently were open and closed comedones, papules were reported less frequently, and pustules and nodules were almost zero frequency. About 50% of women were diagnosed with 52 or more lesions at baseline, while at 6 months the range of lesions was between 6 and 27, and 4–29 lesions in the last month of follow-up (Table 2). The reduction in lesion counts appeared early in the follow-up (Figures 2 and 3). The reduction in the median was observed between start-up and the first month of use of ethinylestradiol/dienogest. After 6 months of follow-up, it was observed that no substantial changes in the median of lesions were presented, and it remained at low levels as those achieved in the first semester.
  | Table 2 Median and ranges of acne lesions in the study population |
  | Figure 2 Distribution of the number of acne lesions in the study population by month of follow-up. |
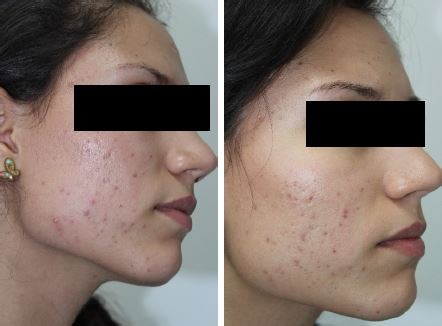
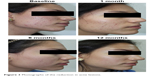  | Figure 3 Photographs of the reduction in acne lesions. |
At least 50% of the population had decreases of at least 45 acne lesions up to the sixth month of follow-up, corresponding to 90% of the lesions from baseline. At the end of follow-up, the median of the relative difference increased by 3% (Table 3). Of the 74 women who attended the sixth-month follow-up appointment, 26% showed a reduction of 100% of lesions compared to their baseline at start-up and 23% showed a reduction of 90%–99%, 32% showed a reduction of 76%–89%, 16% showed a reduction of 50%–75%, and 3% showed a reduction between 30% and 49%. While at the end of follow-up of the 79 women who attended, 23% of them reduced all of the acne lesions. In 48% of women, lesions were reduced by 90%−99%, and in the remaining 28%, acne lesions were reduced by 50%–89% (Table 4).
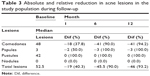  | Table 3 Absolute and relative reduction in acne lesions in the study population during follow-up |
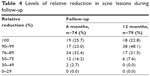  | Table 4 Levels of relative reduction in acne lesions during follow-up |
In a bivariate analysis, we found that the median relative difference in total acne lesions at 6 and 12 months of follow-up was greater than 80% for all groups. In the sixth month, the median relative difference was greater in women with no family history of acne compared to those without such a history, while at the end of follow-up the median relative difference was greater in non-African descendant women compared to that in African descendant. In addition, the data suggest that in women with no history of polycystic ovary and in those with a BMI of <20 kg/m2 median relative difference was greater, but the findings were statistically significant (Table 5).
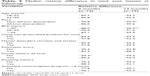  | Table 5 Median relative difference in total acne lesions at follow-up |
Regarding the adverse side effects, 24 women (20%) reported adverse events: eight had headaches, 15 women reported small breakthrough bleeding (spotting), and one woman reported weight gain.
Discussion
This study showed statistically significant reductions in clinical symptoms regarding facial lesions of mild-to-moderate acne in women between 18 and 30 years old. This reduction was evident in the relative reduction in the total number of comedones, papules, and pustules. The results were 92% and 94% of lesion reduction at 6 months and at the end of follow-up, respectively. In addition, 80% of patients improved with a reduction of more than 75% at 6 months, with an increase of 12% at 12 months. Finally, 23% of women completed the follow-up without lesions.
These results are aligned to those described in an uncontrolled, Phase III, multicenter, clinical trial conducted in Poland, with ethinylestradiol 30 μg/dienogest 2 mg, in which women aged 18–35 years participated during 12-month follow-up. A total of 50 women with acne showed 80% improvement at 6 months, while at 12 months 54% showed improvement and 37% were cured.8 Another multicenter, double-blind, randomized study, which included women between 16 and 45 years with mild-to-moderate acne, in which 525 patients received ethinylestradiol 30 μg/dienogest 2 mg, reported that the percentage of reduction in total lesions was −54.7%±26.3% at 6 months and for inflammatory lesions was −64.6%±31.2%; the percentage of patients with improvement was 90.2% at 6 months of follow-up and was superior to placebo.22
Other studies have similar results in the tendency of reducing lesions, although not to the same extent. In a Cochrane systematic review on the effectiveness of oral contraceptives in acne treatment, it was found that in 31 trials, contraceptives had an effect on the reduction in acne lesions. Regarding dienogest, it was reported that the group using this compound reached a percentage of reduction in total lesions of −15.3 (95% CI −19.9 to −10.6) as for the inflammatory lesions.23 Another study in women aged 15–45 years with low doses of ethinylestradiol 20 μg and other progestogen (drospirenone 3 mg) also had an effect in reducing inflammatory and non-inflammatory lesions.24 The ethinylestradiol dose of 20 μg was selected in combination with dienogest in this study.
