Back to Journals » Drug Design, Development and Therapy » Volume 15
Clinical Application of Cytokines in Cancer Immunotherapy
Authors Qiu Y, Su M, Liu L , Tang Y , Pan Y , Sun J
Received 2 March 2021
Accepted for publication 20 April 2021
Published 27 May 2021 Volume 2021:15 Pages 2269—2287
DOI https://doi.org/10.2147/DDDT.S308578
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Yi Qiu,1– 3 Mengxi Su,1,3 Leyi Liu,1,3 Yiqi Tang,1,3 Yuan Pan,1,3 Jianbo Sun1,3
1Guanghua School of Stomatology, Hospital of Stomatology, Sun Yat-Sen University, Guangzhou, People’s Republic of China; 2Zhongshan School of Medicine, Sun Yat-Sen University, Guangzhou, People’s Republic of China; 3Guangdong Provincial Key Laboratory of Stomatology, Guangzhou, People’s Republic of China
Correspondence: Jianbo Sun
Guanghua School of Stomatology, Hospital of Stomatology, Sun Yat-Sen University, No. 74, Zhongshan Er Road, Guangzhou, Guangdong, 510080, People’s Republic of China
Tel +86-20-87330589
Email [email protected]
Abstract: Cytokines are key components of the immune system and play pivotal roles in anticancer immune response. Cytokines as either therapeutic agents or targets hold clinical promise for cancer precise treatment. Here, we provide an overview of the various roles of cytokines in the cancer immunity cycle, with a particular focus on the clinical researches of cytokine-based drugs in cancer therapy. We review 27 cytokines in 2630 cancer clinical trials registered with ClinicalTrials.gov that had completed recruitment up to January 2021 while summarizing important cases for each cytokine. We also discuss recent progress in methods for improving the delivery efficiency, stability, biocompatibility, and availability of cytokines in therapeutic applications.
Keywords: cytokine therapy, cancer immunity cycle, cancer immunotherapy, clinical trial, nanomedicine
Introduction
Cancer is a disease characterized by the abnormalities in the regulation of cell proliferation and differentiation. Many factors contribute to cancer development including genetics,1 lifestyle, and environmental carcinogens, among others.2 Cancer is the second leading cause of death worldwide after cardiovascular disease, accounting for 9.6 million deaths in 2018 according to data from the International Agency for Research on Cancer. Lung cancer is the leading cause of cancer death (18.4%), followed by breast cancer (11.6%), and prostate cancer (7.1%).3 Clinical manifestations include pain, bleeding, lumps and ulcers at the site of disease, along with systemic symptoms such as weight loss and fatigue leading to cachexia. Traditional treatment modalities including surgery, radiotherapy, and chemotherapy have various disadvantages and cause side effects that are in some cases severe. Immunotherapies such as blockade of programmed death (PD)-1 and programmed death ligand 1 (PD-L1) immune checkpoints; chimeric antigen receptor T cell immunotherapy (CAR-T); using the monoclonal antibody against cancer antigen; and cytokine therapy offer a promising alternative to the conventional treatment approaches for cancer.4 In particular, cytokine therapy has shown encouraging results in both basic and clinical research settings.5
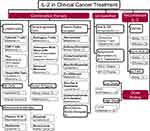
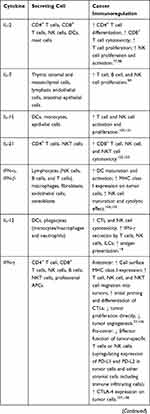
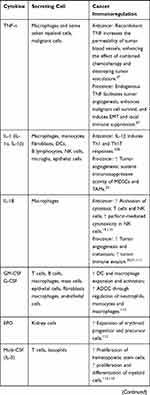
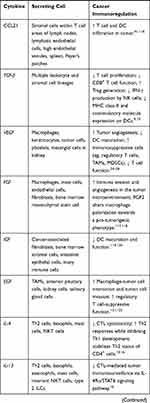
Cytokines are small proteins produced by various cells (immunocytes and non-immunocytes) as molecular messengers to communicate with each other or with other cells. Cytokines have versatile roles in several steps of the cancer immunity cycle including cancer antigen presentation, T cell priming and activation, effector T cell infiltration in cancer site, and cancer cell death, as shown in Figure 1. More importantly, cytokine-mediated signaling pathways control the direction of naïve CD4+ T cell differentiation and thus determine the effects of anticancer immunity (Figure 2 and Table 1). Briefly, transforming growth factor β (TGF-β) signaling in naïve CD4+ T cells is required for the differentiation of regulatory T cells (Tregs) and T helper type 17 (Th17) cells, both of which promote tumor progression. Additionally, Th17 cell differentiation and clonal expansion require a cocktail of cytokines (IL-6, IL-21, IL-23, IL-1β, and TGF-β).6–10 IL-17 secreted by Th17 cells guides macrophages and neutrophils to cancer sites and induces cancer-promoting inflammation. Th17 cells themselves also exert antitumor effects in the melanoma microenvironment by potentiating the functions of CD8+ T cells and T helper type 1 cells (Th1 cells).11 IL-10, IL-11, IL-4, and IL-13 are critical for the differentiation and development of T helper type 2 cells (Th2 cells),12–17 whereas IL-12, IL-18, IL-1β, and interferon (IFN)-γ promote Th1 cell development and activity.18–23 Th1 cells modulate tumor-suppressing pathways by stimulating IFN-γ secretion and enhancing the cytotoxicity of natural killer (NK) cells and CD8+ T cells, while Th2 cells inhibit the anticancer immune responses by blocking Th1 cell differentiation and the release of IFN-γ. Vascular endothelial growth factor (VEGF) and tumor necrosis factor (TNF)-α promote cancer progression by directly facilitating angiogenesis, although recombinant TNF-α has been shown to enhance the effect of combined chemotherapy regimens by increasing the permeability of tumor blood vessels.24–27
 |
 |
 |
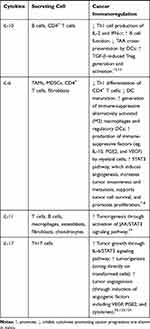 |
Table 1 Varied Roles of Cytokines in Anticancer Immunity |
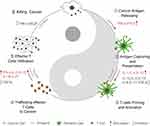 |
Figure 1 Cytokines in the cancer immunity cycle.6 1) Antigens from dead cancer cells are captured by APCs, mainly by DCs. 2–3) DCs present cancer antigens to T cells to prime the adaptive immune response. 4–5) Activated effector T cells infiltrate cancer cells and then 6) kill cancer cells. Dead cancer cells release cancer antigens to continue the immune cycle. Cytokines that have been shown to promote or inhibit the anticancer immune responses are highlighted. Abbreviations: IFN, interferon; IL, interleukin; TGF-β, transforming growth factor β; TNF, tumor necrosis factor. |
Because the roles of cytokines are diverse and precise applications of cytokines are greatly needed, it is urgent to update the progresses of cancer immunotherapy with cytokines. Here, we review total 2339 clinical trials using or by targeting cytokines for precise treatment of cancers registered with ClinicalTrials.gov; summarize the therapeutic efficacy of typical cytokines based on clinical data; and highlight progress in the development and application of nanomaterials for cytokine-based therapy.
Screening for and General Status of Clinical Trials with Cytokine-Based Drugs in Cancer Therapy
In order to review clinical application of cytokines in cancer therapy, we have searched all the known cytokines in ClinicalTrials.gov. In the advanced search page of ClinicalTrials.gov, we entered “cytokine name”, such as “IL-2”, in other term section and chose “completed” in the recruitment status section. Then, we download the search results and screen the trials item by item to make sure that the intervention of the trial includes cytokine-based drugs and the condition of the trial is cancer. As a result, we got 25 cytokines with clinical trials that had completed recruitment in ClinicalTrials.gov, and we also checked 2 cytokines (IL-10 and IL-17) without published clinical studies for cancers because of their crucial roles in anticancer immunity. Finally, we screen out 2630 clinical trials using cytokines as either therapeutic agents or targets in treating cancers registered with ClinicalTrials.gov that had completed recruitment up to January 2021.
