Back to Journals » Degenerative Neurological and Neuromuscular Disease » Volume 9
Clinical and research applications of neuromuscular ultrasound in amyotrophic lateral sclerosis
Received 12 May 2019
Accepted for publication 14 June 2019
Published 16 July 2019 Volume 2019:9 Pages 89—102
DOI https://doi.org/10.2147/DNND.S215318
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Thomas Müller
Stephanie L Barnes1,2, Neil G Simon3
1Department of Neurology, Concord Repatriation General Hospital, Concord, NSW, Australia; 2St Vincent’s Clinical School, School of Medicine, The University of Notre Dame Australia, Sydney, NSW, Australia; 3St Vincent’s Clinical School, Faculty of Medicine, University of New South Wales, Sydney, NSW, Australia
Abstract: Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disorder characterized by dysfunction at multiple levels of the neuraxis. It remains a clinical diagnosis without a definitive diagnostic investigation. Electrodiagnostic testing provides supportive information and, along with imaging and biochemical markers, can help exclude mimicking conditions. Neuromuscular ultrasound has a valuable role in the diagnosis and monitoring of ALS and provides complementary information to clinical assessment and electrodiagnostic testing as well as insights into the underlying pathophysiology of this disease. This review highlights the evidence for ultrasound in the evaluation of bulbar, limb and respiratory musculature and peripheral nerves in ALS. Further research in this evolving area is required.
Keywords: amyotrophic lateral sclerosis, ultrasound, clinical neurophysiology, biomarker, clinical trials
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder affecting both upper and lower motor neurons that is inexorably progressive and universally fatal. Variability of clinical features, particularly early in the course of the disease, and the lack of a definitive biomarker may make diagnosis of ALS challenging. The revised El Escorial criteria1,2 define the need for evidence of lower and upper motor neuron degeneration with progressive spread of symptoms or signs within or between body regions. Electrodiagnostic testing can provide supportive evidence, as described in the Awaji criteria,3 which increases the sensitivity of diagnosis without reducing the specificity.4,5 This, and other forms of diagnostic testing, such as neuroimaging, serology and cerebrospinal fluid (CSF) analysis, remain important to exclude potential mimicking conditions.
Neuromuscular ultrasound has an evolving role in the assessment of patients with ALS (Table 1). It is a readily accessible, noninvasive modality that can be used across multiple regions of the body to provide important information to support a diagnosis of ALS. It has potential roles in structural assessment and dynamic evaluation of bulbar, limb and respiratory musculature as well as peripheral nerves, which currently complements conventional testing modalities in these areas. It is also ideally placed to be used in a serial fashion to monitor disease progression without concerns about radiation exposure. Neuromuscular ultrasound therefore has great potential to facilitate early diagnosis, exclude mimicking disorders and monitor progression of disease. Its use in a clinical trial setting is particularly promising to increase confidence in the underlying diagnosis of “ALS” patients and monitor response to novel treatments.
 |
Table 1 The sonographic characteristics of ALS |
Muscle ultrasound
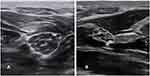
Muscle ultrasonography (MUS) is the most well established application of ultrasound in ALS cohorts. It is a particularly useful technique for screening for abnormalities in large muscle regions across multiple body segments relevant to ALS diagnosis. This includes the detection of fasciculations, muscle atrophy and structural changes such as increase in muscle echogenicity and other textural parameters (Figure 1).
Fasciculations
The role of MUS in the detection of fasciculations in ALS has been clearly demonstrated. This is of diagnostic significance in ALS now that fasciculations are included in the Awaji criteria as evidence of active denervation and are of similar weighting to muscle fibrillations on electromyography (EMG) to support diagnosis, in addition to signs of motor unit loss and reinnervation.6–10 Fasciculations are a marker of denervation,3 which occurs prior to development of muscle weakness detectable on manual examination,11,12 and can be identified even in clinically unaffected muscles.6 Fasciculations may thereby act as a useful marker in ALS with several studies suggesting these as the first sign of disease and an indicator of spread of ALS to uninvolved body regions.13,14 MUS is more sensitive than EMG or visual inspection in detecting fasciculations, increasing identification from 88% to 98%.9 MUS provides a sensitive and reproducible screening tool for subclinical regional lower motor neuron (LMN) dysfunction, including in cranial muscles.6,8,15–18 Optimal scan time is 30 seconds in most muscles and 60 seconds in first dorsal interosseous.19 Using MUS and EMG in combination significantly increases the diagnostic sensitivity for ALS using the Awaji criteria, giving 25% of patients greater diagnostic certainty, likely because MUS enables assessment of a larger area of muscle, including deeper muscle layers, compared to EMG.7,9,16 It also has a potential role in guiding choice of muscles for EMG, reducing the number of muscles examined and increasing diagnostic yield.20,21 This is a particularly useful technique in evaluation of the tongue because it allows screening of large areas of muscle and is not influenced by lack of muscle relaxation in response to (or anticipation of) painful needle examination.6 Fasciculations can be readily distinguished from artefacts based on visual assessment of MUS and the technique is easily learnt with high inter-rater agreement.17,22
The pattern of fasciculations, predominant in upper limb and proximal muscle groups, can be identified with MUS and is specific for ALS diagnosis.23,24 Harding et al25 have also demonstrated high accuracy in identifying fasciculations using computational analysis, which would have particular value as an objective measure in clinical trials and to assist less experienced sonographers. MUS can be used to characterize fasciculation potentials, assess firing rate and, in conjunction with measures of cortical silent period, demonstrate central influences on fasciculation frequency.26 However, MUS still remains potentially inferior to EMG in assessing the complexity of fasciculation potentials, which would provide further support for ALS diagnosis in the Awaji criteria.27,28
Fasciculation analysis with MUS also has a potential role in excluding differential diagnoses in possible ALS cases. Arts et al15 demonstrated the diagnostic utility of MUS in differentiating ALS from mimicking disorders with 96% sensitivity and 84% specificity. However, mimics that are hardest to distinguish from ALS such as multifocal motor neuropathy (MMN) and peripheral nerve hyperexcitability syndromes (PNHS) were not included in their prospective sample. Grimm et al6 confirmed these findings and included relevant mimicking disorders, specifically axonal polyneuropathy, myasthenia gravis, cervical myelopathy, cramps, inclusion body myositis, Kennedy syndrome, spinal muscular atrophy, polymyositis, plexus neuritis and MMN, although numbers of patient with each alternative diagnosis were small. The pattern and distribution of fasciculations and number of muscles involved is highly sensitive and specific in differentiating ALS from mimics, and fasciculations are less prominent in advanced disease.16,18 A MUS fasciculation score has been devised to facilitate diagnosis.24 When used in concert with measures of cortical hyperexcitability, fasciculation intensity, that is, the number of fasciculations occurring in 60 seconds, can also provide important prognostic information on rate of disease progression.29
MUS can also assist in distinguishing ALS from benign fasciculation syndromes due to the predominantly distal distribution of fasciculations in the latter disorders.30 MUS has shown fasciculations to be more widespread and frequent in ALS than in PNHS, which can be an important diagnostic differentiator.26 There is some evidence that this reflects central influences (ie cortical hyperexcitability) on fasciculations occurring in ALS.23,31,32
Detection of fibrillation potentials with high resolution MUS has also been described,6,33,34 although EMG remains the more sensitive modality.6
Fasciculation assessment and monitoring may also have relevance in treatment and prognostication. Vazquez-Costa et al23 showed an association between fasciculation number and reduced body mass index (BMI), which is known to be a poor prognostic marker. Treatment options which reduce fasciculation frequency may therefore improve prognosis. Indeed higher riluzole levels in serum correlate with reduced fasciculation intensity.35 Quantification of fasciculations may also be relevant to disease monitoring as fasciculation number inversely correlates with disability, likely related to attrition of hyperexcitable motor units as ALS progresses.23
MUS therefore acts as a complementary technique to EMG in identifying fasciculations as an early marker of LMN dysfunction. It also has an important role as a diagnostic tool and can facilitate differentiation of ALS from mimicking disorders.
