Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Clinical and Radiological Features of COPD Patients Living at ≥3000 m Above Sea Level in the Tibet Plateau
Authors Liang Y, Yangzom D, Tsokyi L, Ning Y, Su B, Luo S , Ma Cuo B, ChuTso M, Ding Y, Chen Y, Sun Y
Received 16 June 2021
Accepted for publication 16 August 2021
Published 26 August 2021 Volume 2021:16 Pages 2445—2454
DOI https://doi.org/10.2147/COPD.S325097
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Ying Liang,1,2 Drolma Yangzom,2 Lhamo Tsokyi,2 Yanping Ning,2 Baiyan Su,3,4 Shuai Luo,4 Bian Ma Cuo,2 Meilang ChuTso,2 Yanling Ding,1 Yahong Chen,1 Yongchang Sun1
1Department of Respiratory and Critical Care Medicine, Peking University Third Hospital, Beijing, 100191, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Tibet Autonomous Region People’s Hospital, Lhasa, 850000, People’s Republic of China; 3Radiology Department, Peking Union Medical College Hospital, Beijing, 100730, People’s Republic of China; 4Radiology Department, Tibet Autonomous Region People’s Hospital, Lhasa, 850000, People’s Republic of China
Correspondence: Yongchang Sun
Department of Respiratory and Critical Care Medicine, Peking University Third Hospital, North Garden Road 49, Haidian District, Beijing, People’s Republic of China
Tel +86 139 1097 9132
Fax +86 108 226 6989
Email [email protected]
Background: COPD at high altitude may have different risk factors and unique clinical and radiological phenotypes. We aimed to investigate the demographic data, clinical and radiological features of COPD patients permanently residing at the Tibet Plateau (≥ 3000 meters above sea level).
Methods: We conducted an observational cross-sectional study which consecutively enrolled COPD patients visiting the outpatient of Respiratory Medicine at Tibet Autonomous Region People’s Hospital from January 2018 to March 2021. All patients were Tibetan permanent residents aging ≥ 40 years and met the diagnosis of COPD according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines. Data including demographic characteristics, altitude of residence, risk factors, respiratory symptoms, comorbidities and medications, as well as computed tomography (CT) measurements were collected.
Results: Eighty-four patients with definite COPD were enrolled for analysis. Their mean age was 64.7 (± 9.1) years. All patients lived at ≥ 3000 m above sea level and 34.5% of them lived at ≥ 4000 m. About 8.3% of the patients were current smokers and 44.0% were ex-smokers. Up to 88.1% of the patients reported long-term exposure to indoor biomass fuels. Most of the patients were classified as having mild-to-moderate (GOLD I: 27.4%; GOLD II: 51.2%) COPD, while 89.3% had a CAT score ≥ 10. Only 36.9% of the patients received regular long-term medications for COPD in the past year, in whom ICS/LABA and oral theophylline were the most common used pharmacological therapy. On CT scanning, the majority of our patients (70.7%) showed no or minimal emphysema, while signs of previous tuberculosis were found in 45.1% of the patients.
Conclusion: COPD patients living at the Tibet Plateau had a heavy respiratory symptom burden, but most of them did not receive adequate pharmacological treatment. Indoor biomass fuel exposure and previous tuberculosis were prevalent, while the emphysema phenotype was less common in this population.
Keywords: chronic obstructive pulmonary disease, high altitude, phenotype, computed tomography
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable and treatable airway inflammatory disease characterized by persistent respiratory symptoms and airflow limitation.1 According to Global Burden of Disease Study, COPD is the third leading cause of death worldwide in 2017. Moreover, the morbidity and mortality of COPD were the highest among all the chronic respiratory diseases.2 There are approximately 99.9 million patients with COPD in China and the prevalence of COPD among population aged ≥40 years is 13.7%.3 To date, the economic and societal burden of COPD is still substantial and great.4
Globally, there are around 400 million people living at high altitude (>1500 m above sea level).5 Low barometric and hypoxia environment at high altitude may aggravate gas exchange and hypoxia of patients with COPD and potentiate pulmonary hypertension and development of cor pulmonale, leading to higher mortality.6–8 The average altitude of the Tibet Plateau is over 3000 meters above sea level, with many regions even at an average altitude of >3500 meters (very high altitude).6 A population-based cross-sectional survey conducted in Xinjiang and Tibet Autonomous Regions showed that the overall prevalence of COPD in residents living at 2100–4700 meters above sea level was 8.2% and the prevalence decreased with increasing altitude. Being≥40 years of age, household air pollution and a history of tuberculosis were the independent risk factors for COPD in these regions.9 Around 3.5 million people permanently live at the Tibet Plateau and Tibetans are the major ethnics. Ethnic difference and special environmental setting may affect the clinical phenotypes of COPD. However, studies regarding the clinical and radiological features of COPD patients permanently residing at the Tibet Plateau (≥3000 meters above sea level) are limited.