It is interesting that G-CSF, GM-CSF, VEGF, IL-2 and IFN-γ are the five most studied cytokines (Figure 3A and Supplementary Table 1), which could be explained by the fact that they have been discovered and clinically studied very early (Figure 4A and B) and they play very important roles in cancer treatment. VEGF is the most studied target for the treatment of most types of cancer because the role of VEGF in angiogenesis induction, cell proliferation and promoting vascular permeability is extremely important for cancer growth, migration and infiltration. CSF can promote proliferation and differentiation of multiple immune cells such as macrophage, granulocytes, and mononuclear phagocytes, and thus is widely used as medication to stimulate the production of white blood cells following chemotherapy. Similarly, IL-2 is used to stimulate T cell production for enhancing anti-cancer immunity. IFN-γ can directly inhibit tumor cell proliferation and augment anti-tumor immunity by promoting MHC expression, antigen presentation, and the function of tumor-infiltrating Th1 cells, CTLs and macrophages. The clinical trials of cytokines cover nearly all cancer types (Figure 3B) but most of the clinical trials are done on melanoma and hematological malignancies because the two cancer types have better responses and outcomes than other cancers in the immune therapy.28,29
 |
Figure 4 Historical timelines of cytokine research. (A) Timeline of cytokine discovery. The time point is the year in which the cytokines, EPO,69 IFNs,70,71 EGF,72 G-CSF,73,74 FGF,75 IL-1,76 IL-2,77 IGF,78 TNF,79 GM-CSF,80 TGF-β,81 IL-3,82 IL-4,83 IL-6,84 IL-7,85 IL-10,86 IL-12,87 IL-13,88 VEGF,89 IL-11,90 IL-15,91 IL-17,92 IL-18,93 IL-21,94,95 and CCL21,96,97 were first described. (B) Timeline of the first clinical trials of cytokines for cancer treatment. The time point is the year that the trial was first registered with ClinicalTrials.gov. Clinical trial registry (NCT) numbers are shown. |
The year of discovery of each cytokine and the year of the first clinical trial with the cytokine for cancer treatment are shown, respectively, in Figure 4A and B, which gives a visualized understanding of research progresses of cytokines in certain years. The main cytokines were discovered in the last 3 decades of last century and the clinical trials were carried out intensively between 1998 and 2008. The interval time from the discovery to the first clinical trial of certain cytokine is varied with the maximum of 95 years (EPO) and minimum of 7 years (IL-21). The cytokine-based drugs could be grouped into two types: cytokine drugs and drugs targeting cytokines.
Clinical Application of Cytokines as Cancer Therapeutic Agents
IL-2, type I IFN, IL-12, chemokine (C-C motif) ligand (CCL) 21, and colony-stimulating factors (CSF) family cytokines are known to promote anticancer immunity. Although IFN-γ, TNF-α, and IL-1 families play a dual role in the cancer immunity cycle, they are widely studied for their anticancer activity. In this section, we present the efficacy of these cytokine-based drugs in cancer treatment.
IL-2
There are 268 trials registered with ClinicalTrials.gov using IL-2 for cancer treatment. Of the 52 trials for which results are available, 7 treated cancer with IL-2 alone, including 3 trials using IL-2 and 4 using IL-2 derivatives (hu14.18-IL12, denileukin diftitox [ONTAK®], and ALT-801) for the treatment of melanoma, breast cancer, metastatic renal cell carcinoma (mRCC), and neuroblastoma. There were 45 trials investigating the effects of IL-2 combined with other therapies. In general, melanoma and leukemia responded better than other types of cancer to IL-2 treatment and IL-2 performed more outstanding when combined with other therapies in cancer treatment. The objectives of clinical studies using IL-2 in cancer treatment are summarized in Figure 5.
In 1992, high-dose aldesleukin became the first cytokine approved by the US Food and Drug Administration (FDA) for the treatment of mRCC based on an objective response rate (ORR) of 14% in 255 patients.30 In 2006, a new trial using high-dose aldesleukin for the treatment of mRCC was conducted by the Cytokine Working Group to evaluate the clinical utility of PD-L1, B7 homolog 3 protein, carbonic anhydrase 9, plasma VEGF, and fibronectin levels as biomarkers for therapeutic response monitoring. PD-L1 and B7 homolog 3 protein were identified as candidate markers but require independent validation.31 The IL-2 derivative hu14.18-IL-2, which consists of 2 molecules of IL-2 covalently linked via the Fc region, has demonstrated long-term tumor control in animal models.32 In Phase I and II trials, hu14.18-IL-2 prolonged the tumor-free survival period in some patients with recurrent stage III or stage IV melanoma following resection.33
The anticancer efficacy of IL-2 may be enhanced when it is used in combination with other immunotherapies and chemotherapy agents. In one trial, 6 of 11 patients with non-Hodgkin lymphoma treated with IL-2 plus rituximab achieved complete or at least partial remission (NCT00994643). A Phase III trial reported that IL-2 combined with other immunotherapeutic reagents, including dinutuximab and granulocyte/macrophage (GM)-CSF, enhanced the efficacy of isotretinoin in the treatment of neuroblastoma after stem cell transplantation; the 3-year event-free survival rates for isotretinoin with and without immunotherapy is 62.9% against 48.1%, respectively (NCT00026312). Results from 3 other trials supported the survival benefits of combination treatment (NCT01334515, NCT01592045, and NCT01041638). In addition to immunotherapy, data from 27 trials suggest that chemotherapy drugs such as ONTAK®, etoposide, cyclophosphamide can increase the antitumor activity of IL-2.
Other IL-2 Family Members
Given the therapeutic effects of IL-2, other members of the IL-2 family including IL-7, IL-15, and IL-21, that are known to act independently or synergistically with IL-2 in the anticancer immune response have been investigated for the treatment of breast cancer, renal cell cancer, melanoma, and leukemia. However, in a trial of IL-7 in patients with metastatic castration-resistant prostate cancer (NCT01881867), the number of T cells per 300,000 peripheral blood mononuclear cell was not higher than in the comparator group. In trials investigating the efficacy of intravenous (NCT01385423 [phase I]) or subcutaneous (NCT02395822 [phase II]) recombinant human IL-15 in enhancing the effects of NK cell therapy in patients with acute myelogenous leukemia, 32% of patients in the phase I trial and 40% of those in the Phase II trial achieved complete remission.34 In a phase II trial evaluating the efficacy and safety of IL-21 in the treatment of malignant melanoma (NCT01152788), IL-21 did not demonstrate a clinical benefit over dacarbazine, with a progression-free survival (PFS) of 1.87 vs 2.04 years, although IL-21 was associated with fewer adverse events.
Type I IFNs
Type I IFNs including IFN-α and IFN-β play an essential role in the presentation of cancer antigens by mediating the maturation and activation of dendritic cells (DCs) and inducing the expression of major histocompatibility complex I molecules on tumor cells.35,36 Since 1996, there have been 248 trials investigating the therapeutic potential of IFN-α in the treatment of cancers including melanoma and leukemia, with results for 76 available on ClinicalTrials.gov. Although there is in vitro evidence that IFN-β more potently inhibits tumor cell proliferation than IFN-α, there have been no clinical trials demonstrating its efficacy in cancer therapy.
A study conducted from 1988 to 2010 evaluating the efficacy of high-dose IFN-α-2b in 1150 patients who had undergone resection for stage II or III melanoma (NCT00003641) found no improvements in 5-year relapse-free survival and overall survival (OS) rates. In addition to treating melanoma, IFN-α has been used as first-line treatment for mRCC, but was found to be less effective than the tyrosine kinase inhibitor su011248 in a phase III trial (NCT00083889). Various forms of IFN-α including pegylated (PEG)-IFN-α and recombinant adenovirus (rAd)-IFN (encoding IFN-α2b) have been evaluated in clinical studies. Two trials compared the efficacy of PEG-IFN-α and IFN-α in different types of cancer; in patients with melanoma, the median OS was 25.63 months with PEG-IFN-α vs 20.67 months with IFN-α (NCT03552549), whereas in chronic myelogenous leukemia, the 12-month survival rate was slightly higher in the IFN-α group than in the PEG-IFN-α group (91.3% [158/173] vs 90.1% [154/171]) (NCT03547154). In both trials, more severe adverse effects were reported in patients receiving PEG-IFN-α treatment. In another phase II study (NCT01687244), rAd-IFN showed promising results in patients with Bacillus Calmette-Guérin-refractory or relapsed bladder cancer.
The antitumor activity of IFN-α can be dramatically enhanced by including other types of immunotherapy in the treatment regimen. In a phase III trial initiated in 2004 (NCT00738530), 649 patients with mRCC received IFN-α alone or with bevacizumab; PFS was 5.5 and 10.2 months, respectively, and ORR was 12.5% and 32.4%, respectively. When the chemotherapy drug vinblastine was added to the regimen, the PFS was increased to 274 days (NCT00520403). Results from 5 other trials supported the effects of IFN-α in combination with bevacizumab. A trial assessing the efficacy of pembrolizumab (anti-PD-1) plus sylatron (PEG–IFN-α-2b) for the treatment of advanced cholangiocarcinoma was initiated in 2017, but no patients completed the study due to adverse effects (NCT02982720).