Structural assessment of muscle
Muscle atrophy, an expected finding in ALS, can also be clearly demonstrated with MUS (Figure 1).10,36 Decreased muscle thickness, even in early phase disease, has been demonstrated. However, it is important to recognize that compensatory reinnervation may facilitate maintenance of muscle size at least initially.10,37 Significant variability in muscle thickness across muscle groups has been observed, which fits with the patchy nature of ALS pathology.10
MUS is ideal for screening multiple anatomic sites for abnormality. This was utilized by Pathak et al38 who surveyed a series of muscles of relevance in ALS and demonstrated a mean 20.25% decrease in muscle thickness over a six-month period in an ALS cohort. However, thinning in only certain muscles correlated with ALS Functional Rating Scale—Revised (ALSFRS-R)39 and/or forced vital capacity (FVC) scores, likely related to the impacts of upper motor neuron (UMN) dysfunction on clinical scoring measures and also compensatory use or hypertrophy of muscles to compensate for paretic ones.38 Given the evolution of changes in muscle thickness over time, this also has a potential role as a marker of disease progression. Appropriate muscles for monitoring could be selected depending on the site of ALS onset.38 Multiple potential technical issues were identified in measuring muscle thickness including mild discrepancies of transducer position or pressure on muscle that can significantly affect results. In addition, changes in muscle echogenicity and heterogeneity that develop with ALS progression could impair visualization of reference points for transducer positioning.
MUS can demonstrate morphological changes in the muscle structure, specifically quantitative changes in muscle echo intensity (EI), echovariation (EV) and gray-level co-occurrence matrix (GLCM) parameters (Figure 1). EI, the brightness of the ultrasound image, is a product of ultrasound waves reflected off interfaces between tissues with different acoustical impedance. EV measures the range of brightness seen, which may be homogeneous or heterogeneous, and GLCM parameters assess the relationship between intensity of adjacent pixels, as described by Martinez-Paya et al.40
Increased muscle EI occurs as ALS progresses due to replacement of denervated muscle fibers with intramuscular fibrous and fatty tissue, and is evident even prior to development of clinical weakness.6,10,12,37,41 A clinicopathologic correlation study42 identified increased intramuscular fibrous tissue as the primary determinant of increased muscle EI in ALS. EI tends to be more abnormal than muscle thickness and so may act as a better measure of muscle pathology.10 EI measures can also be used to predict survival,43 with even higher predictive value when used in conjunction with ALSFRS-R, which is known to correlate with survival.44,45 EI of the thenar and hypothenar muscles, characteristically preferentially affected in ALS causing dissociated small hand muscle atrophy, can be used to create a compound measure, the split hand index, which has value as a potentially objective diagnostic marker with greater diagnostic accuracy than electrodiagnostic measures.46 EI of individual muscles could not differentiate ALS from mimics, rather the relative difference in atrophy between medial and lateral hand muscles was most significant, which also correlated with disease severity based on ALSFRS-R score.46 EI, in combination with muscle thickness, has also been demonstrated as a reliable and reproducible indicator of denervation in hand muscles.47 The reliability of EI, however, can be problematic due to both technical factors such as anisotropy resulting from minor changes in transducer angle47,48 and lack of consistency between ultrasound systems.15,47
Evaluation of EI can be facilitated by quantitative computer-based analysis, which minimizes variability in assessment based on examiner experience, ultrasound machine settings and is more sensitive than visual analysis.49,50 However, the argument against using these intrinsic muscle properties as markers is that they require time-consuming offline image analysis.38
Muscle textural parameters also have characteristic features in ALS patients. EV, a marker of tissue homogeneity, has been shown to decrease in ALS-affected muscles and strongly correlates with muscle strength and ALSFRS-R score.37 It is proposed as a more reliable ALS marker than muscle thickness or EI because it is unaffected by non-disease factors such as age, exhibits less interindividual variability and is not observed in other neuromuscular diseases, although ALS mimics were not included in this study.37,50 GLCM parameters demonstrate reduced granularity in ALS-affected muscles compared to controls, which can be used in combination with other quantitative MUS variables to increase diagnostic accuracy in ALS.40
Besides assessment of the echotexture of muscle, MUS may also provide information about its biophysical properties through ultrasound elastography applications. Elastography measures the extent of tissue deformation from an externally applied force and provides information about the mechanical properties of muscle. In ALS, increased muscle stiffness is seen, as expected due to progressive muscle infiltration with fibro-fatty tissue.42,51 Strain elastography involves application of a mechanical stimulus, for example, pressure to the tissue applied by the examiner, but the utility of this technique is limited by lack of standardization.51 Shear wave elastography measures speed of propagation of shear waves through tissue to provide an absolute measurement of tissue stiffness that is operator-independent, reproducible and more reliable that strain elastography.52,53 It has not yet been used to investigate changes in ALS but several studies in other neurological disorders such as cerebral palsy54 and Duchenne muscular dystrophy55 demonstrate its potential in this area.
Disease progression
MUS has also been investigated as a marker of disease progression in ALS with variable results.56 Consistent changes across multiple parameters with disease progression have been recorded by some57,58 but not others.40,43,57 Further study is required with larger longitudinal samples.
Multiple MUS parameters have been identified which can provide supportive information in ALS diagnosis. MUS can be used to screen large areas of muscle across multiple body regions and also to guide electrodiagnostic examination. Sonographic abnormalities may be evident in muscles with preserved strength, enabling demonstration of additional body regions subclinically involved in the underlying disease process, which can also facilitate accurate diagnosis of ALS in limited disease presentations. MUS parameters may also be relevant in monitoring disease progression. Real-time MUS in the neurophysiology clinic therefore has a role in the diagnosis and monitoring of the disease.
