Back to Journals » Clinical Audit » Volume 9
Clinical audit for the adherence to safety monitoring for pioglitazone use in patients with type 2 diabetes in the Al-Wazarat primary care center, Saudi Arabia
Authors Tourkmani AM, Alharbi TJ, Abdelhay O , Alkhashan HI, Alobaikan AH, Alaboud AF, Bakhiet A, Alqahtani H, Albattal SM, Abanami NM
Received 4 December 2016
Accepted for publication 7 June 2017
Published 6 July 2017 Volume 2017:9 Pages 25—31
DOI https://doi.org/10.2147/CA.S122952
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Marietta Stanton
Ayla M Tourkmani,1 Turki J Alharbi,1 Osama Abdelhay,2 Hesham I Alkhashan,1 Aljoharah H Alobaikan,1 Aboud F Alaboud,1 Ahmed Bakhiet,1 Hala Alqahtani,1 Saad M Albattal,1 Nawaf M Abanami1
1Family and Community Medicine Department, Prince Sultan Military Medical City, 2Research Unit, MSD, Riyadh, Saudi Arabia
Aim: Despite the safety warnings issued for pioglitazone, its utilization continued in several countries. However, an individualized patient risk–benefit assessment has been advised before the use of pioglitazone. The objectives of the current study are to assess the adherence to pioglitazone safety monitoring and determine the reasons for pioglitazone discontinuation.
Methods: Retrospective cohort study of patients with type 2 diabetes was carried out for patients who have been started on oral pioglitazone at the Al-Wazarat primary care center between January 2011 until the end of 2014. Adverse events and pioglitazone discontinuation were monitored during patient visits scheduled in 12-month period following the start of pioglitazone. The Chronic Disease Unit at the Al-Wazarat primary care center designed a monitoring data sheet. The safety-monitoring sheet included patients’ demographics (age and gender), history of bladder cancer, current bladder cancer, and current bladder diseases. The patient’s weight, edema status, liver function test (LFT), electrocardiogram (ECG), bone mass density (BMD), and hematuria were recorded at pioglitazone initiation and weeks 12, 24, and 48.
Results: A total of 183 patients (78 males and 105 females) were included in the cohort. The side effect that was associated with highest monitoring adherence (two or more times during the study) was weight gain (94.5%), followed by LFTs (47.5%), edema (31.1%), bone mineral density (18.0%), and ECG (16.4%). Approximately 47.4% had one or more adverse events, with the most common side effect being weight gain (35.1%), followed by osteoporosis (10.5%), edema (8.8%), hypoglycemia (7.0%), elevated liver enzymes (3.5%), and osteopenia (3.5%). Approximately 63.9% discontinued pioglitazone, with the common reasons being shifting to insulin therapy (28.2%), weight gain (25.6%), loss to follow-up (15.4%), hypoglycemia (10.2%), and osteoporosis (8.4%). This reports an appropriate initiation of pioglitazone but suboptimal adherence to pioglitazone safety monitoring in a primary care setting.
Keywords: hypoglycemia, primary care, adverse effects, diabetes care, follow-up
Background
Saudi Arabia has one of the highest prevalences of type 2 diabetes (T2D) worldwide.1 The prevalence of T2D was estimated at ~24% among Saudi adults, which is considered three times higher than the average global prevalence (8.3%).1,2 As T2D is characterized by a progressive decline in pancreatic beta-cell function and increased insulin resistance, the majority of patients with T2D are using oral hypoglycemic drugs to achieve or sustain glycemic control.3 Thiazolidinediones, such as pioglitazone, are synthetic oral hypoglycemic drugs that improve glycemic control by improving the sensitivity of hepatic and peripheral (muscle and adipose) tissue to insulin.4
Pioglitazone was shown to be effective in reducing HbA1c level (by up to 1.5) in patients with T2D when used as monotherapy or in combination with other oral hypoglycemic drugs as well as with insulin.5,6 In addition, pioglitazone was shown to have some benefits on lipid metabolism and cardiovascular risk.7 However, a consensus statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) advised in 2009 to exercise caution in using pioglitazone by its association with fluid retention, congestive heart failure, and fractures, especially in older women.8 A couple of years later, additional concern on pioglitazone use was sparked after its possible association with bladder cancer,9 which was recently confirmed in a meta-analysis of six studies.10
Despite the safety warnings issued for pioglitazone, its utilization continued in several countries without significant reduction.11,12 In addition, pioglitazone is still considered by ADA as a useful alternative to nontolerated metformin monotherapy, with modest cardiovascular benefits.13,14 However, individualized patient risk–benefit assessment has been advised before the use of pioglitazone.15 Therefore, we developed a pioglitazone safety monitoring sheet to choose the optimum candidate patient and to limit the impact of its adverse events. The objective of the current study is to assess the adherence of treating physicians in our primary care center with the components of the safety monitoring sheet and determine the reasons for pioglitazone discontinuation.