Type II IFN
As the sole type II IFN, IFN-γ is a typical pro-inflammatory cytokine that exerts antitumor effects by suppressing proliferation and promoting apoptosis in tumor cells and inducing necrotic death and inhibiting angiogenesis in tumors. However, IFN-γ was shown to upregulate PD-L1 expression on tumor cells, which suppressed anticancer immunity through the binding of PD-L1 to its receptor PD-1 on lymphocytes.23 Despite these conflicting roles in cancer, the therapeutic potential of recombinant or adenovirus-delivered IFN-γ is being investigated in 22 trials, although only 2 have posted results. In a phase II trial (NCT00501644), 59 patients with ovarian or fallopian tube cancer or primary peritoneal cancer were treated with subcutaneous GM-CSF and IFN-γ before and after intravenous carboplatin; the ORR was 56% and median time to progression was 6 months. However, there was no control group in this trial. Another phase II trial assessed the efficacy of IFN-γ combined with 5-fluorouracil (FU), leucovorin, and bevacizumab in patients with metastatic colorectal cancer (CRC) (NCT00786643), but the specific contribution of IFN-γ to the treatment effect was not investigated. In summary, the efficacy of IFN-γ in cancer therapy has yet to be established.
IL-12
IL-12, which is mainly produced by antigen-presenting cells, plays an important role in regulating innate and adaptive immune responses. There are 47 registered Phase I–III trials evaluating the efficacy and safety of intratumoral IL-12 administration either alone (22 trials) or with other immunotherapies (eg, DCs, T cells, and vaccines; 17 trials) for the treatment of melanoma, Merkel cell carcinoma, ovarian carcinoma, head and neck squamous cell carcinoma, and other cancers. In most cases a plasmid encoding IL-12 was used. A phase III trial that enrolled 51 patients with melanoma optimized the therapeutic strategy (NCT01502293): patients underwent 5 treatment cycles at 3-month intervals consisting of 3 intratumoral injections of IL-12 plasmid immediately followed by in vivo electroporation, which resulted in an ORR of 32.1% higher than the other two groups (underwent 9 cycles [25.0%] and 2 cycles [25.0%] at 6-week intervals, respectively). On the other hand, tumor-infiltrating CD8+T cells expressing IL-12 showed unsatisfactory results for the treatment of metastatic melanoma in a phase I/II trial (NCT01236573).
TNF
TNF was initially recognized as an antitumor cytokine. However, endogenous TNF induces the expression of multiple cytokines that act on M2 macrophages to stimulate the extracellular matrix remodeling as well as the differentiation of myeloid endothelial progenitor cells, which promotes tumor angiogenesis.27 These findings suggest that TNF can serve as either therapeutic target or agent. The first clinical trial of TNF for cancer treatment was initiated in February 1992; to date, there have been 20 trials involving at least 1152 participants in which TNF or related biological agents were used to treat 3 main tumor types—namely, melanoma, CRC, and head and neck cancer. Only one study has published results. Etanercept, a TNF inhibitor, was investigated for the treatment of idiopathic pneumonia in patients with leukemia and lymphoma after stem cell transplantation (NCT00309907), but the results did not reflect the effect of TNF inhibitor.
CSF Family
The anticancer efficacy of CSF family cytokines including GM-CSF, granulocyte (G)-CSF, erythropoietin (EPO), and IL-3, has been widely studied in clinical settings. To date, 1311 clinical trials enrolling over 200,000 cancer patients treated with GM-CSF and G-CSF alone or in combination have been registered at ClinicalTrials.gov; of these, 96% and 94% studied the effects of GM-CSF and G-CSF in combination therapy, respectively (Figure 6A).
EPO exhibits pro-proliferative and anti-apoptotic activities in multiple nonhematopoietic cell types including tumor cells.37 EPO has been used to alleviate cancer- and chemotherapy-related anemia. The first clinical trial of EPO for cancer treatment was initiated in 2003 and to date, 15 trials without results have been published at ClinicalTrials.gov.
IL-3, also known as multi-CSF and hematopoietic cell growth factor, has been the focus of 7 clinical trials. A single-arm trial study evaluating the efficacy of DT388IL3 fusion protein for the treatment of patients with acute myeloid leukemia or myelodysplastic syndromes reported an overall response rate of 81.8% (NCT00397579).
IL-1 Family
IL-1 and IL-18 are members of the IL-1 family; IL-1 is an important regulator in innate immunity,38 and both cytokines stimulate IFN-γ production by T cells and NK cells. IL-1 has dual roles in anticancer immune response. Clinically, patients with high IL-1 concentrations in tumors have poor prognoses.39 Anakinra is an IL-1 receptor antagonist that is commonly used to treat rheumatoid arthritis; its antitumor efficacy has been assessed in 8 trials. In a phase II trial, anakinra combined with dexamethasone was used to treat multiple myeloma and plasma cell neoplasm (NCT00635154); the 6-month progression-free rate was 90.7%. 6 clinical trials are investigating a recombinant human IL-18, namely SB-485232, for the treatment of patients with melanoma, lymphoma, and ovarian neoplasms, but no results have been published.
CCL21
Chemokines and their receptors mediate immunocytes trafficking into the cancer microenvironment, playing roles in promoting or inhibiting cancers. CCL21, together with CCL19, regulates the migration of DCs and T cells to secondary lymphoid organs when binding to their receptor CCR7, thus plays an important role in adaptive immunity and immune tolerance.40 Intratumoral injection of CCL21 enhances the infiltration of T cells and DCs in tumor.41 To date, 3 cancer clinical trials using CCL21 have been registered at ClinicalTrials.gov. In a phase II trial (NCT01433172), CCL21 combined with GM.CD40L vaccine (tumor antigen expressing GM-CSF and CD40L) was used to treat lung adenocarcinoma; the 6-month progression-free survival rate was higher in the combination group than in the GM.CD40L group (15.2% [5/33] vs 9.4% [3/32]).42 For chemokine (C-X-C motif) ligand (CXCL)12, CXCL8, CCL2, CCL3 and CCL5 which are involved in cancer progression and metastasis, few clinical trials studied drugs targeting their receptors, CXCR4, CXCR 1/2 and CCR2, and their effects for cancers have not been verified.43
Clinical Application of Drugs Targeting Cytokines in Cancer Therapy
TGF-β, VEGF, epidermal growth factor (EGF), insulin-like growth factor (IGF) and fibroblast growth factor (FGF), IL-4, IL-13, IL-10, IL-6, IL-11, and IL-17 are known to inhibit anticancer immune response. In this section, we present efficacy of cancer therapy by targeting these cytokines.
TGF-β Inhibitors
TGF-β is an oncogenic factor that facilitates evasion of systemic immune surveillance.44 The clinical efficacy of various inhibitors of TGF-β signaling including GC1008 (fresolimumab, anti-TGF-β monoclonal antibody), TEW-7197 (TGF-β receptor activin-like kinase [ALK]4/ALK5), and AP 12009 (TGF-β2 antisense oligodeoxynucleotide) has been investigated in metastatic breast cancer, RCC, recurrent or refractory high-grade glioma, and advanced melanoma. In a phase II trial examining the efficacy and safety of combined fresolimumab (1 or 10 mg/kg) and local radiotherapy in the treatment of metastatic breast cancer (NCT01401062), overall response rates were 100% with both low and high drug doses and the rate of serious adverse events was 27% and 25%, respectively. The results of 7 other trials of TGF-β inhibitors in cancer treatment have yet to be reported.
Inhibitors of Growth Factor Receptors
Angiogenesis is a vital step in tumor progression and metastasis. Sustained expression of VEGF during tumor development induces the formation of tumor vasculature.45 Various VEGF receptor (VEGFR) inhibitors either alone or in combination with other drugs have been investigated for cancer treatment. These inhibitors include antibodies against VEGFR (eg, bevacizumab, ramucirumab, and ranibizumab); inhibitors of receptor protein kinases (eg, axitinib and vandetanib); soluble decoy receptors containing VEGFR domains (eg, aflibercept); and small molecules that interfere with the binding sites of VEGFR (eg, vatalanib). There are 301 trials registered at ClinicalTrials.gov for the treatment of various cancers (CRC, breast cancer, ovarian cancer, non-small-cell-lung cancer, lymphoma, etc) using VEGFR inhibitors, of which 35% have examined the effects of VEGFR inhibitor monotherapy (Figure 6A).