Diaphragm ultrasound
Respiratory dysfunction eventually occurs in all ALS patients, often leading to neuromuscular respiratory failure as a cause of death.59 This can be due to a combination of factors including weakness of respiratory and bulbar muscles and abnormalities of central respiratory control, potentially exacerbated by intercurrent respiratory tract infection or aspiration.60,61 Monitoring for respiratory dysfunction is essential because it has implications for prognosis and also plays a role in therapeutic decision-making since interventions such as noninvasive ventilation may improve life expectancy and quality of life.59,62,63
Current measures of respiratory function assessment include pulmonary function tests (PFTs), electrodiagnostic measures and arterial blood gas (ABG) analysis, although ABGs are not routinely used in the setting of ALS. PFTs are the gold standard to evaluate development of respiratory muscle weakness. However the value of conventional PFTs may be limited due to mechanical factors in the setting of significant facial and/or bulbar muscle weakness64 and due to lack of cooperation or volition where there is comorbid depression or cognitive impairment.65
Electrodiagnostic evaluation of the diaphragm is occasionally used. Phrenic nerve conduction studies correlate with other respiratory function measures,66 however, care must be taken to standardize electrode position and respiratory phase during stimulation.67 Needle EMG of the diaphragm may also have a role in demonstrating early subclinical respiratory muscle dysfunction in ALS68 and is the definitive test to detect diaphragmatic paralysis. However, electrodiagnostic testing of the diaphragm is mildly invasive, may be technically challenging, carries a risk of complications, particularly pneumothorax, and is prone to large interindividual differences.69
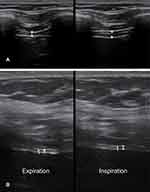
Involvement of respiratory musculature in ALS, specifically the diaphragm, can be assessed using dynamic muscle ultrasound. The diaphragm can be clearly identified using ultrasound with minimal interobserver variability in its assessment (Figure 2).70 Several studies have shown a relationship between dynamic diaphragm thickness and respiratory function in ALS patients, specifically a positive correlation with impaired vital capacity and inverse correlation with pCO2, indicating atrophy and impaired diaphragmatic contractility in those patients with hypoventilation.70–74 Dynamic diaphragm thickness also correlated with functional assessments in ALS using the ALSFRS-R.71 Diaphragm thickness also significantly correlates with FVC and compound muscle action potential (CMAP) amplitude on phrenic nerve stimulation, which suggests it reflects the number of functional motor units in the diaphragm of ALS patients.75 However, over short periods of follow-up, phrenic nerve stimulation appears more sensitive than diaphragm ultrasound in detecting change in ALS patients, which has implications for its use in a trial setting.76 Overall, dynamic ultrasound measures of the diaphragm correlate strongly with currently available measures of assessing respiratory function in ALS and provide a novel method of evaluation.
Diaphragmatic ultrasound has several advantages over the currently available methods of evaluating respiratory dysfunction in ALS. It is a noninvasive bedside test that is easily accessible and can be readily repeated to allow serial monitoring of respiratory muscle weakness and therefore potentially has a role in assessing progression of disease. It also allows dynamic assessment of diaphragmatic motion throughout the respiratory cycle and enables rapid, high sensitivity detection of significant respiratory dysfunction (represented by FVC ≤50% predicted).72 It is possible to visualize the diaphragm even in obese patients because the muscle is located relatively superficially, although this is unlikely to be a limiting factor in an ALS cohort.75 This technique may enable better assessment of ALS-related respiratory dysfunction beyond traditional PFTs given the observation of a strong negative correlation between degree of diaphragm excursion and disease duration,71 whereas similar relationships were not seen with forced expiratory volume (FEV1) or FVC. Ultrasound can also be used in conjunction with electrodiagnostic measures to increase accuracy of phrenic nerve conduction studies (NCS) by allowing direct vision of diaphragm twitch77 and to guide needle placement and reduce pneumothorax risk with EMG.
There are several limitations in the existing body of literature around diaphragmatic ultrasound in respiratory function assessment. It is unclear if sonographic measures of the diaphragm are reliable in ALS patients with significant bulbar involvement. Firstly, diaphragmatic ultrasound measurements did not correlate with PFTs in the bulbar-onset subgroup in Pinto et al (2016), perhaps because the PFTs in this group were themselves unreliable and therefore diaphragm ultrasound may represent a superior marker of respiratory dysfunction. Secondly, patients with severe bulbar weakness were excluded from the majority of studies. Finally, there is some evidence for abnormalities of central respiratory drive in bulbar-onset ALS which would not be reflected in direct muscle-based measurement and may be partial drivers of hypoventilation.61,78 Establishing the diagnostic accuracy of diaphragm ultrasound in assessing respiratory muscle weakness would require a reference standard beyond those currently available, for example, simultaneous diaphragmatic EMG and esophageal manometry, which would be both invasive and time-consuming.79 Further studies of ultrasound evaluation of respiratory function are required to more fully characterize the role of respiratory muscle ultrasonography in ALS patient evaluation. However, the advantages of using ultrasound over other techniques are clear.
Bulbar muscle dysfunction and dysphagia
Dysphagia occurs commonly in ALS due to bulbar motor neuron dysfunction. Accurate assessment of swallowing is important because it has implications for assessing aspiration risk and need for tube feeding, monitoring progression,80 and determining prognosis.81,82 Videofluoroscopy (VFS) has been traditionally used to evaluate dysphagia and is currently regarded as the gold standard.83 However, ultrasonographic evaluation of swallowing provides several advantages over VFS. It is a repeatable noninvasive rapid bedside test, avoids ionizing radiation and can assess reflex swallowing in uncooperative patients.84 It also avoids risk of aspiration of barium contrast media which has a twofold benefit since patients report a preference to water ingestion over barium based agents84 and aspiration confers high mortality in ALS patients.85 Electrodiagnostic measures can also be used but these are invasive, poorly tolerated and often technically difficult due to inadequate tongue relaxation.86
To this end, three studies have assessed the role of bulbar muscle ultrasound in assessment of dysphagia in ALS. Tamburrini et al84 demonstrated the role of video ultrasonography (VUS) in comparison to VFS to allow morphological and dynamic assessment of the oral phase of swallowing. They showed that VUS was more sensitive than VFS in evaluating abnormalities in early dysphagia including bolus position, oral bolus transport and dynamic tongue movements. Notably abnormal bolus position, a static measure, may represent a particularly useful marker of swallowing dysfunction because it was consistently associated with dynamic abnormalities in the oral phase of deglutition. While stagnation of ingested material in the oral cavity could not be visualized with VUS due to lack of bone penetration, this was invariably associated with other swallowing abnormalities and so did not represent an important limiting factor of VUS. Tongue atrophy was also noted in association with evidence of dynamic swallowing abnormality and thus suggested a relationship between structural and dynamic abnormalities of bulbar muscles, although this tends to occur later in the ALS disease process. This study therefore demonstrated the value of VUS as an alternative technique to VFS that additionally allows early and sensitive identification of dynamic swallowing dysfunction in the oral phase.