Dienogest25 has a significant anti-androgenic activity, which ameliorates the symptoms of acne vulgaris, hirsutism, seborrhea, alopecia, greasy skin, and hair. In addition, it inhibits ovulation, stabilizes the menstrual cycle and has anti-proliferative effects on the endometrium. As a combined oral contraceptive, dienogest/ethinylestradiol inhibits ovulation, improves menstrual cycle control, reduces the intensity and duration of menstrual bleeding, and improves dysmenorrhea. It is a well-tolerated product, which can induce adverse events such as breast pain, headache, intermenstrual bleeding, nausea, or vomit.25 This study reported expected adverse events such as headache and intermenstrual bleeding, which was also reported as the most frequent in another study.22
In addition, the findings in the relative reduction in acne lesions in users of dienogest/ethinylestradiol are similar to those effects reported by other non-contraceptive drug used in acne treatment, such as isotretinoin. A study in patients with moderate-to-severe acne found that a 92% of women with moderate acne showed a reduction in lesions at sixth month.26 In this study, the papule and pustule lesions reduced almost in total. However, this study was not intended to compare the COCP effects vs isotretinoin; because the participants included did not have severe acne, use of isotretinoin was not recommended.
According to the guidelines for the management of acne vulgaris of the American Academy of Dermatology1 and the European guide for the management of acne,27 oral contraceptives are part of alternative treatment for women combined with other medicines for moderate acne, including papulopustular acne. However, this study shows evidence that women’s oral contraceptive ethinylestradiol 20 μg/dienogest 2 mg may be considered for the treatment of mild and moderate comedonian and papulopustular acne or may be an alternative choice as an adjunct to topical treatment within the first line of treatment in women, as proposed by the Department of Family and Community Medicine at Hershey Medical Center and Mayo Clinic in the USA.28
Among the possible limitations of this study is that the percentage of loss to follow up was greater than 20%, while women who did not complete follow-up were similar to those who continued in the study (Table 1). Most losses were voluntary withdrawals; additionally, incomplete tracking for all women, in particular those who were withdrawn because of improvement or no improvement, being more the “improved” group, may had an impact on an underestimation of the effect of COCPs in relative reduction in acne lesions. High attrition rates were also found in different studies.23 In bivariate analysis, it was found that those women not being an afro-descendant and not having family history of acne are more likely to reduce lesions; however, different studies have demonstrated that there are no strong relationships between different age groups and family history acne.18
Another limitation was not having standardized the information about the topical products (self-prescribed) used for acne. However, bivariate analysis of reduced acne lesions and use of these products showed that the median of relative difference of lesions to the end of follow up was not different between users and nonusers of acne products. A strength of the study was tracking up to 12 months of oral contraceptive use; this identified that reducing lesions occurs mainly in the first 6 months and that in later periods the benefit is maintained. Further studies to estimate the probability and time to full or partial improvement of acne lesions and possible predictors are required.
Conclusion
The continued use of COCPs, ethinylestradiol 20 μg/dienogest 2 mg, reduced inflammatory and non-inflammatory acne lesions in women in childbearing age with nonsevere acne, who participated in this study. For women with acne, in which this type of treatment is not contraindicated and who wish to use COCPs, ethinylestradiol 20 μg/dienogest 2 mg could be a beneficial alternative.
Acknowledgments
The authors thank the college women who participated in this study; these women were enrolled in the Program of Sexual and Reproductive Health “Talking with our pants off” of the Santiago de Cali University and Support for Research, Academic, Scientific and Technological Services (SEACIT). This study was funded by Santiago de Cali University and Abbott-Lafrancol.
The student appearing in Figure 3 has provided her written informed consent for this publication.
Disclosure
The study sponsors were not involved in design, implementation, or analysis of project information. The authors report no conflicts of interest in this work.