In the present study, we aimed to describe the risk factors, clinical features and computed tomography (CT) findings in an outpatient cohort of patients with COPD living at very high altitude in the Tibet Plateau.
Methods
Study Design and Subjects
We conducted an observational cross-sectional study in the outpatient of Respiratory Medicine in Tibet Autonomous Region People’s Hospital and consecutively enrolled patients with COPD from January 2018 to March 2021. All the patients met the diagnosis of COPD according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines and had definite airflow limitation with a post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC)<0.7. The patients were Tibetan residents living at the Tibet Plateau from their childhood and came from seven districts in Tibet Autonomous Region of the People’s Republic of China: Lhasa (~3650 m), Qamdo (~3500 m), Linzhi (~3000 m), Shannan (~3700 m), Nakchu (~5200 m), Shigatse (~3900 m) and Ali (~4500 m). Tibet Autonomous Region People’s Hospital is located in the City of Lhasa (~3650 m above sea level).
Exclusion criteria included: 1) age <40 years; 2) subjects with airway diseases other than COPD; 3) acute exacerbation of COPD in the past 3 months; 4) active tuberculosis; 5) cardiovascular or cerebrovascular events in the past 3 months; 6) cognitive dysfunction such as vascular dementia or Alzheimer’s disease; 7) refusal to participate in this study.
The study protocol was approved by the Independent Ethics Committee of the Peking University Third Hospital (IRB00006761-M2020430) which was the primary research institution of this study and the Ethics Committee of Tibet Autonomous Region People’s Hospital (ME-TBHP-20-KJ-036). Written informed consents were obtained from the patients or their close relatives. Each subject was recruited once and data were analyzed anonymously.
Questionnaire and Data Collection
A standardized questionnaire based on the Screening Questionnaire FRESH AIR Uganda10,11 was used, including demographic data, education level, living condition, respiratory symptoms, exacerbations, tobacco use, biomass fuel use, history of tuberculosis, comorbidities, medications and spirometry results. Besides, altitude of current residence, duration of current residence, current or past occupation, and the COPD Assessment Test (CAT) score12 were included in this questionnaire. According to GOLD guidelines, the patients were classified into Group A to D, based on CAT score and exacerbation history in the past year.1 The questionnaire was translated to Chinese and strictly discussed by respiratory specialists from Peking University Third Hospital and Tibet Autonomous Region People’s Hospital.
Patient visit was completed by the physicians who were from Department of Respiratory and Critical Care Medicine in Tibet Autonomous Region People’s Hospital. A centralized training session for these physicians was performed prior to the interview. Upon explaining the aim of this study and the amount of time required, the interviewer carried on asking the questions from the above questionnaire in Chinese or Tibetan, trying their best to make the patients to fully understand the meaning of each question. Data in the questionnaire were inputted into a specialized database and any identifier of the patients was not collected.
Spirometry Test
Standardized spirometry tests were performed according to current ATS/ERS recommendations,13 using a MasterScreen PFT Analyzer Unit (Jaeger, Hoechberg, Germany). Post-bronchodilator FEV1% predicted, FVC percent predicted, and ratio of FEV1/FVC were recorded.
Blood Routine Examination
Blood routine examination was performed in the same period. The results were also valid if the patient received this examination within one month. Blood routine examination was analyzed by Clinical Laboratory of Tibet Autonomous Region People’s Hospital. Count of white blood cells, red blood cells and platelets, hemoglobin, percentage of eosinophils and neutrophils were collected.