Nakamori et al80 focused specifically on sonographic measurement of tongue thickness, measured from the surface of the mylohyoid muscle to the tongue dorsum, and demonstrated significantly reduced tongue thickness in ALS patients compared to controls. This was associated with both BMI and onset type in ALS patients with more pronounced thinning in bulbar-onset patients. As in Tamburrini et al84 reduced tongue thickness was associated with dynamic impairment in the oral phase of deglutition, which suggests that tongue thickness can be used as a surrogate marker for bulbar muscle dysfunction. In addition, in serial measurements tongue thickness decreased over time in the ALS cohort, so this measure could also be used to monitor disease progression. However, the relationship of tongue thickness to BMI and the virtually universal and multifactorial weight loss that occurs as ALS progresses will also affect this measure. Nutritional support to maintain body weight has been previously shown to prolong survival87 and tongue thickness may be used as a surrogate marker of survival potential, which may potentially add prognostic value. Unsurprisingly, tongue ultrasonography also improved detection of fasciculations compared to clinical examination.80
Noto et al86 specifically aimed to quantify UMN-related bulbar dysfunction in ALS patients using dynamic M-mode ultrasound of the mylohyoid-geniohyoid muscle complex. Thickness ratio in the mylohyoid-geniohyoid muscle complex was significantly lower in the ALS cohort than controls. Sub-analysis revealed that this was specifically the case in the bulbar-onset group. Thickness ratio and maximal thickness during swallowing negatively correlated with clinical UMN scores. Thus dynamic bulbar muscle ultrasound is a marker of UMN dysfunction in ALS, although its sensitivity was relatively low at 66.7% and reproducibility of the method requires further evaluation.86 Pathophysiologically, these findings may reflect spasticity and reduced bulbar muscle contractility due to UMN dysfunction in ALS.86 There is potential to develop this technique as a noninvasive objective marker of bulbar UMN dysfunction in ALS, which can be difficult to identify clinically in patients with bulbar onset ALS.
Tongue ultrasonography in the assessment of dysphagia in ALS is a noninvasive and better tolerated alternative to traditional VFS, and represents a potentially useful measure to complement clinical and electrodiagnostic assessment. The optimal methodology to maximize tolerability and reproducibility is yet to be established so further investigation is required. Ultrasonography can act as a noninvasive marker of morphological and dynamic abnormalities in the oral phase of swallowing. Current limitations include difficulties in imaging interpretation due to motion artefact from active accessory muscles of respiration in established ALS, lack of utility in evaluating the pharyngeal phase of swallowing and inability to perform the procedure safely even with small water boluses in severe dysphagia, although such assessment is unlikely to be necessary in such established disease.80,86 Tongue ultrasonography in assessment of dysphagia and bulbar dysfunction in ALS may therefore provide a convenient adjunct to traditional clinical and electrodiagnostic assessment provided its limitations are recognized.
Nerve ultrasound
Ultrasonographic evaluation of peripheral nerves in ALS has also been examined. Several studies have demonstrated a reduction in cross-sectional area (CSA) of peripheral nerves and longitudinal diameter of cervical nerve roots in ALS patients,36,88–90 reflecting nerve atrophy as a result of LMN dropout. This technique has good intra- and interobserver reliability.36 As expected, no difference in pure sensory peripheral nerve CSA was observed.36 Schreiber et al90 demonstrated the utility of ulnar nerve distal CSA as a marker of LMN involvement and potential use as a diagnostic marker to differentiate primary lateral sclerosis, in which nerve CSA was similar to controls, from UMN-dominant ALS, in which nerve CSA was significantly reduced, a demarcation which is difficult to achieve clinically but has significant prognostic consequences. A single case study91 has also demonstrated vagus nerve atrophy in ALS. This result requires further evaluation and validation in larger cohorts.
Cervical nerve root measures have also been investigated. These provide information about innervation of larger proximal muscle groups and the reduction in nerve root diameter in ALS patients compared to controls tends to be greater than the reduction in peripheral nerve CSA.88 This correlates with pathological evidence of motor nerve fiber predominance in nerve roots compared to mixed peripheral nerves.92,93 Nerve root measures may therefore provide a better marker of motor axonal loss than peripheral nerves. There was also a trend towards an inverse correlation of cervical nerve root diameter with degree of disability, with thinner nerve roots observed in ALS patients with more impaired upper limb function.89 However in other studies, no correlation was found with disease severity based on ALSFRS-R or duration.
Peripheral nerve ultrasound can also be used to distinguish ALS from mimicking disorders, particularly MMN and PNHS, where accurate diagnosis has significant treatment and prognostic implications. MMN is significantly more likely to be associated with nerve/root enlargement, particularly in settings where electrodiagnostic testing is nonconfirmatory ie in the absence of conduction block.94–98 Jongbloed et al95 also suggested a comparable role of MRI, however this modality remains less easily accessible and more expensive than ultrasonography. PNHS can also be differentiated from ALS using peripheral nerve ultrasound with some evidence supporting a preserved ulnar CSA distal:proximal ratio in PNHS compared to an increased ratio in ALS.98
The aforementioned studies have used various methods and sites of assessing CSA/nerve root diameter. Noto et al98 extended these approaches by examining ratios of distal and proximal peripheral nerve CSA. Like the previous studies, they demonstrated a particular propensity for reduction in upper arm median nerve CSA, as well as an increase in CSA distal:proximal ratio in ALS, which may reflect a particular vulnerability of this nerve to involvement in the neurodegenerative process of ALS. Reduced CSA was more profound in the proximal portion of the nerve, which pathophysiologically fits with the known predominance of motor axons in the median nerve in the upper arm compared to the distal portion,99 thereby creating a distinctive pattern of asymmetric atrophy in a primary motor neuronopathy like ALS. These findings were present across all ALS subtypes which suggests a role diagnostically, regardless of site of disease onset. The authors postulated that peripheral nerve CSA distal:proximal ratios may be a more sensitive marker of ALS by reflecting a characteristic pattern of motor neuron atrophy.98
In these studies, peripheral nerve and nerve root CSA have not shown a clear relationship to clinical severity, disease duration or electrodiagnostic measures. The lack of correlation with ALSFRS-R scores is unsurprising given this composite measure reflects both UMN and LMN involvement across multiple body regions and there is significant clinical heterogeneity of patients receiving the same score with variable inputs from different levels of the neuraxis in comparison to sonographic changes recorded in a single peripheral nerve.
Interestingly, Schreiber et al100 demonstrate an association between sonographic peripheral nerve atrophy and increased CSF levels of progranulin, normalized to CSF:serum albumin ratio in ALS. Progranulin is a neuritic growth factor previously shown to be upregulated in brain and spinal cord microglia in the setting of nervous system axonal injury, including ALS.101 Motor neuron loss is known to occur prior to central nervous system (CNS) progranulin upregulation.102 The authors therefore propose that the observed relationship of CSF levels to peripheral nerve CSA reflects compensatory progranulin upregulation aimed to maintain structure and function of neurons, but unsuccessful in inhibiting progressive peripheral nerve atrophy.
Peripheral nerve ultrasound has also provided information about the pathophysiology underlying electrophysiological abnormalities in ALS. Specifically, Mori et al103 demonstrated that “secondary carpal tunnel syndrome” (ie a compressive etiology) is not the cause of increased median distal motor latency in ALS due to lack of sonographic evidence of focal median nerve enlargement at the wrist. Rather the authors suggested that the median nerve in ALS may be uniquely vulnerable, resulting in selective conduction abnormalities, particularly distally, although the underlying mechanism remains unclear. These insights into the pathophysiological underpinnings of ALS are important because of their potential role in developing new therapeutic options.