Methods
The study was conducted in chronic diseases center (Ch.D.C.) at the Al-Wazarat Healthcare Center (WHC) at a large family medicine center in Riyadh, Saudi Arabia. The total monthly number of diabetic patients served in the unit is ~23,460 patients. Diabetic patients are served by family medicine clinics (six clinics in the morning session and five clinics in the afternoon session) which are run by family medicine physicians. A pharmacy clinic (covers nine sessions per week) is run by a clinical pharmacist specialist, and other clinics are run by diabetic educator, health educator, dietitian, and social worker. The current data were collected in the clinical pharmacist clinic.
Patients with T2D who were receiving care at chronic diseases unit since 2011 and who were using pioglitazone (irrespective of the dose) were potential candidates for the current study. Those who continued their care at another center (N=9) and those who did not return to continue treatment after pioglitazone prescription (N=3) were excluded from the study. As per recommendations of international agencies,13,16 local policies at chronic diseases unit required not to initiate pioglitazone therapy in patients with heart failure, highly elevated liver enzymes (>2.5-fold higher than the upper limit of normal), severe osteoporosis without adequate treatment, and history of cancer bladder.
The study design is a retrospective chart review of patients with T2D, who have been started on oral pioglitazone since January 2011 until the end of December 2014. The study outcomes were monitored during patient visits scheduled during the first 12 months following the start of pioglitazone. The first visit was for determining pioglitazone eligibility and is considered as the baseline. Informed consents were not obtained from the patients as it was considered as monitoring of routine care. The study was approved by the Research Committee of primary health care center in Prince Sultan Military Cardiac Center (PSMMC). The Research Committee at PSMMC waived the requirement for informed consent from patients because this study was a chart review for standard care procedures.
The main study outcomes included the physicians’ adherence to pioglitazone safety monitoring as well as frequency and reasons for pioglitazone discontinuation. Pioglitazone adverse events monitored include weight gain (≥3 kg increase between the first and the last visits), peripheral edema, elevated liver enzymes (threefold higher than upper limit of normal), electrocardiogram (ECG) abnormalities, bone mineral density (BMD) abnormalities (osteoporosis), and hematuria (using urine dipstick). Pioglitazone discontinuation was defined as stopping taking pioglitazone after its initiation, irrespective of the development of adverse events. As per the local policy, a physician should consider discontinuing pioglitazone therapy if any of the above pioglitazone adverse events develops.
Data collection tools
Pioglitazone safety monitoring sheet was used to collect the data. This sheet was locally developed at the chronic disease unit and is required (as per the local policy) to be filled before and following the prescription of pioglitazone. In addition to pioglitazone adverse events described above, patient demographics (age and gender), eligibility to initiate pioglitazone therapy, and the dose of pioglitazone were included.
Statistical methods
Patient demographics, glycemic control, and pioglitazone adverse events were presented as frequency and percentages for categorical variables and mean and standard deviation (SD) for continuous variables. Paired t-test was used to examine the changes in weight and glycemic control between the baseline and last visit. Missing data at a specific visit were defined as withdrawal due to loss to follow-up or lack of documentation of pioglitazone monitoring sheet.