Chest CT Scanning
High-resolution chest CT examination was performed using SOMATOM Drive (Siemens, Erlangen, Germany) at full-inspiration when the patients held their breath. The thickness of each slice was 1.00–1.25 mm. Lung parenchymal and airway measurements were performed with the use of software package SYNAPSE 3D (FUJIFILM, Japan).
The area with CT attenuation value of ≤−950 Hu in full-inspiration, ie, low attenuation area (LAA), was considered to be emphysema. The percentage of the LAA divided by total lung volumes (LAA%) was used as an index of the severity of emphysema.14 LAA% could be calculated automatically by the SYNAPSE 3D software. According to the LAA%, the severity of emphysema was classified as no emphysema (LAA% <6%), moderate emphysema (LAA% ≥6% and <14%), and severe emphysema (LAA% ≥14%), as described previously.15,16
For airway analysis, a bronchial tree model was extracted automatically by the SYNAPSE 3D software. The opening of apical segment of right upper lobe was set manually, where bronchial wall thickness (WT), luminal area (LA) and bronchial wall area (WA) could be calculated automatically by the software.17–19 The three measurements were corrected by body surface area (BSA). The percentage WA (WA%) was calculated as WA/(WA+LA)×100%. The WA% was a good surrogate parameter for small airway disease.20,21 The thickness-to-diameter ratio (TDR) of the airways was automatically calculated as bronchial WT/outer diameter.
The presence of previous pulmonary tuberculosis on chest CT scans was recorded. The definition of previous pulmonary tuberculosis by radiological imaging was described previously.22
Imaging analysis was performed by two radiological specialists who were unaware of the clinical data of the patients.
Statistical Analysis
All data were analyzed anonymously. Statistical analyses were performed using SPSS software, version 19.0 (IBM, Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as numbers (%). Unpaired t-test was used to evaluate the differences between continuous variables. Chi-square test or Fisher exact test was used for categorical variables. Results were considered statistically significant at P-value <0.05.
Results
During the study period, 113 subjects with a previous diagnosis of COPD or probable COPD visited the outpatient of Department of Respiratory Medicine in Tibet Autonomous Region People’s Hospital. Ten subjects refused to participate in this study. Nineteen subjects were excluded as their post-bronchodilator FEV1/FVC ratios were ≥0.7. Finally, 84 COPD patients who completed the questionnaire with valid data were included in the analysis (Figure 1).
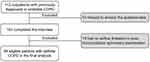 |
Figure 1 Flow chart of the study. |
The mean age of the patients was 64.7 (±9.1) years and 59.5% were male. The educational levels of the patients were generally low, with 75.0% only having primary school education or lower. All patients lived at ≥3000 m above sea level for a long-term residence and 34.5% of them lived at ≥4000 m above sea level. More than one half were rural residents or living in pastoral areas. 8.3% of the patients were current smokers and 44.0% were ex-smokers. Indoor biomass exposure was very common and up to 88.1% had long-term indoor biomass fuel exposure. 22.6% of the patients reported a definite tuberculosis history in the past. Approximately 10% reported recurrent respiratory infection in their childhood (Table 1).
 |
Table 1 Demographic Characteristics and Risk Factors Relevant to COPD (n = 84) |
In these patients, shortness of breath or wheezing (75.0%) and dyspnea in daily life (83.3%) were the most common respiratory symptoms. The mean CAT score was 17.7, with nearly 90.0% of the patients having a CAT score ≥10, 58.3% having a CAT score 10–19 and 31.0% having a CAT score over 20. Hypertension was the most common comorbidity. The mean FEV1%predicted and FVC %predicted were 67.08 (±21.90) % and 93.58 (±24.26) %, respectively. Most of the patients were in GOLD Stage I and II. 19.0% of the patients experienced ≥2 exacerbations in the past year, but 47.6% were ever hospitalized due to exacerbation (Table 2). According to the CAT score and exacerbation history, the proportions of patients in GOLD Group A-D were 7.1%, 44.0%, 3.6% and 45.2%, respectively. Only 36.9% (31/84) of the patients received regular long-term medications for COPD in the past 12 months. Inhaled corticosteroids plus long-acting β2-agonists (ICS plus LABA) (90.3%, 28/31) and oral theophylline (41.9%, 13/31) were the most commonly used pharmacological therapy in these patients. Long-acting muscarinic antagonist (LAMA) was only prescribed in 16.1% (5/28) of them (Table 3).