Similar issues are common to all nerve ultrasonography studies in ALS. They have been limited by relatively small sample sizes and substantial heterogeneity in disease severity. Analysis by region of onset and ALS subtype was not possible in some studies due to size limitations. In addition, patients in the “ALS” cohort in these studies typically meet criteria for probable or definite ALS. Results therefore may not apply to those patients who present the greatest diagnostic challenge—those with possible ALS or other ALS mimics. Furthermore, the majority of studies recorded significant overlap in sonographic measures between ALS and control groups, which potentially limits the utility of this technique in the individual patient setting, despite the population differences recorded.
Nerve ultrasound in ALS provides a potential method of preclinical detection of motor neuron loss superior to manual muscle testing and CMAP because axonal degeneration can be clinically masked in the early phases by relative preservation of muscle strength via reinnervation by surviving axons.89,104 However, the changes observed are typically sub-millimeter and this measure is therefore too insensitive to small degrees of change to be of significant clinical use. This also applies to the longitudinal assessment of nerve CSA in which statistically significant changes were observed over time but these were 20 times below currently detectable limits of ultrasonography.105 There is a need for further work in this area, particularly with the use of higher frequency probes to evaluate measures at the neuronal level such as fascicular diameter105 and careful standardization of ultrasonographic methodology to reliably capture small changes in CSA.
These studies demonstrate the potential role for nerve ultrasound as an adjunctive diagnostic modality in ALS to complement electrodiagnostic testing, as well as providing insights into ALS pathogenesis. It is likely to have a particular role in diagnosis of early disease when electrodiagnostic studies may be inconclusive106 but peripheral nerve/nerve root morphological changes may be already present and alsodifferentiating from potential ALS mimics. Its role in monitoring disease progression is not yet well established.
Limitations of neuromuscular ultrasound
Despite its obvious advantages of accessibility, noninvasiveness and ability to screen and detect abnormalities in multiple regions of the body relevant to ALS, the use of neuromuscular ultrasound in this patient group has several limitations. It is an operator-dependent and machine-dependent imaging modality so appropriate training and a sufficiently high-quality machine is required. Fortunately, these techniques are relatively easy to learn.17,22 Interrater reliability of measurements is always a consideration in such settings, however several robust measures in ALS patients have now been established.37,94 Patient factors such as movement or poor cooperation in the setting of cognitive dysfunction can also influence scan quality. Other technical factors such as transducer position and pressure must also be considered in interpretation of results.
Conclusions
Overall, neuromuscular ultrasound has a valuable role in the diagnosis and monitoring of patients with ALS and also has the potential to offer important insights into the underlying pathophysiology of this devastating disease. The validation of a composite sonographic marker of nerve and muscle dysfunction seems feasible in the near future to complement clinical and electrodiagnostic assessment techniques. Future technological advances, such as the development of ultra high-frequency transducers, as well as novel techniques such as ultrasound elastography, will also enable additional applications of these techniques. The wide ranging applications of neuromuscular ultrasound in ALS and other neuromuscular diseases make it an indispensable piece of equipment in all clinical neurophysiology departments.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–299.
2. Ludolph A, Drory V, Hardiman O, et al. A revision of the El Escorial criteria - 2015. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(5–6):291–292. doi:10.3109/21678421.2015.1049183
3. de Carvalho M, Dengler R, Eisen A, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008;119(3):497–503. doi:10.1016/j.clinph.2007.09.143
4. Boekestein WA, Kleine BU, Hageman G, Schelhaas HJ, Zwarts MJ. Sensitivity and specificity of the ‘Awaji’ electrodiagnostic criteria for amyotrophic lateral sclerosis: retrospective comparison of the Awaji and revised El Escorial criteria for ALS. Amyotroph Lateral Scler. 2010;11(6):497–501. doi:10.3109/17482961003777462
5. Schrooten M, Smetcoren C, Robberecht W, Van Damme P. Benefit of the Awaji diagnostic algorithm for amyotrophic lateral sclerosis: a prospective study. Ann Neurol. 2011;70(1):79–83. doi:10.1002/ana.22380
6. Grimm A, Prell T, Decard BF, et al. Muscle ultrasonography as an additional diagnostic tool for the diagnosis of amyotrophic lateral sclerosis. Clin Neurophysiol. 2015;126(4):820–827. doi:10.1016/j.clinph.2014.06.052
7. Boekestein WA, Schelhaas HJ, van Dijk JP, et al. Ultrasonographic detection of fasciculations markedly increases diagnostic sensitivity of ALS. Neurology. 2012;78(5):370. author reply 370-371. doi:10.1212/WNL.0b013e3182475368
8. O’Gorman CM, Weikamp JG, Baria M, et al. Detecting fasciculations in cranial nerve innervated muscles with ultrasound in amyotrophic lateral sclerosis. Muscle Nerve. 2017;56(6):1072–1076. doi:10.1002/mus.25676
9. Misawa S, Noto Y, Shibuya K, et al. Ultrasonographic detection of fasciculations markedly increases diagnostic sensitivity of ALS. Neurology. 2011;77(16):1532–1537. doi:10.1212/WNL.0b013e318233b36a
10. Arts IM, van Rooij FG, Overeem S, et al. Quantitative muscle ultrasonography in amyotrophic lateral sclerosis. Ultrasound Med Biol. 2008;34(3):354–361. doi:10.1016/j.ultrasmedbio.2007.08.013
11. Swash M, Schwartz MS. A longitudinal study of changes in motor units in motor neuron disease. J Neurol Sci. 1982;56(2–3):185–197.