Results
A total of 183 patients (78 males and 105 females) were included in the current analysis. Table 1 shows the demographics and monitoring results during different visits. The average age was 54.6±9.4 years. The baseline weight was 83.6±15.2 kg. The average duration of follow-up was 11.0±3.3 months. The pioglitazone dose received at initiation was 30 mg in 69 (37.7%) patients and 15 mg in 63 (34.4%) patients. During the follow-up period, 9 (4.9%) patients reduced their dose from 30 to 15 mg and 3 (1.6%) patients increased the dose from 15 to 30 mg. No information about the dosage given at initiation was found for 51 (27.9%) patients. The average baseline HbA1c was 9.1±1.7. The patients experienced a significant reduction in HbA1c during the study, with the mean difference between the first and last visits being –1.22±0.79 (p<0.001). At baseline, 3 (1.6%) patients had edema, 6 (3.3%) had abnormal ECG, 9 (4.9%) had elevated liver function tests (LFTs), 6 (3.3%) had osteopenia, 3 (1.6%) had osteoporosis, and 3 (1.6%) had hematuria (Table 1). Table 2 shows the contraindications for the initiation of pioglitazone that have been found in the patients. Only 4 patient records show elevated liver enzymes. However, the patients were on medications.
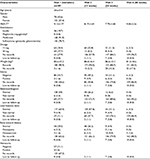  | Table 1 Baseline and follow-up characteristics of the study patients (N=183) Notes: Data presented as mean ± standard deviation or n (%). *Included in the monitoring sheet but no users recorded. |
  | Table 2 Contraindications for the initiation of pioglitazone in the patients records (N=183) |
As shown in Figure 1, the side effect of pioglitazone that was associated with high monitoring adherence (at least two records) was weight gain 173 (94.5%). The side effects with moderate monitoring adherence are LFTs (47.5%) and edema (31.1%) and with low monitoring adherence are BMD (18.0%) and ECG (16.4%). There were no follow-up records for hematuria. Among the 173 patients whose weight was monitored at least twice, the average weight change between the first and last visits was 2.36 kg gain (95% confidence interval of the change was 1.71 to 3.01 kg). This change was statistically significant (p<0.001). As shown in Figure 2, the distribution of weight change shows increasing trend with two-thirds of the observations >0.
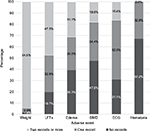  | Figure 1 Percentages of monitoring adverse events among pioglitazone users in the study (N=183). Abbreviations: LFT, liver function test; BMD, bone mineral density; ECG, electrocardiogram. |
  | Figure 2 Distribution of weight change among the study patients (N=173). |
Adverse events of pioglitazone were monitored in 171 out of the 183 patients, with 81 (44.3%) having one or more adverse events. As shown in Figure 3, the most common side effect was weight gain (35.6%) defined as ≥3 kg gain between the first and last visits, followed by osteoporosis (10.5%), edema (8.8%), hypoglycemia (7.0%), elevated liver enzymes (3.5%), and osteopenia (3.5%). There were no reported ECG abnormalities.
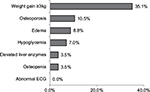  | Figure 3 Percentages of developing adverse events after pioglitazone use among the study patients (N=171). Abbreviation: ECG, electrocardiogram. |
Out of the 183 patients included, 117 (63.9%) discontinued pioglitazone during the study. As shown in Figure 4, the most common reasons for discontinuation of pioglitazone were shifting to insulin therapy (28.2%), significant weight gain (25.6%), lost to follow-up (15.4%), hypoglycemia (10.2%), and osteoporosis (8.4%). The remaining 12.8% of the patients discontinued pioglitazone without reason for discontinuation being reported by their physician.