A large phase III clinical trial that enrolled 1690 participants investigated the efficacy of docetaxel alone or with vandetanib in non-small-cell-lung cancer (NSCLC) (NCT00312377). Median PFS was longer with the combination therapy than with docetaxel alone (17.3 vs 14 weeks), although median OS was comparable between the 2 groups (10.6 vs 10 months). In another phase III trial of 913 patients with NSCLC (NCT00532155), aflibercept increased the median OS of docetaxel from 10.05 to 10.41 months and prolonged median PFS from 4.11 to 5.19 months. In a phase III trial examining the efficacy of aflibercept vs a placebo in 1226 patients with metastatic CRC who had failed to respond to the FOLFIRI regimen (irinotecan, 5-FU, and leucovorin) (NCT00561470), median OS was increased from 12.6 to 13.5 months while median PFS was increased from 4.67 to 6.90 months. Besides combination with chemotherapy, the efficacy of VEGF inhibitors combined with other immunotherapies has been evaluated in 40 clinical trials. As described in the paragraph of IFN-α, bevacizumab in conjunction with IFN-α showed clinical benefits in mRCC and melanoma patients. Thus, VEGFR inhibition is an effective therapeutic strategy for the treatment of multiple cancers.
In addition to VEGF, growth factors such as EGF, IGF and FGF, have been shown to be crucial for the development and progression of certain cancers. Clinically, human epidermal growth factor receptor 1 and 2 (HER1 and HER2), IGF-1 receptor (IGF-1R) and FGF receptor (FGFR) have been found to be overexpressed in various cancers, particularly in breast and lung cancers.46–48 There are 206, 71 and 14 trials for blocking EGFR, IGF-1R and FGFR, respectively, with small molecule inhibitors or monoclonal antibodies in treating cancers registered with ClinicalTrials.gov that had completed recruitment. Unsurprisingly, most of these trials are for lung and breast cancers: 71/206 trials of HER1/2 inhibitors and 16/71 of IGF-1R inhibitors are for treating lung cancers; and 44/206 trials of HER 1/2 inhibitors and 9/71 trials are for treating breast cancers. Their effects in combination of chemotherapeutics have been generally studied. A phase II trial (NCT00986674) demonstrated that carboplatin and paclitaxel are more effective when given with cixutumumab (anti-EGFR antibody) and cetuximab (anti-IGF-1R antibody) than with cetuximab alone in treating advanced non-small cell lung cancer, with overall response rates of 22%, 21.7% and 11%, respectively. However, a phase II trial (NCT00684983) showed that cixutumumab did not enhance the effects of capecitabine and lapatinib ditosylate (EGFR and HER2 inhibitors) in treating HER2-positive stage IIIB-IV breast cancers. Five trials with published results showed limited effects of FGFR inhibitors. A phase II trial studied the effects of dovitinib (a multitargeted inhibitor of FGFR and VEGFR) for patients with advanced lung cancer or CRC who have progressed on anti-VEGF treatment, and the overall response rate was 14.3%. Overall, blockade of growth factor receptors brings considerable therapeutic effects when combining with chemotherapy in treating certain cancers.
IL-4, IL-13, and IL-10
IL-4 and IL-13 function as immunosuppressive cytokines that inhibit antitumor immunity by enhancing the Th2 cell response and blocking Th1 cell differentiation.16 Mutated forms of IL-4 and IL-13 receptors highly expressed in multiple human tumor cell lines.49,50 Based on these observations, targeted drugs were developed by linking Pseudomonas exotoxin to IL-4 or IL-13 (IL4-PE38KDEL and IL13-PE38QQR, respectively).
Since 2001, there have been 7 cancer trials of IL-4 registered at ClinicalTrial.gov. It was shown in vitro that IL-4 can inhibit the growth of Kaposi sarcoma cells,51 and one trial assessed the efficacy of IL-4 in the treatment of 48 patients with Kaposi sarcoma (NCT00000769) although no findings have been published. IL-4 was also administered as an adjuvant to enhance the effect of a DC vaccine in the treatment of Wilms’ tumor (NCT00001564) and Ewing sarcoma (NCT00923910),52 but the outcome of these trials is unknown.
The first clinical trial using IL13-PE38QQR (for the treatment of malignant gliomas) was initiated in 2000. Since then, there have been 10 clinical trials involving over 500 participants with malignant gliomas who were treated with IL-13-PE38QQR. It is difficult to conclude these trials as the results have not been published. In one phase III trial of 300 patients with recurrent malignant gliomas (NCT00064779), IL13-PE38QQR was directly infused into the tumor tissue for 96 hours. After 15 days, patients underwent surgery to excise the recurrent tumors and received another infusion. However, no results have been posted for this or any other trial investigating IL13-PE38QQR.
IL-10 functions as an immune suppressor that inhibits the cancer immunity cycle.53 To date, there have been no reports from ClinicalTrial.gov evaluating the efficacy of IL-10 for cancer treatment, although many trials have examined the use of IL-10 for the treatment of autoimmune disease such as rheumatic arthritis.
IL-6, IL-11, and IL-17
The IL-6 cytokine family, which includes IL-6 and IL-11, participates in the activation of oncogenic signal transducer and activator of transcription (STAT)3.54 Twenty trials of IL-6 for cancer treatment (multiple myeloma, lymphoma, mRCC, and prostate cancer) have been registered at ClinicalTrials.gov, mostly involving siltuximab, an IL-6 antagonist approved by the FDA for the treatment of multicentric Castleman disease. In a phase II trial of 88 patients with myeloma (NCT00911859), siltuximab combined with VELCADE (a prescription medication for myeloma) resulted in a higher complete response rate (26.5% vs 22.4%) and overall response rate (87.8% vs 79.6%) than VELCADE alone; in the second part of this trial (286 patients; NCT00401843), PFS of the 2 groups was 245 and 232 days, respectively. Given its role in hematopoiesis, IL-11 has been investigated for its potential to increase platelet counts in patients with chronic myelogenous leukemia in 2 trials.
IL-17 is a pro-oncogenic cytokine that is mainly produced by Th17 cells and induces the production of IL-6 by tumor cells to activate the IL-6/STAT3 signaling pathway.55 Elevated IL-17 expression is related to poor prognosis in patients with invasive ductal carcinoma. But no cancer clinical trial using or targeting IL-17 has been published in ClinicalTrial.gov.
Recent Progress in Enhancing the Efficacy of Cytokines
According to review of hundreds of clinical trials, we know that efficacy of cytokines as therapeutic drugs on clinical outcomes are limited. One possible reason for this is that because of the short half-life of cytokines in the blood, frequent high doses are required to achieve lasting therapeutic effects. For example, the effective dosage of IL-2 is 600,000 IU/kg administered every 8 hours for 5 days; moreover, 3 treatment cycles are needed for its activity. Because of this, adverse events generally occur in patients receiving cytokine therapy, include fatigue, chills, fever, chest pain, and musculoskeletal pain.56 More serious adverse events are gastrointestinal disorders (eg, stomachache, diarrhea, and gastritis), cardiac abnormalities (eg, myocardial infarction, nodal tachycardia), and disorders of the immune system (eg, anaphylaxis) and blood and lymphatic systems (eg, anemia and febrile neutropenia).