12. Wohlfart G. Collateral regeneration from residual motor nerve fibers in amyotrophic lateral sclerosis. Neurology. 1957;7(2):124–134. doi:10.1212/wnl.7.2.124
13. de Carvalho M, Kiernan MC, Swash M. Fasciculation in amyotrophic lateral sclerosis: origin and pathophysiological relevance. J Neurol Neurosurg Psychiatry. 2017;88(9):773–779. doi:10.1136/jnnp-2017-315574
14. Eisen A, Vucic S. Fasciculation potentials: a diagnostic biomarker of early ALS? J Neurol Neurosurg Psychiatry. 2013;84(9):948. doi:10.1136/jnnp-2013-305036
15. Arts IM, Overeem S, Pillen S, et al. Muscle ultrasonography: a diagnostic tool for amyotrophic lateral sclerosis. Clin Neurophysiol. 2012;123(8):1662–1667. doi:10.1016/j.clinph.2011.11.262
16. Johansson MT, Ellegaard HR, Tankisi H, Fuglsang-Frederiksen A, Qerama E. Fasciculations in nerve and muscle disorders - A prospective study of muscle ultrasound compared to electromyography. Clin Neurophysiol. 2017;128(11):2250–2257. doi:10.1016/j.clinph.2017.08.031
17. Regensburger M, Tenner F, Mobius C, Schramm A. Detection radius of EMG for fasciculations: empiric study combining ultrasonography and electromyography. Clin Neurophysiol. 2017. doi:10.1016/j.clinph.2017.06.228
18. Takamatsu N, Nodera H, Mori A, et al. Which muscle shows fasciculations by ultrasound in patients with ALS? J Med Invest. 2016;63(1–2):49–53. doi:10.2152/jmi.63.49
19. Noto YI, Shibuya K, Shahrizaila N, et al. Detection of fasciculations in amyotrophic lateral sclerosis: the optimal ultrasound scan time. Muscle Nerve. 2017;56(6):1068–1071. doi:10.1002/mus.25607
20. Shen J, Cartwright MS. Neuromuscular ultrasound in the assessment of polyneuropathies and motor neuron disease. J Clin Neurophysiol. 2016;33(2):86–93. doi:10.1097/WNP.0000000000000241
21. Caress JB. Optimal muscle selection in amyotrophic lateral sclerosis and the end of the 4-limb EMG. Muscle Nerve. 2017;56(1):4–6. doi:10.1002/mus.25662
22. Kramer HH, Vlazak A, Doring K, Tanislav C, Allendorfer J, Kaps M. Excellent interrater agreement for the differentiation of fasciculations and artefacts - a dynamic myosonography study. Clin Neurophysiol. 2014;125(12):2441–2445. doi:10.1016/j.clinph.2014.04.009
23. Vazquez-Costa JF, Campins-Romeu M, Martinez-Paya JJ, et al. New insights into the pathophysiology of fasciculations in amyotrophic lateral sclerosis: an ultrasound study. Clin Neurophysiol. 2018;129(12):2650–2657. doi:10.1016/j.clinph.2018.09.014
24. Tsuji Y, Noto YI, Shiga K, Teramukai S, Nakagawa M, Mizuno T. A muscle ultrasound score in the diagnosis of amyotrophic lateral sclerosis. Clin Neurophysiol. 2017;128(6):1069–1074. doi:10.1016/j.clinph.2017.02.015
25. Harding PJ, Loram ID, Combes N, Hodson-Tole EF. Ultrasound-based detection of fasciculations in healthy and diseased muscles. IEEE Trans Biomed Eng. 2016;63(3):512–518. doi:10.1109/TBME.2015.2465168
26. Noto YI, Simon NG, Selby A, et al. Ectopic impulse generation in peripheral nerve hyperexcitability syndromes and amyotrophic lateral sclerosis. Clin Neurophysiol. 2018;129:974–980. doi:10.1016/j.clinph.2018.01.061
27. Swash M, de Carvalho M. Muscle ultrasound detects fasciculations and facilitates diagnosis in ALS. Neurology. 2011;77(16):1508–1509. doi:10.1212/WNL.0b013e318233b3c4
28. de Carvalho M, Swash M. Fasciculation potentials and earliest changes in motor unit physiology in ALS. J Neurol Neurosurg Psychiatry. 2013;84(9):963–968. doi:10.1136/jnnp-2012-304545
29. Tsugawa J, Dharmadasa T, Ma Y, Huynh W, Vucic S, Kiernan MC. Fasciculation intensity and disease progression in amyotrophic lateral sclerosis. Clin Neurophysiol. 2018;129:2149–2154. doi:10.1016/j.clinph.2018.07.015
30. Walker FO. Ultrasonography in peripheral nervous system diagnosis. Continuum (Minneap Minn). 2017;23(5,Peripheral Nerve and Motor Neuron Disorders):1276–1294. doi:10.1212/CON.0000000000000522
31. de Carvalho M, Swash M. Physiology of the fasciculation potentials in amyotrophic lateral sclerosis: which motor units fasciculate? J Physiol Sci. 2017;67(5):569–576. doi:10.1007/s12576-016-0484-x
32. Kaji R, Kohara M, Kimura J. Fasciculations evoked by magnetic cortical stimulation in patients with amyotrophic lateral sclerosis. Neurology. 1993;43:A277–A278.
33. Pillen S, Nienhuis M, van Dijk JP, Arts IM, van Alfen N, Zwarts MJ. Muscles alive: ultrasound detects fibrillations. Clin Neurophysiol. 2009;120(5):932–936. doi:10.1016/j.clinph.2009.01.016
34. van Alfen N, Nienhuis M, Zwarts MJ, Pillen S. Detection of fibrillations using muscle ultrasound: diagnostic accuracy and identification of pitfalls. Muscle Nerve. 2011;43(2):178–182. doi:10.1002/mus.21863
35. Groeneveld GJ, Van Kan HJ, Kalmijn S, et al. Riluzole serum concentrations in patients with ALS: associations with side effects and symptoms. Neurology. 2003;61(8):1141–1143. doi:10.1212/01.wnl.0000090459.76784.49
36. Cartwright MS, Walker FO, Griffin LP, Caress JB. Peripheral nerve and muscle ultrasound in amyotrophic lateral sclerosis. Muscle Nerve. 2011;44(3):346–351. doi:10.1002/mus.22035
37. Martinez-Paya JJ, Del Bano-Aledo ME, Rios-Diaz J, Tembl-Ferrairo JI, Vazquez-Costa JF, Medina-Mirapeix F. Muscular echovariation: a new biomarker in amyotrophic lateral sclerosis. Ultrasound Med Biol. 2017;43(6):1153–1162. doi:10.1016/j.ultrasmedbio.2017.02.002
38. Pathak S, Caress JB, Wosiski-Kuhn M, Milligan C, Williams D, Cartwright MS. A pilot study of neuromuscular ultrasound as a biomarker for amyotrophic lateral sclerosis. Muscle Nerve. 2019;59(2):181–186. doi:10.1002/mus.26360