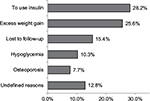  | Figure 4 Reasons for pioglitazone discontinuation among the study patients (N=117). |
Discussion
We are reporting the adherence of physicians to appropriate initiation of pioglitazone and monitoring of its adverse events among patients with T2D seeking care at a large primary care center in Saudi Arabia. The physicians were moderately adherent to filling the safety monitoring sheet. They were adherent to proper pioglitazone initiation. For example, no patients were started on pioglitazone with heart failure or a history of bladder cancer. Even the few patients with elevated liver enzymes or osteoporosis at baseline were found to be mild cases or already on medication. Pioglitazone in practice was shown to be prescribed for patients poorly controlled by other oral hypoglycemic drugs and who have multiple comorbidities, sometimes contraindicated ones.17 However, appropriate choice of patients treated with pioglitazone is critical to reducing the burden of adverse events and to increasing the drug tolerability. For example, in a postmarketing surveillance study conducted in a primary care setting in the UK, it was found that patients who developed adverse events of pioglitazone had high frequency of preexisting medical conditions such as liver problems (6.3%), cardiovascular conditions (43.6%), and obesity (49.5%) and are at higher risk of discontinuation.18
Weight gain was the most frequent adverse event monitored in follow-up visits. This can be attributed to the fact that it is easily measured, routinely done during clinic visits, and it can be self-reported. Other than weight, other adverse events were monitored moderately (such as liver enzymes and edema) or poorly (such as BMD, ECG, and hematuria). We could not identify a similarly designed study to compare the current findings. However, the unsatisfactory frequency of monitoring observed in the current study may be partially explained by the high percentage of pioglitazone discontinuation. In addition, the poor monitoring of BMD and ECG may indicate that these measures were requested for high-risk groups rather than all patients. Interestingly, there were no follow-up records for hematuria. The current recommendations for preventing bladder cancer among those who receive pioglitazone focus on the patient history of the disease or its risk factors (such as age, smoking, and exposure to certain chemicals or treatment) before initiating the drug and patient reports of bladder cancer symptoms (pain and blood in urine) during the follow-up.19,20 Therefore, the physicians probably used patient history rather than using urine dipstick.
Weight gain (≥3 kg) was the most common side effect in the current study, affecting approximately one-third of our patients. This frequency was much higher than that reported in previous studies (<4%).18,21,22 However, when comparing the average amount of weight change, the weight gain in the current study (2.36 kg) is lower than the weight gain reported in other studies (3.0–4.3 kg).18,22,23 The average weight gain over 48-week period was 3 kg in pioglitazone users only and 1.4 kg when dapagliflozin was used with pioglitazone.23 The results of our study may report lower average weight gain due to higher dropout rates among our patients who gained >3 kg in the same period. Weight gain caused by pioglitazone is probably a combination of fluid retention and fat accumulation.24 The rest of the adverse events were comparable to the rates reported before.7,21
Although glycemic control in the current study was significant and comparable to previous studies,6 >60% of our patients discontinued pioglitazone. This issue was considerably higher than that seen in postmarketing surveillance studies where ~30% of the patients discontinue pioglitazone.21 Moreover, it was markedly higher than that seen in controlled studies where only 6%–16% of the patients discontinue pioglitazone.22,25 This discrepancy may be at least partially explained by the fact that two-thirds of our patients discontinued pioglitazone for causes other than adverse events, including physician advice to shift to insulin which was the most frequent cause for discontinuation. Previous studies conducted in a similar setting showed that approximately one-third of the patients who discontinue pioglitazone do that for reasons related to poor glycemic control.18,21 Excess weight gain was the most frequent side effect to cause discontinuation of pioglitazone in our patients. Previous studies showed conflicting findings for the role of weight gain in discontinuation of pioglitazone, with some reporting a major role21 and others reporting a minor role.22
The current study had the advantage of being the first local study to monitor the safety of pioglitazone, in a real time general practice setting among a group of poorly controlled T2D patients. Nevertheless, we acknowledge some limitations such as the retrospective design and possible selection bias. In addition, being a single-center study, the results of the current study should be generalized cautiously to similar populations. However, we believe that sharing our experience may enhance the awareness of pioglitazone safety in a primary care setting and could be used as a basis for future multicenter prospective studies.
Conclusion
This study reports an appropriate initiation of pioglitazone but suboptimal adherence to pioglitazone safety monitoring in a primary care setting. Weight gain was the most frequent side effect and was the most frequently monitored side effect. Also, weight gain was the leading cause of drug discontinuation among adverse effects. The current finding points to the critical need to enforce better the adherence of physicians to pioglitazone safety monitoring by increasing their awareness as well as adding electronic flagging of treated patients.