References
Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e33. | ||
Dréno B. Treatment of adult female acne: a new challenge. J Eur Acad Dermatol Venereol. 2015;29(suppl 5):14–19. | ||
Gollnick HPM. From new findings in acne pathogenesis to new approaches in treatment. J Eur Acad Dermatol Venereol. 2015;29(S5):1–7. | ||
Bettoli V, Zauli S, Virgili A. Is hormonal treatment still an option in acne today? Br J Dermatol. 2015;172(suppl 1):37–46. | ||
Asociación Colombiana de Dermatología y Cirugía Dermatológica. Guías colombianas para el manejo del acné: Una revisión basada en la evidencia por el Grupo Colombiano de Estudio en Acné [Colombian guidelines for managing acne: an evidence-based review by the Colombian Acne Study Group]. Rev Asoc Colomb Dermatol. 2011;19(2):129–158. Spanish. | ||
Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71(3):450–459. | ||
Raudrant D, Rabe T. Progestogens with antiandrogenic properties. Drugs. 2003;63(5):463–492. | ||
Golbs S, Domhardt R, Radowicky S, Kałuzny Z, Wisser KH, Zimmermann T. Clinical findings with the oral contraceptive combination Ethinylestradiol/Dienogest in Poland. Methods Find Exp Clin Pharmacol. 2002;24(9):585–592. | ||
Antonio C-AJ, Camila Á-MM, Marcela M-VD, María C-TA, Manuela V-PM. Prevalencia de acné en adolescentes de un municipio colombiano y percepciones asociadas [Prevalence of acne in adolescents of a Colombian municipality and associated perceptions]. Arch Med. 2014;10(1):1–11. Spanish. | ||
Instituto de Vigilancia de Medicamentos y alimentos – Invima. Resolución No. 2014027083, Registro Sanitario del Bellaface [Resolution number 2014027083, Bellaface Health Registry]. Bogotá: Ministerio de Salud y Protección social Colombia; 2014. Spanish. | ||
Mango D, Ricci S, Manna P, Miggiano GAD, Serra GB. Clinical and hormonal effects of ethinylestradiol combined with gestodene and desogestrel in young women with acne vulgaris. Contraception. 1996;53(3):163–170. | ||
Morales-Cardona CA, Sánchez-Vanegas G. Acne relapse rate and predictors of relapse following treatment with oral isotretinoin. Actas Dermosifiliogr. 2013;104(1):61–66. | ||
Gallo MF, Nanda K, Grimes DA, Schulz KF. 20 mcg versus >20 mcg estrogen combined oral contraceptives for contraception (protocol). Cochrane Database Syst Rev. 2003;3:CD003989. | ||
Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298. | ||
Pérez-Campos EF. Ethinylestradiol/dienogest in oral contraception. Drugs. 2010;70(6):681–689. | ||
Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002. | ||
Jensen JT, Parke S, Mellinger U, Serrani M, Mabey RG. Hormone withdrawal-associated symptoms: comparison of oestradiol valerate/dienogest versus ethinylestradiol/norgestimate. Eur J Contracept Reprod Health Care. 2013;18(4):274–283. | ||
Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474–485. | ||
Dréno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27(9):1063–1070. | ||
Tan JKL, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol. 2015;172(suppl 1):3–12. | ||
Shapiro SS, Wilk MB, Chen HJ. A comparative study of various tests for normality. J Am Stat Assoc. 1968;63(324):1343–1372. | ||
Palombo-Kinne E, Schellschmidt I, Schumacher U, Gräser T. Efficacy of a combined oral contraceptive containing 0.030 mg Ethinylestradiol/2 mg Dienogest for the treatment of papulopustular acne in comparison with placebo and 0.035 mg Ethinylestradiol/2 mg cyproterone acetate. Contraception. 2009;79(4):282–289. | ||
Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. | ||
Koltun W, Maloney JM, Marr J, Kunz M. Treatment of moderate acne vulgaris using a combined oral contraceptive containing ethinylestradiol 20 μg plus drospirenone 3 mg administered in a 24/4 regimen: a pooled analysis. Eur J Obstet Gynecol Reprod Biol. 2011;155(2):171–175. | ||
Foster RH, Wilde MI. Dienogest. Drugs. 1998;56(5):825–833. | ||
Rao PK, Bhat RM, Nandakishore B, Dandakeri S, Martis J, Kamath GH. Safety and efficacy of low-dose isotretinoin in the treatment of moderate to severe acne vulgaris. Indian J Dermatol. 2014;59(3):316. | ||
Nast A, Dréno B, Bettoli V, et al; European Dermatology Forum. European evidence-based (S3) guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012;26(suppl 1):1–29. | ||
Botros PA, Tsai G, Pujalte GG. Evaluation and management of acne. Prim Care. 2015;42(4):465–471. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