39. Cedarbaum J, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169(1–2):13–21.
40. Martinez-Paya JJ, Rios-Diaz J, Del Bano-Aledo ME, Tembl-Ferrairo JI, Vazquez-Costa JF, Medina-Mirapeix F. Quantitative muscle ultrasonography using textural analysis in amyotrophic lateral sclerosis. Ultrason Imaging. 2017;39(6):357–368. doi:10.1177/0161734617711370
41. Pillen S, Tak RO, Zwarts MJ, et al. Skeletal muscle ultrasound: correlation between fibrous tissue and echo intensity. Ultrasound Med Biol. 2009;35(3):443–446. doi:10.1016/j.ultrasmedbio.2008.09.016
42. Arts IM, Schelhaas HJ, Verrijp KC, et al. Intramuscular fibrous tissue determines muscle echo intensity in amyotrophic lateral sclerosis. Muscle Nerve. 2012;45(3):449–450. doi:10.1002/mus.22254
43. Arts IMP, Overeem S, Pillen S, Jurgen Schelhaas H, Zwarts MJ. Muscle changes in amyotrophic lateral sclerosis: a longitudinal ultrasonography study. Clin Neurophysiol. 2011;122(3):623–628. doi:10.1016/j.clinph.2010.07.023
44. Arts IM, Overeem S, Pillen S, Schelhaas HJ, Zwarts MJ. Muscle ultrasonography to predict survival in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2011;82(5):552–554. doi:10.1136/jnnp.2009.200519
45. Kimura F, Fujimura C, Ishida S, et al. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology. 2006;66(2):265–267. doi:10.1212/01.wnl.0000194316.91908.8a
46. Seok HY, Park J, Kim YH, Oh K-W, Kim SH, Kim B-J. Split hand muscle echo intensity index as a reliable imaging marker for differential diagnosis of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2018;89:943–948. doi:10.1136/jnnp-2017-317917
47. Simon NG, Ralph JW, Lomen-Hoerth C, et al. Quantitative ultrasound of denervated hand muscles. Muscle Nerve. 2015;52:221–230. doi:10.1002/mus.24519
48. Crass JR, van de Vegte GL, Harkavy LA. Tendon echogenicity: ex vivo study. Radiology. 1988;167(2):499–501. doi:10.1148/radiology.167.2.3282264
49. Pillen S, van Keimpema M, Nievelstein RA, Verrips A, van Kruijsbergen-Raijmann W, Zwarts MJ. Skeletal muscle ultrasonography: visual versus quantitative evaluation. Ultrasound Med Biol. 2006;32(9):1315–1321. doi:10.1016/j.ultrasmedbio.2006.05.028
50. Pillen S, Arts IM, Zwarts MJ. Muscle ultrasound in neuromuscular disorders. Muscle Nerve. 2008;37(6):679–693. doi:10.1002/mus.21015
51. Martinez-Paya JJ, Del Bano-Aledo ME, Rios-Diaz J, Fornes-Ferrer V, Vazquez-Costa JF. Sonoelastography for the assessment of muscle changes in amyotrophic lateral sclerosis: results of a pilot study. Ultrasound Med Biol. 2018;44(12):2540–2547. doi:10.1016/j.ultrasmedbio.2018.08.009
52. Sowa Y, Numajiri T, Itsukage S, Nishino K. Comparison of shear-wave and strain ultrasound elastography for evaluating fat induration after breast reconstruction. Plast Reconstr Surg Glob Open. 2016;4(4):e677. doi:10.1097/GOX.0000000000000790
53. Ryu J, Jeong WK. Current status of musculoskeletal application of shear wave elastography. Ultrasonography. 2017;36(3):185–197. doi:10.14366/usg.16053
54. Lee SSM, Gaebler-Spira D, Zhang L, Rymer WZ, Steele KM. Use of shear wave ultrasound elastography to quantify muscle properties in cerebral palsy. Clin Biomech. 2016;31(31):20–28. doi:10.1016/j.clinbiomech.2015.10.006
55. Lacourpaille L, Hug F, Guevel A, et al. Non-invasive assessment of muscle stiffness in patients with duchenne muscular dystrophy. Muscle Nerve. 2015;51(2):284–286. doi:10.1002/mus.24445
56. Simon NG, Turner MR, Vucic S, et al. Quantifying disease progression in amyotrophic lateral sclerosis. Ann Neurol. 2014;76(5):643–657. doi:10.1002/ana.24273
57. Lee CD, Song Y, Peltier AC, Jarquin-Valdivia AA, Donofrio PD. Muscle ultrasound quantifies the rate of reduction of muscle thickness in amyotrophic lateral sclerosis. Muscle Nerve. 2010;42(5):814–819. doi:10.1002/mus.21779
58. Martinez-Paya JJ, Rios-Diaz J, Medina-Mirapeix F, Vazquez-Costa JF, Del Bano-Aledo ME. Monitoring progression of amyotrophic lateral sclerosis using ultrasound morpho-textural muscle biomarkers: a pilot study. Ultrasound Med Biol. 2018;44(1):102–109. doi:10.1016/j.ultrasmedbio.2017.09.013
59. Andersen PM, Abrahams S, Borasio GD, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)–revised report of an EFNS task force. Eur J Neurol. 2012;19(3):360–375. doi:10.1111/j.1468-1331.2011.03501.x
60. Howard RS, Orrell RW. Management of motor neurone disease. Postgrad Med J. 2002;78(926):736–741. doi:10.1136/pmj.78.926.736
61. Hadjikoutis S, Wiles CM. Respiratory complications related to bulbar dysfunction in motor neuron disease. Acta Neurol Scand. 2001;103(4):207–213.
62. Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006;5(2):140–147. doi:10.1016/S1474-4422(05)70326-4
63. Kleopa KA, Sherman M, Neal B, Romano GJ, Heiman-Patterson T. Bipap improves survival and rate of pulmonary function decline in patients with ALS. J Neurol Sci. 1999;164(1):82–88.
64. Lyall RA, Donaldson N, Polkey MI, Leigh PN, Moxham J. Respiratory muscle strength and ventilatory failure in amyotrophic lateral sclerosis. Brain. 2001;124(Pt 10):2000–2013.
65. Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007;6(11):994–1003. doi:10.1016/S1474-4422(07)70265-X
66. Evangelista T, Carvalho M, Pinto A, Luis Mde L. Phrenic nerve conduction in amyotrophic lateral sclerosis. J Neurol Sci. 1995;129:
67. Bolton CF. AAEM minimonograph #40: clinical neurophysiology of the respiratory system. Muscle Nerve. 1993;16(8):809–818. doi:10.1002/mus.880160802
68. Stewart H, Eisen A, Road J, Mezei M, Weber M. Electromyography of respiratory muscles in amyotrophic lateral sclerosis. J Neurol Sci. 2001;191(1–2):67–73.
69. Cruz-Martinez A, Armijo A, Fermoso A, Moraleda S, Mate I, Marin M. Phrenic nerve conduction study in demyelinating neuropathies and open-heart surgery. Clin Neurophysiol. 2000;111(5):821–825.
70. Hiwatani Y, Sakata M, Miwa H. Ultrasonography of the diaphragm in amyotrophic lateral sclerosis: clinical significance in assessment of respiratory functions. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(2):127–131. doi:10.3109/17482968.2012.729595
71. Aliberti S, Messinesi G, Gramegna A, Tremolizzo L, Susani E, Pesci A. Diaphragm ultrasonography in the management of patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(2):154–156. doi:10.3109/21678421.2012.762931
72. Carrie C, Bonnardel E, Vally R, Revel P, Marthan R, Marthan R. Vital capacity impairment due to neuromuscular disease and its correlation with diaphragmatic ultrasound: a preliminary study. Ultrasound Med Biol. 2016;42(1):143–149. doi:10.1016/j.ultrasmedbio.2015.09.020
73. Fantini R, Mandrioli J, Zona S, et al. Ultrasound assessment of diaphragmatic function in patients with amyotrophic lateral sclerosis. Respirology. 2016;21(5):932–938. doi:10.1111/resp.12759
74. Pinto S, Alves P, Pimentel B, Swash M, de Carvalho M. Ultrasound for assessment of diaphragm in ALS. Clin Neurophysiol. 2016;127(1):892–897. doi:10.1016/j.clinph.2015.03.024
75. Noda Y, Sekiguchi K, Kohara N, Kanda F, Toda T. Ultrasonographic diaphragm thickness correlates with compound muscle action potential amplitude and forced vital capacity. Muscle Nerve. 2016;53(4):522–527. doi:10.1002/mus.24902
76. Pinto S, Alves P, Swash M, de Carvalho M. Phrenic nerve stimulation is more sensitive than ultrasound measurement of diaphragm thickness in assessing early ALS progression. Neurophysiol Clin. 2017;47(1):69–73. doi:10.1016/j.neucli.2016.08.001
77. Johnson NE, Utz M, Patrick E, et al. Visualization of the diaphragm muscle with ultrasound improves diagnostic accuracy of phrenic nerve conduction studies. Muscle Nerve. 2014;49(5):669–675. doi:10.1002/mus.24059
78. de Carvalho M, Costa J, Pinto S, Pinto A. Percutaneous nocturnal oximetry in amyotrophic lateral sclerosis: periodic desaturation. Amyotroph Lateral Scler. 2009;10(3):154–161. doi:10.1080/17482960802382305
79. Simon NG, Kiernan MC. Diaphragm ultrasound in amyotrophic lateral sclerosis and other neuromuscular disorders. Clin Neurophysiol. 2016;127(1):28–30. doi:10.1016/j.clinph.2015.04.066
80. Nakamori M, Hosomi N, Takaki S, et al. Tongue thickness evaluation using ultrasonography can predict swallowing function in amyotrophic lateral sclerosis patients. Clin Neurophysiol. 2016;127(2):1669–1674. doi:10.1016/j.clinph.2015.07.032
81. Del Aguila M, Longstreth W
82. Zoccolella S, Beghi E, Palagano G, et al. Analysis of survival and prognostic factors in amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2008;79:33–37. doi:10.1136/jnnp.2007.118018
83. Rofes L, Arreola V, Almirall J, et al. Diagnosis and management of oropharyngeal dysphagia and its nutritional and respiratory complications in the elderly. Gastroenterol Res Pract. 2011;2011: 818979.