Nanomaterials used as carriers to deliver cytokines to target tissues can improve the stability of cytokines in blood and reduce their toxicity. At the same time, the unique features of nanomaterials have advantages for the therapeutic application of cytokines including aqueous solubility, prolonged circulation time, and preferential accumulation at tumor sites.57 Recent studies on nanomaterials used for cytokine loading are summarized in Table 2.
 |
Table 2 Nanomaterials for Therapeutic Delivery of Cytokines |
Nanomaterials can improve the stability and bioactivity of cytokines. For example, sustained released over a period of 1 month was achieved for IFN-α encapsulated in poloxamer-blend microspheres.58 Chitosan coated with pJME/GM-CSF (plasmid DNA) was more effective than naked pJME/GM-CSF in promoting DC recruitment.59 Nanoscale liposomal polymeric gels loaded with TGF-β inhibitor and IL-2 delayed tumor growth and increased NK cell activity and the number of tumor-infiltrating T cells.60 Nanomaterials can also reduce the toxic effects of cytokine therapy; for instance, encapsulation in PEG liposomes abrogated the diarrhea induced by TNF‐α in rats with subcutaneous BN175 sarcomas.61 Gold nanoparticles were found to enhance the accumulation of TNF around blood vessels in a mouse model of epithelial carcinoma, leading to a significant decrease in tumor volume.62 Magnetic nanoparticles carrying human IFN-α2b were enriched in the liver upon application of a magnetic field and compared to the control group, the volume of human liver cancer cell-derived tumors in nude mice was reduced by about 30%.63 Nanomaterials are good auxiliaries for cytokine gene therapy. Chitosan coated with plasmids encoding the cytokines IL-15 and IL-21 suppressed tumor growth and prolonged survival in mice.64,65 PEG–poly (lactic-co-glycolic acid)–PEG nanoparticles were shown to be effective carriers for IL18 gene delivery.66 Magnetic nanoparticles carrying a plasmid encoding a small interfering RNA targeting gene encoding epidermal growth factor receptor reduced endogenous epidermal growth factor receptor expression in U251 glioma cells, resulting in tumor regression in vivo.67 Two clinical trials involving 168 patients have investigated the efficacy of colloidal gold-bound TNF for the treatment of primary or metastatic cancer (NCT00356980 and NCT00436410, respectively), but the results have yet to be reported.
Besides, artificial oncolytic viruses are well-established carriers for cytokine gene therapy. There are 17 cytokines including CCL2, CCL5, CCL19, CXCL11, FGF2, FLT3L, GM-CSF, IFN-α/β, IFN-γ, IL-2, IL-7, IL-12, IL-15, IL-18, IL-23, IL-24, and TNF-α that are delivered by artificial oncolytic viruses derived from adenovirus, herpesvirus, paramyxovirus, poxvirus, or rhabdovirus. More details could be found in the recent review wrote by Pol et al.68
Although animal studies showed that properties and efficacy of cytokine-based drugs can be improved by nanomaterials, sufficient clinical studies are required to support the conclusion. Two ongoing clinical trials involving 168 patients aim to investigate the efficacy of colloidal gold-bound TNF for the treatment of primary or metastatic cancer (NCT00356980 and NCT00436410, respectively), but the results have yet to be reported.
Conclusions
As important immune regulators, cytokine-based drugs offer many possibilities for cancer treatment. Large amounts of cytokines can be readily produced using eukaryotic or prokaryotic expression systems as the cDNA sequences of most cytokines are available, which makes cytokines attractive to new drug development. However, our statistical results (Supplementary Table 1) indicate that a large number of clinical trials of cytokine-based drugs ended up without published results mainly because of the low efficacy, serious adverse effects, and antagonistic roles in immunoregulation. These problems are partly overcome by delivering with nanomaterials or oncolytic viruses in animal experiments, or combining with immunotherapies or chemotherapeutic agents or both (Figure 6B and C) in clinic. Such strategies would be used and improved in the future clinical trials. Moreover, clarifying the immune-regulatory mechanisms of cytokines can improve their efficacy and safety in cancer therapy.
Abbreviations
APC, antigen-presenting cell; B7-H3, B7 homolog 3 protein; CAR-T, chimeric antigen receptor T cell; CCL, C-C motif chemokine ligand; CRC, colorectal cancer; CSF, colony-stimulating factor; CTL, cytotoxic T lymphocyte; CTLA-4, cytotoxic T lymphocyte-associated protein 4; CXCL, chemokine (C-X-C motif) ligand; DC, dendritic cell; EGF, epidermal growth factor; EMT, epithelial-to-mesenchymal transition; EPO, erythropoietin; FDA, US Food and Drug Administration; FGF, fibroblast growth factor; Fu, fluorouracil; G, granulocyte; GM, granulocyte/macrophage; IFN, interferon; IGF, insulin-like growth factor; IL, interleukin; ILC, innate lymphoid cell; i.v., intravenous injection; IU, international unit; JAK, Janus kinase; MDSC, myeloid-derived suppressor cell; MHC, major histocompatibility complex; mRCC, metastatic renal cell carcinoma; NK, natural killer; NKT, natural killer T; NSCLC, non-small-cell-lung cancer; ORR, objective response rate; OS, overall survival; PD-1, programmed death 1; PD-L1, programmed death ligand 1; PEG, pegylated; PFS, progression-free survival; PGE2, prostaglandin E2; PLGA, poly (lactic-co-glycolic acid); rAd, recombinant adenovirus; STAT, signal transducer and activator of transcription; s.c., subcutaneous injection. TAA, tumor-associated antigen; TAM, tumor-associated macrophage; TGF-β, transforming growth factor β; Th, T helper cell; TNF, tumor necrosis factor; Treg, regulatory T cell; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Grant No. 31800006) to YQ; Natural Science Foundation of Guangdong Province (Grant No. 18zxxt26) to YQ; Guangzhou Basic and Applied Basic Research Foundation (Grant No. 202002030127) to JS; Guangdong Basic and Applied Basic Research Foundation (Grant No. 2021A1515012324) to JS; the Fundamental Research Funds for the Central Universities (Grant No. 20ykzd08) to JS; Natural Science Foundation of Guangdong Province (Grant No. 2018A030313563) to JS; Program for Guangdong Introducing Innovative and Entrepreneurial Teams (Grant No. 2016ZT06S252) to JS; Guangdong Financial Fund for High-Caliber Hospital Construction to JS.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare they have no competing financial interests and other onflicts of interest in this work.
References
1. Sondka Z, Bamford S, Cole CG, et al. The COSMIC cancer gene census: describing genetic dysfunction across all human cancers. Nat Rev Cancer. 2018;18(11):696–705. doi:10.1038/s41568-018-0060-1
2. Vineis P, Wild CP. Global cancer patterns: causes and prevention. Lancet. 2014;383(9916):549–557. doi:10.1016/s0140-6736(13)62224-2
3. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
4. Ferris RL. Immunology and immunotherapy of head and neck cancer. J Clin Oncol. 2015;33(29):3293–3304. doi:10.1200/jco.2015.61.1509
5. Haddad R, Wirth L, Posner M. Emerging drugs for head and neck cancer. Expert Opin Emerg Drugs. 2006;11(3):461–467. doi:10.1517/14728214.11.3.461
6. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi:10.1016/j.immuni.2013.07.012
7. Tsukamoto H, Fujieda K, Senju S, et al. Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity. Cancer Science. 2018;109(3):523–530. doi:10.1111/cas.13433
8. Fisher DT, Appenheimer MM, Evans SS. The two faces of IL-6 in the tumor microenvironment. Semin. Immunol. 2014;26(1):38–47. doi:10.1016/j.smim.2014.01.008
9. Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13(18):5262–5270. doi:10.1158/1078-0432.Ccr-07-1157
10. Geissmann F, Revy P, Regnault A, et al. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162(8):4567–4575.
11. Chen C, Gao F-H. Th17 cells paradoxical roles in melanoma and potential application in immunotherapy. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.00187
12. Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukocyte Biol. 2005;78(5):1043–1051. doi:10.1189/jlb.0705358
13. Seo N, Hayakawa S, Takigawa M, Tokura Y. Interleukin-10 expressed at early tumour sites induces subsequent generation of CD4+ T-regulatory cells and systemic collapse of antitumour immunity. Immunology. 2001;103(4):449–457. doi:10.1046/j.1365-2567.2001.01279.x
14. Xu DH, Zhu Z, Wakefield MR, et al. The role of IL-11 in immunity and cancer. Cancer Lett. 2016;373(2):156–163. doi:10.1016/j.canlet.2016.01.004
15. Li Z, Chen L, Qin Z. Paradoxical roles of IL-4 in tumor immunity. Cell Mol Immunol. 2009;6(6):415–422. doi:10.1038/cmi.2009.53
16. Terabe M, Matsui S, Noben-Trauth N, et al. NKT cell–mediated repression of tumor immunosurveillance by IL-13 and the IL-4R–STAT6 pathway. Nat Immunol. 2000;1(6):515–520. doi:10.1038/82771
17. Terabe M, Park JM, Berzofsky JA. Role of IL-13 in regulation of anti-tumor immunity and tumor growth. Cancer Immunol Immunother. 2004;53(2):79–85. doi:10.1007/s00262-003-0445-0
18. Tugues S, Burkhard SH, Ohs I, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2014;22(2):237–246. doi:10.1038/cdd.2014.134
19. Micallef MJ, Tanimoto T, Kohno K, Ikeda M, Kurimoto M. Interleukin 18 induces the sequential activation of natural killer cells and cytotoxic T lymphocytes to protect syngeneic mice from transplantation with Meth A sarcoma. Cancer Res. 1997;57(20):4557–4563.