Acknowledgment
The efforts of Ms Hessa Al Almuqati and Huda Al Enazi in helping Pioglitazone Study literature review are acknowledged and appreciated.
Disclosure
The authors report no conflicts of interest in this work.
References
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. | ||
Al-Nozha MM, Al-Maatouq MA, Al-Mazrou YY, et al. Diabetes mellitus in Saudi Arabia. Saudi Med J. 2004;25(11):1603–1610. | ||
Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65(3):385–411. | ||
Smith U. Pioglitazone: mechanism of action. Int J Clin Pract Suppl. 2001; (121):13–18. | ||
Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000;23(11):1605–1611. | ||
Derosa G. Efficacy and tolerability of pioglitazone in patients with type 2 diabetes mellitus: comparison with other oral antihyperglycaemic agents. Drugs. 2010;70(15):1945–1961. | ||
Desouza CV, Shivaswamy V. Pioglitazone in the treatment of type 2 diabetes: safety and efficacy review. Clin Med Insights Endocrinol Diabetes. 2010;3:43–51. | ||
Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. | ||
Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34(4):916–922. | ||
Ferwana M, Firwana B, Hasan R, et al. Pioglitazone and risk of bladder cancer: a meta-analysis of controlled studies. Diabet Med. 2013;30(9):1026–1032. | ||
Niyomnaitham S, Page A, La Caze A, Whitfield K, Smith AJ. Utilisation trends of rosiglitazone and pioglitazone in Australia before and after safety warnings. BMC Health Serv Res. 2014;14(1):151. | ||
Jain R, Mullins CD, Lee H, Wong W. Use of rosiglitazone and pioglitazone immediately after the cardiovascular risk warnings. Res Social Adm Pharm. 2012;8(1):47–59. | ||
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55(6):1577–1596. | ||
Schernthaner G, Currie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk–benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155–S161. | ||
Govindan J, Evans M. Pioglitazone in clinical practice: where are we now? Diabetes Ther. 2012;3(1):1–8. | ||
Marks DH. Drug utilization, safety and clinical use of Actos and Avandia. Int J Risk Saf Med. 2013;25(1):39–51. | ||
Schofl C, Lubben G. Postmarketing surveillance study of the efficacy and tolerability of pioglitazone in insulin-resistant patients with type 2 diabetes mellitus in general practice. Clin Drug Investig. 2003;23(11):725–734. | ||
Fogg C, Kasliwal R, Shakir SA. Risk management and outcomes of adverse events to pioglitazone in primary care in the UK: an observational study. Drug Saf. 2009;32(3):229–237. | ||
European Medicines Agency. Questions and answers on the review of pioglitazone-containing medicines (Actos, Glustin, Competact, Glubrava and Tandemact); 2011. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2011/07/WC500109179.pdf. Accessed July 1, 2015. | ||
U.S. Food and Drug Administration (FDA). FDA drug safety communication: ongoing safety review of actos (pioglitazone) and potential increased risk of bladder cancer after two years exposure; 2010. Available from: http://www.fda.gov/drugs/drugsafety/ucm226214.htm. Accessed July 1, 2015. | ||
Kasliwal R, Wilton LV, Shakir SA. Monitoring the safety of pioglitazone: results of a prescription-event monitoring study of 12,772 patients in England. Drug Saf. 2008;31(10):839–850. | ||
Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf. 2009;32(3):187–202. | ||
Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on Hb(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35(7):1473. | ||
Basu A, Jensen MD, McCann F, Mukhopadhyay D, Joyner MJ, Rizza RA. Effects of pioglitazone versus glipizide on body fat distribution, body water content, and hemodynamics in type 2 diabetes. Diabetes Care. 2006;29(3):510–514. | ||
Belcher G, Lambert C, Edwards G, Urquhart R, Matthews DR. Safety and tolerability of pioglitazone, metformin, and gliclazide in the treatment of type 2 diabetes. Diabetes Res Clin Pract. 2005;70(1):53–62. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