84. Tamburrini S, Solazzo A, Sagnelli A, et al. Amyotrophic lateral sclerosis: sonographic evaluation of dysphagia. Radiol Med. 2010;115(5):784–793. doi:10.1007/s11547-010-0523-2
85. Sorenson EJ, Crum B, Stevens JC. Incidence of aspiration pneumonia in ALS in Olmsted County, MN. Amyotroph Lateral Scler. 2007;8(2):87–89. doi:10.1080/17482960601147461
86. Noto YI, Simon N, Shibuya K, Matamala JM, Dharmadasa T, Kiernan MC. Dynamic muscle ultrasound identifies upper motor neuron involvement in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(5–6):404–410. doi:10.1080/21678421.2017.1286355
87. Korner S, Hendricks M, Kollewe K, et al. Weight loss, dysphagia and supplement intake in patients with amyotrophic lateral sclerosis (ALS): impact on quality of life and therapeutic options. BMC Neurol. 2013;13:84. doi:10.1186/1471-2377-13-202
88. Nodera H, Takamatsu N, Shimatani Y, et al. Thinning of cervical nerve roots and peripheral nerves in ALS as measured by sonography. Clin Neurophysiol. 2014;125(9):1906–1911. doi:10.1016/j.clinph.2014.01.033
89. Mori A, Nodera H, Takamatsu N, et al. Sonographic evaluation of cervical nerve roots in ALS and its clinical subtypes. J Med Invest. 2016;63:54–57. doi:10.2152/jmi.63.54
90. Schreiber S, Abdulla S, Debska-Vielhaber G, et al. Peripheral nerve ultrasound in amyotrophic lateral sclerosis phenotypes. Muscle Nerve. 2015;51:669–675. doi:10.1002/mus.24431
91. Tawfik EA. Vagus nerve ultrasound in a patient with amyotrophic lateral sclerosis. Muscle Nerve. 2016;54(5):978–979. doi:10.1002/mus.25126
92. Heads T, Pollock M, Robertson A, Sutherland WH, Allpress S. Sensory nerve pathology in amyotrophic lateral sclerosis. Acta Neuropathol. 1991;82(4):316–320.
93. Sobue G, Matsuoka Y, Mukai E, Takayanagi T, Sobue I. Pathology of myelinated fibers in cervical and lumbar ventral spinal roots in amyotrophic lateral sclerosis. J Neurol Sci. 1981;50(3):413–421.
94. Grimm A, Decard BF, Athanasopoulou I, Schweikert K, Sinnreich M, Axer H. Nerve ultrasound for differentiation between amyotrophic lateral sclerosis and multifocal motor neuropathy. J Neurol. 2015;262(4):870–880. doi:10.1007/s00415-015-7648-0
95. Jongbloed BA, Haakma W, Goedee HS, et al. Comparative study of peripheral nerve Mri and ultrasound in multifocal motor neuropathy and amyotrophic lateral sclerosis. Muscle Nerve. 2016;54(6):1133–1135. doi:10.1002/mus.25391
96. Loewenbruck KF, Liesenberg J, Dittrich M, et al. Nerve ultrasound in the differentiation of multifocal motor neuropathy (MMN) and amyotrophic lateral sclerosis with predominant lower motor neuron disease (ALS/LMND). J Neurol. 2016;263(1):35–44. doi:10.1007/s00415-015-7927-9
97. Nodera H, Izumi Y, Takamatsu N, Kaji R. Cervical root sonography to differentiate multifocal motor neuropathy from ALS. J Med Invest. 2016;63(1–2):104–107. doi:10.2152/jmi.63.104
98. Noto YI, Garg N, Li T, et al. Comparison of cross-sectional areas and distal-proximal nerve ratios in amyotrophic lateral sclerosis. Muscle Nerve. 2018;58(6):777–783. doi:10.1002/mus.26301
99. Watchmaker GP, Gumucio CA, Crandall RE, Vannier MA, Weeks PM. Fascicular topography of the median nerve: a computer based study to identify branching patterns. J Hand Surg Am. 1991;16(1):53–59.
100. Schreiber S, Debska-Vielhaber G, Abdulla S, et al. Peripheral nerve atrophy together with higher cerebrospinal fluid progranulin indicate axonal damage in amyotrophic lateral sclerosis. Muscle Nerve. 2018;57(2):273–278. doi:10.1002/mus.25682
101. Irwin D, Lippa CF, Rosso A. Progranulin (PGRN) expression in ALS: an immunohistochemical study. J Neurol Sci. 2009;276(1–2):9–13. doi:10.1016/j.jns.2008.08.024
102. Philips T, De Muynck L, Thu HN, et al. Microglial upregulation of progranulin as a marker of motor neuron degeneration. J Neuropathol Exp Neurol. 2010;69(12):1191–1200. doi:10.1097/NEN.0b013e3181fc9aea
103. Mori A, Nodera H, Takamatsu N, et al. Focal nerve enlargement is not the cause for increased distal motor latency in ALS: sonographic evaluation. Clin Neurophysiol. 2015;126(8):1632–1637. doi:10.1016/j.clinph.2014.10.152
104. Dengler R, Konstanzer A, Hesse S, Schubert M, Wolf W. Collateral nerve sprouting and twitch forces of single motor units in conditions with partial denervation in man. Neurosci Lett. 1989;97(1–2):118–122.
105. Schreiber S, Dannhardt-Stieger V, Henkel D, et al. Quantifying disease progression in amyotrophic lateral sclerosis using peripheral nerve sonography. Muscle Nerve. 2016;54(3):391–397. doi:10.1002/mus.25066
106. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–955. doi:10.1016/S0140-6736(10)61156-7
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.