20. Kim J, Kim C, Kim TS, et al. IL-18 enhances thrombospondin-1 production in human gastric cancer via JNK pathway. Biochem Biophys Res Commun. 2006;344(4):1284–1289. doi:10.1016/j.bbrc.2006.04.016
21. Kim KE, Song H, Kim TS, et al. Interleukin-18 is a critical factor for vascular endothelial growth factor-enhanced migration in human gastric cancer cell lines. Oncogene. 2006;26(10):1468–1476. doi:10.1038/sj.onc.1209926
22. Mantovani A, Barajon I, Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev. 2018;281(1):57–61. doi:10.1111/imr.12614
23. Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol. 2018;9:847. doi:10.3389/fimmu.2018.00847
24. Tamura R, Tanaka T, Akasaki Y, et al. The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: perspectives for therapeutic implications. Med Oncol. 2019;37(1):2. doi:10.1007/s12032-019-1329-2
25. Aguiar RBD, Moraes JZD. Exploring the immunological mechanisms underlying the anti-vascular endothelial growth factor activity in tumors. Front Immunol. 2019;10:1023. doi:10.3389/fimmu.2019.01023
26. Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;9:978. doi:10.3389/fimmu.2018.00978
27. Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9(5):361–371. doi:10.1038/nrc2628
28. Herzberg B, Fisher DE. Metastatic melanoma and immunotherapy. Clin Immunol. 2016;172:105–110. doi:10.1016/j.clim.2016.07.006
29. Im A, Pavletic SZ. Immunotherapy in hematologic malignancies: past, present, and future. J Hematol Oncol. 2017;10(1):94. doi:10.1186/s13045-017-0453-8
30. Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688–696. doi:10.1200/jco.1995.13.3.688
31. McDermott DF, Cheng S-C, Signoretti S, et al. The high-dose aldesleukin “select” trial: a trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2015;21(3):561–568. doi:10.1158/1078-0432.Ccr-14-1520
32. Neal ZC, Yang JC, Rakhmilevich AL, et al. Enhanced activity of hu14.18-IL2 immunocytokine against murine NXS2 neuroblastoma when combined with interleukin 2 therapy. Clin Cancer Res. 2004;10(14):4839–4847. doi:10.1158/1078-0432.Ccr-03-0799
33. Albertini MR, Yang RK, Ranheim EA, et al. Pilot trial of the hu14.18-IL2 immunocytokine in patients with completely resectable recurrent stage III or stage IV melanoma. Cancer Immunol Immunother. 2018;67(10):1647–1658. doi:10.1007/s00262-018-2223-z
34. Cooley S, He F, Bachanova V, et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 2019;3(13):1970–1980. doi:10.1182/bloodadvances.2018028332
35. Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):119–134. doi:10.1016/s1359-6101(01)00022-3
36. Borden EC. Interferons alpha and beta in cancer: therapeutic opportunities from new insights. Nat Rev Drug Discov. 2019;18(3):219–234. doi:10.1038/s41573-018-0011-2
37. Szenajch J, Wcislo G, Jeong JY, Szczylik C, Feldman L. The role of erythropoietin and its receptor in growth, survival and therapeutic response of human tumor cells from clinic to bench - a critical review. Biochim Biophys Acta. 2010;1806(1):82–95. doi:10.1016/j.bbcan.2010.04.002
38. Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281(1):8–27. doi:10.1111/imr.12621
39. Lewis AM, Varghese S, Xu H, Alexander HR. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med. 2006;4(1):48. doi:10.1186/1479-5876-4-48
40. Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8(5):362–371. doi:10.1038/nri2297
41. Sharma S, Stolina M, Luo J, et al. Secondary lymphoid tissue chemokine mediates T cell-dependent antitumor responses in vivo. J Immunol. 2000;164(9):4558–4563. doi:10.4049/jimmunol.164.9.4558
42. Gray JE, Chiappori A, Williams CC, et al. A phase I/randomized phase II study of GM.CD40L vaccine in combination with CCL21 in patients with advanced lung adenocarcinoma. Cancer Immunol Immunother. 2018;67(12):1853–1862. doi:10.1007/s00262-018-2236-7
43. Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–572. doi:10.1038/nri.2017.49
44. Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi:10.1038/ng1001-117
45. Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409–426. doi:10.1007/s10456-017-9562-9
46. Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19(56):6550–6565. doi:10.1038/sj.onc.1204082
47. Denduluri SK, Idowu O, Wang Z, et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015;2(1):13–25. doi:10.1016/j.gendis.2014.10.004
48. Katoh M, Nakagama H. FGF receptors: cancer biology and therapeutics. Med Res Rev. 2014;34(2):280–300. doi:10.1002/med.21288
49. Joshi BH, Leland P, Asher A, et al. In situ expression of interleukin-4 (IL-4) receptors in human brain tumors and cytotoxicity of a recombinant IL-4 cytotoxin in primary glioblastoma cell cultures. Cancer Res. 2001;61(22):8058–8061.
50. Joshi BH, Leland P, Puri RK. Identification and characterization of interleukin-13 receptor in human medulloblastoma and targeting these receptors with interleukin-13-pseudomonas exotoxin fusion protein. Croat Med J. 2003;44(4):455–462.
51. Miles SA, Testa M, Huang J, Wade M, Carden J, Scadden DT. Lack of antitumor activity and intolerance of interleukin-4 in patients with advanced HIV disease and Kaposi’s sarcoma. J Interferon Cytokine Res. 2002;22(11):1143–1148. doi:10.1089/10799900260442575
52. Khajeh H, Bahari A, Lagzian M, Sabbagh SK. Functional and key gene expression analyses of chicken monocyte-derived dendritic cells with recombinant interleukin 4. Iran J Allergy Asthma Immunol. 2016;15(6):508–514.
53. Mannino MH, Zhu Z, Xiao H, et al. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015;367(2):103–107. doi:10.1016/j.canlet.2015.07.009
54. Grivennikov SI. IL-11: a prominent pro-tumorigenic member of the IL-6 family. Cancer Cell. 2013;24(2):145–147. doi:10.1016/j.ccr.2013.07.018
55. Wang L, Yi T, Kortylewski M, et al. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206(7):1457–1464. doi:10.1084/jem.20090207
56. Baldo BA. Side effects of cytokines approved for therapy. Drug Saf. 2014;37:921–943. doi:10.1007/s40264-014-0226-z
57. Li YL, Zhu L, Liu Z, et al. Reversibly stabilized multifunctional dextran nanoparticles efficiently deliver doxorubicin into the nuclei of cancer cells. Angew Chem Int Ed Engl. 2009;48(52):9914–9918. doi:10.1002/anie.200904260
58. Sánchez A, Tobío M, González L, Fabra A, Alonso MJ. Biodegradable micro- and nanoparticles as long-term delivery vehicles for interferon-alpha. Eur J Pharm Sci. 2003;18(3–4):221–229. doi:10.1016/s0928-0987(03)00019-8
59. Zhai Y-Z, Zhou Y, Ma L, Feng G-H. Effects of cell-mediated immunity induced by intramuscular chitosan-pJME/GM-CSF nano-DNA vaccine in BAlb/c mice. Bing Du Xue Bao. 2014;30(4):423–428.
60. Park J, Wrzesinski SH, Stern E, et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11(10):895–905. doi:10.1038/nmat3355
61. Ten Hagen TL, Seynhaeve AL, van Tiel ST, Ruiter DJ, Eggermont AM. Pegylated liposomal tumor necrosis factor-alpha results in reduced toxicity and synergistic antitumor activity after systemic administration in combination with liposomal doxorubicin (Doxil) in soft tissue sarcoma-bearing rats. Int J Cancer. 2002;97(1):115–120. doi:10.1002/ijc.1578
62. Shao J, Griffin RJ, Galanzha EI, et al. Photothermal nanodrugs: potential of TNF-gold nanospheres for cancer theranostics. Sci Rep. 2013;3(1):1293. doi:10.1038/srep01293
63. Ye H, Tong J, Wu J, et al. Preclinical evaluation of recombinant human IFNα2b-containing magnetoliposomes for treating hepatocellular carcinoma. Int J Nanomedicine. 2014;9:4533–4550. doi:10.2147/IJN.S67228
64. Yan C, Jie L, Yongqi W, et al. Delivery of human NKG2D-IL-15 fusion gene by chitosan nanoparticles to enhance antitumor immunity. Biochem Biophys Res Commun. 2015;463(3):336–343. doi:10.1016/j.bbrc.2015.05.065
65. Tan L, Han S, Ding S, et al. Chitosan nanoparticle-based delivery of fused NKG2D-IL-21 gene suppresses colon cancer growth in mice. Int J Nanomedicine. 2017;12:3095–3107. doi:10.2147/IJN.S128032
66. Nie Y, Zhang Z-R, He B, Gu Z. Investigation of PEG-PLGA-PEG nanoparticles-based multipolyplexes for IL-18 gene delivery. J Biomater Appl. 2012;26(8):893–916. doi:10.1177/0885328210384889
67. Han L, Zhang A, Wang H, et al. Tat-BMPs-PAMAM conjugates enhance therapeutic effect of small interference RNA on U251 glioma cells in vitro and in vivo. Hum Gene Ther. 2010;21(4):417–426. doi:10.1089/hum.2009.087
68. Pol JG, Workenhe ST, Konda P, Gujar S, Kroemer G. Cytokines in oncolytic virotherapy. Cytokine Growth Factor Rev. 2020;56:4–27. doi:10.1016/j.cytogfr.2020.10.007
69. Cantarelli C, Angeletti A, Cravedi P. Erythropoietin, a multifaceted protein with innate and adaptive immune modulatory activity. Am J Transplant. 2019;19(9):2407–2414. doi:10.1111/ajt.15369
70. Wheelock EF. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science. 1965;149(3681):310–311. doi:10.1126/science.149.3681.310
71. Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. doi:10.1098/rspb.1957.0048
72. Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962;237(5):1555–1562. doi:10.1016/S0021-9258(19)83739-0
73. Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966;44(3):287–299. doi:10.1038/icb.1966.28
74. Ichikawa Y, Pluznik DH, Sachs L. In vitro control of the development of macrophage and granulocyte colonies. Proc Natl Acad Sci U S A. 1966;56(2):488–495. doi:10.1073/pnas.56.2.488
75. Rinderknecht E, Humbel RE. Amino-terminal sequences of two polypeptides from human serum with nonsuppressible insulin-like and cell-growth-promoting activities: evidence for structural homology with insulin B chain. Proc Natl Acad Sci U S A. 1976;73(12):4379–4381. doi:10.1073/pnas.73.12.4379
76. Gery I, Gershon RK, Waksman BH. Potentiation of cultured mouse thymocyte responses by factors released by peripheral leucocytes. J Immunol. 1971;107(6):1778–1780.
77. Morgan D, Ruscetti F, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193(4257):1007–1008. doi:10.1126/science.181845
78. Armelin HA. Pituitary extracts and steroid hormones in the control of 3T3 cell growth. Proc Natl Acad Sci U S A. 1973;70(9):2702–2706. doi:10.1073/pnas.70.9.2702
79. Carswell EA, Old LJ, Kassel RL, et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci. 1975;72(9):3666–3670. doi:10.1073/pnas.72.9.3666
80. Burgess AW, Camakaris J, Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977;252(6):1998–2003. doi:10.1016/S0021-9258(18)71855-3
81. de Larco JE, Todaro GJ. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978;75(8):4001–4005. doi:10.1073/pnas.75.8.4001
82. Ihle JN, Pepersack L, Rebar L. Regulation of T cell differentiation: in vitro induction of 20 alpha-hydroxysteroid dehydrogenase in splenic lymphocytes from athymic mice by a unique lymphokine. J Immunol. 1981;126(6):2184–2189.
83. Howard M, Farrar J, Hilfiker M, et al. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982;155(3):914–923. doi:10.1084/jem.155.3.914
84. Hirano T, Yasukawa K, Harada H, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324(6092):73–76. doi:10.1038/324073a0
85. Namen AE, Lupton S, Hjerrild K, et al. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333(6173):571–573. doi:10.1038/333571a0
86. Mosmann TR, Coffman RL. Two types of mouse helper T-cell clone Implications for immune regulation. Immunol Today. 1987;8:223–227. doi:10.1016/0167-5699(87)90171-X
87. Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170(3):827–845. doi:10.1084/jem.170.3.827
88. Cherwinski HM, Schumacher JH, Brown KD, Mosmann TR. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166(5):1229–1244. doi:10.1084/jem.166.5.1229
89. Senger DR, Galli S, Dvorak A, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi:10.1126/science.6823562
90. Paul SR, Bennett F, Calvetti JA, et al. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci U S A. 1990;87(19):7512–7516. doi:10.1073/pnas.87.19.7512
91. Grabstein K, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264(5161):965–968. doi:10.1126/science.8178155
92. Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150(12):5445–5456.
93. Okamura H, Tsutsui H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378(6552):88–91. doi:10.1038/378088a0
94. Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408(6808):57–63. doi:10.1038/35040504
95. Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci. 2000;97(21):11439–11444. doi:10.1073/pnas.200360997
96. Hedrick JA, Zlotnik A. Identification and characterization of a novel beta chemokine containing six conserved cysteines. J Immunol. 1997;159(4):1589–1593.
97. Nagira M, Imai T, Hieshima K, et al. Molecular cloning of a novel human CC chemokine secondary lymphoid-tissue chemokine that is a potent chemoattractant for lymphocytes and mapped to chromosome 9p13. J Biol Chem. 1997;272(31):19518–19524. doi:10.1074/jbc.272.31.19518
98. Gruenbacher G, Gander H, Nussbaumer O, et al. IL-2 costimulation enables statin-mediated activation of human NK cells, preferentially through a mechanism involving CD56 + dendritic cells. Cancer Res. 2010;70(23):9611–9620. doi:10.1158/0008-5472.Can-10-1968
99. ElKassar N, Gress RE. An overview of IL-7 biology and its use in immunotherapy. J Immunotoxicol. 2009;7(1):1–7. doi:10.3109/15476910903453296
100. Marçais A, Cherfils-Vicini J, Viant C, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol. 2014;15(8):749–757. doi:10.1038/ni.2936
101. Dunne J, Lynch S, O’Farrelly C, et al. Selective expansion and partial activation of human NK cells and NK receptor-positive T cells by IL-2 and IL-15. J Immunol. 2001;167(6):3129–3138. doi:10.4049/jimmunol.167.6.3129
102. Skak K, Frederiksen KS, Lundsgaard D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology. 2008;123(4):575–583. doi:10.1111/j.1365-2567.2007.02730.x
103. Frederiksen KS, Lundsgaard D, Freeman JA, et al. IL-21 induces in vivo immune activation of NK cells and CD8+ T cells in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother. 2008;57(10):1439–1449. doi:10.1007/s00262-008-0479-4
104. Cella M, Salio M, Sakakibara Y, et al. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189(5):821–829. doi:10.1084/jem.189.5.821
105. Müller L, Aigner P, Stoiber D. Type I interferons and natural killer cell regulation in cancer. Front Immunol. 2017;8:304. doi:10.3389/fimmu.2017.00304
106. Ivashkiv LB. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat Rev Immunol. 2018;18(9):545–558. doi:10.1038/s41577-018-0029-z
107. Abiko K, Matsumura N, Hamanishi J, et al. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112(9):1501–1509. doi:10.1038/bjc.2015.101
108. Mo X, Zhang H, Preston S, et al. Interferon-γ signaling in melanocytes and melanoma cells regulates expression of CTLA-4. Cancer Res. 2018;78(2):436–450. doi:10.1158/0008-5472.Can-17-1615
109. Baker KJ, Houston A, Brint E. IL-1 family members in cancer; two sides to every story. Front Immunol. 2019;10:1197. doi:10.3389/fimmu.2019.01197
110. Hyodo Y, Matsui K, Hayashi N, et al. IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J Immunol. 1999;162(3):1662–1668.
111. Yoon D-Y, Cho Y-S, Park J-W, Kim S-H, Kim J-W. Up-regulation of reactive oxygen species (ROS) and resistance to Fas-mediated apoptosis in the C33A cervical cancer cell line transfected with IL-18 receptor. Clin Chem Lab Med. 2004;42(5):499–506. doi:10.1515/cclm.2004.085
112. Kim I-K, Koh C-H, Jeon I, et al. GM-CSF promotes antitumor immunity by inducing Th9 cell responses. Cancer Immunol Res. 2019;7(3):498–509. doi:10.1158/2326-6066.Cir-18-0518
113. Hardee ME, Arcasoy MO, Blackwell KL, Kirkpatrick JP, Dewhirst MW. Erythropoietin biology in cancer. Clin Cancer Res. 2006;12(2):332–339. doi:10.1158/1078-0432.Ccr-05-1771
114. Kindler V, Thorens B, de Kossodo S, et al. Stimulation of hematopoiesis in vivo by recombinant bacterial murine interleukin 3. Proc Natl Acad Sci. 1986;83(4):1001–1005. doi:10.1073/pnas.83.4.1001
115. Dougan M, Dranoff G, Dougan SK. GM-CSF, IL-3, and IL-5 family of cytokines: regulators of inflammation. Immunity. 2019;50(4):796–811. doi:10.1016/j.immuni.2019.03.022
116. Middel P, Brauneck S, Meyer W, Radzun H-J. Chemokine-mediated distribution of dendritic cell subsets in renal cell carcinoma. BMC Cancer. 2010;10(1):578. doi:10.1186/1471-2407-10-578
117. Katoh M. FGFR inhibitors: effects on cancer cells, tumor microenvironment and whole-body homeostasis (review). Int J Mol Med. 2016;38(1):3–15. doi:10.3892/ijmm.2016.2620
118. Im JH, Buzzelli JN, Jones K, et al. FGF2 alters macrophage polarization, tumour immunity and growth and can be targeted during radiotherapy. Nat Commun. 2020;11(1):4064. doi:10.1038/s41467-020-17914-x
119. Huang C-T, Chang M-C, Chen Y-L, et al. Insulin-like growth factors inhibit dendritic cell-mediated anti-tumor immunity through regulating ERK1/2 phosphorylation and p38 dephosphorylation. Cancer Lett. 2015;359(1):117–126. doi:10.1016/j.canlet.2015.01.007
120. Yahya MA, Sharon SM, Hantisteanu S, Hallak M, Bruchim I. The role of the insulin-like growth factor 1 pathway in immune tumor microenvironment and its clinical ramifications in gynecologic malignancies. Front Endocrinol (Lausanne). 2018;9:297. doi:10.3389/fendo.2018.00297
121. Zheng Y, Yang W, Aldape K, He J, Lu Z. Epidermal growth factor (EGF)-enhanced vascular cell adhesion molecule-1 (VCAM-1) expression promotes macrophage and glioblastoma cell interaction and tumor cell invasion. J Biol Chem. 2013;288(44):31488–31495. doi:10.1074/jbc.M113.499020
122. MacDonald F, Zaiss DMW. The immune system’s contribution to the clinical efficacy of EGFR antagonist treatment. Front Pharmacol. 2017;8:575. doi:10.3389/fphar.2017.00575
123. Wang KP, Kim M, Di Caro G, et al. Interleukin-17 receptor A signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 2014;41(6):1052–1063. doi:10.1016/j.immuni.2014.11.009
124. Numasaki M, Fukushi JI, Ono M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101(7):2620–2627. doi:10.1182/blood-2002-05-1461
125. Chen F, Huang G, Huang H. Preparation and application of dextran and its derivatives as carriers. Int J Biol Macromol. 2020;145:827–834. doi:10.1016/j.ijbiomac.2019.11.151
126. Cui L, Cohen JA, Broaders KE, Beaudette TT, Frechet JMJ. Mannosylated dextran nanoparticles: a pH-sensitive system engineered for immunomodulation through mannose targeting. Bioconj Chem. 2011;22(5):949–957. doi:10.1021/bc100596w
127. Sabel MS, Su G, Griffith KA, Chang AE. Intratumoral delivery of encapsulated IL-12, IL-18 and TNF-alpha in a model of metastatic breast cancer. Breast Cancer Res Treat. 2010;122(2):325–336. doi:10.1007/s10549-009-0570-3
128. Arora A, Su G, Mathiowitz E, et al. Neoadjuvant intratumoral cytokine-loaded microspheres are superior to postoperative autologous cellular vaccines in generating systemic anti-tumor immunity. J Surg Oncol. 2006;94(5):403–412. doi:10.1002/jso.20572
129. Guan Y, Zheng Z, Liang L, et al. The apoptosis of OVCAR-3 induced by TNF-α plus IFN-γ co-immobilized polylactic acid copolymers. J Mater Chem. 2012;22(29):14746–14755. doi:10.1039/c2jm31972a
130. Liu X, Gao X, Zheng S, et al. Modified nanoparticle mediated IL-12 immunogene therapy for colon cancer. Nanomedicine. 2017;13(6):1993–2004. doi:10.1016/j.nano.2017.04.006
131. Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31(6):220–227. doi:10.1016/j.it.2010.04.002
132. Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnology. 2011;9(1):55. doi:10.1186/1477-3155-9-55
133. Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21(23):2475–2490. doi:10.1016/s0142-9612(00)00115-0
134. Xu Q, Guo L, Gu X, et al. Prevention of colorectal cancer liver metastasis by exploiting liver immunity via chitosan-TPP/nanoparticles formulated with IL-12. Biomaterials. 2012;33(15):3909–3918. doi:10.1016/j.biomaterials.2012.02.014
135. Demento S, Steenblock ER, Fahmy TM. Biomimetic approaches to modulating the T cell immune response with nano- and micro- particles. Annu Int Conf IEEE Eng Med Biol Soc. 2009:1161–1166, doi:10.1109/iembs.2009.5332625
136. Suzuki R, Namai E, Oda Y, et al. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J Control Release. 2010;142(2):245–250. doi:10.1016/j.jconrel.2009.10.027
137. Dass C, Hallaj-Nezhadi S, Lotfipour F. Nanoparticle-mediated interleukin-12 cancer gene therapy. J Pharm Pharm Sci. 2010;13(3):472–485. doi:10.18433/j3630v
138. Gabizon A, Chemla M, Tzemach D, Horowitz AT, Goren D. Liposome longevity and stability in circulation: effects on the in vivo delivery to tumors and therapeutic efficacy of encapsulated anthracyclines. J Drug Target. 1996;3(5):391–398. doi:10.3109/10611869608996830
139. Papahadjopoulos D, Allen TM, Gabizon A, et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci U S A. 1991;88(24):11460–11464. doi:10.1073/pnas.88.24.11460
140. Curnis F, Fiocchi M, Sacchi A, et al. NGR-tagged nano-gold: a new CD13-selective carrier for cytokine delivery to tumors. Nano Res. 2016;9(5):1393–1408. doi:10.1007/s12274-016-1035-8
141. Brzoska K, Gradzka I, Kruszewski M. Impact of silver, gold, and iron oxide nanoparticles on cellular response to tumor necrosis factor. Toxicol Appl Pharmacol. 2018;356:140–150. doi:10.1016/j.taap.2018.08.005
142. Mohseni N, Sarvestani FS, Ardestani MS, Kazemilomedasht F, Ghorbani M. Inhibitory effect of gold nanoparticles conjugated with interferon gamma and methionine on breast cancer cell line. Asian Pac J Trop Biomed. 2016;6(2):173–178. doi:10.1016/j.apjtb.2015.10.014
143. Cai W, Gao T, Hong H, Sun J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol Sci Appl. 2008;1:17–32. doi:10.2147/nsa.S3788
144. Giljohann DA, Seferos D, Daniel W, et al. Gold nanoparticles for biology and medicine. Angew Chem Int Ed. 2010;49(19):3280–3294. doi:10.1002/anie.200904359
145. Choi EW, Shin IS, Chae YJ, et al. Effects of GM-CSF gene transfer using silica-nanoparticles as a vehicle on white blood cell production in dogs. Exp Hematol. 2008;36(7):807–815. doi:10.1016/j.exphem.2008.01.007
146. Allen IC, Shirasuna K, Usui F, et al. Interferon-tau attenuates uptake of nanoparticles and secretion of interleukin-1β in macrophages. PLoS One. 2014;9. doi:10.1371/journal.pone.0113974
147. Gu L, Ruff LE, Qin Z, et al. Multivalent porous silicon nanoparticles enhance the immune activation potency of agonistic CD40 antibody. Adv Mater. 2012;24(29):3981–3987. doi:10.1002/adma.201200776
148. Hu B, Du H-J, Yan G-P, et al. Magnetic polycarbonate microspheres for tumor-targeted delivery of tumor necrosis factor. Drug Deliv. 2014;21(3):204–212. doi:10.3109/10717544.2013.843609
149. Mejias R, Pérez-Yagüe S, Gutiérrez L, et al. Dimercaptosuccinic acid-coated magnetite nanoparticles for magnetically guided in vivo delivery of interferon gamma for cancer immunotherapy. Biomaterials. 2011;32(11):2938–2952. doi:10.1016/j.biomaterials.2011.01.008
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.