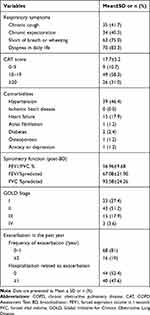 |
Table 2 Clinical Characteristics of COPD Patients Living at High Altitude ≥3000 m Above Sea Level (n = 84) |
 |
Table 3 Pharmacology Therapy in COPD Patients Who Received Regular Medications in High Altitude ≥3000 m Above Sea Level |
The mean percentage of peripheral blood eosinophils was 2.9%. The proportions of patients with an eosinophil count <100/μL, 100–300/μL and ≥300/μL were 44.0%, 44.0% and 12.0%, respectively (Table 4).
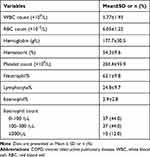 |
Table 4 Blood Routine Characteristics of COPD Patients Living at High Altitude ≥3000 m Above Sea Level (n = 84) |
Eighty-two patients received chest CT scanning and had good image quality for analysis. The mean LAA% of the patients was 5.23% (±7.39%). The proportions of no emphysema, moderate emphysema and severe emphysema were 70.7%, 15.9% and 13.4%, respectively. The mean WA% and TDR at the opening of the right upper apical segment was 50.85% (±11.18%) and 0.30 (±0.08), respectively. Up to 45.1% (37/82) of the patients showed signs of previous tuberculosis on CT scan (Table 5).
 |
Table 5 Imaging Features of COPD Patients Living at High Altitude ≥3000 m Above Sea Level (n = 82) |
We further divided the patients into 2 groups according to their altitude of residence: 3000–4000 m (n = 55) and ≥4000 m (n =29). The age, gender, BMI, smoking status, biomass exposure, CAT score, spirometry and GOLD classification were not statistically different between the 2 groups. However, patients living at 3000–4000m above sea level had a greater LAA% than those living at ≥4000 m, with more patients with moderate and severe emphysema. Airway wall structure was similar between the two groups. The proportions of patients with previous tuberculosis on CT scan were also similar (Table 6).
 |
Table 6 Comparison of Clinical and Imaging Features Between COPD Patients Living at 3000–4000 m and ≥4000 m Above Sea Level |
Discussion
Our study found that COPD patients residing at very high altitude (≥3000 m above sea level) in Tibet had a heavy respiratory symptom burden, with a high proportion of patients having a CAT score ≥10, and having more severe exacerbations leading to hospitalization. However, only a small number of patients could maintain their regular pharmacological treatment. Our patients also showed unique features on chest CT, with a lower percentage of emphysema, but a high prevalence of previous tuberculosis. In addition, most of our patients had a long-term history of exposure to household biomass fuels, rather than cigarette smoking. Our data provide novel insights into the risk factors and phenotypes of COPD in patients living at high altitude.
Tobacco exposure is the major risk factor for COPD across the world.1 In our study, fewer patients were current smokers and the overall rate of cigarette smoking was relatively lower compared with previous studies on patients living at sea level.23–25 However, exposure to biomass fuels was more common, especially indoor biomass fuel use. Nearly 90% of the patients were exposed to long-term household air pollution. In Tibetan rural regions, dry cow dung is generally used as fuel for cooking and heating, leading to very high levels of indoor air pollution. Exposure to biomass fuels is another important risk factor for COPD.26,27 A recent study demonstrated that household air pollution produced by solid biomass fuels was heavier in the highlands, where it became an independent risk factor for the development of COPD.11 Another study showed, interestingly, that compared with cigarette smoking, fewer inhaled biomass particles deposited in the alveolar region, which could explain why biomass fuel exposure is associated with less emphysema.28 Switching solid biomass fuels to clean fuels may be helpful to reduce the incidence of COPD at high altitudes.29
In our study, although the majority of the patients had mild-to-moderate COPD, breathlessness was the predominant symptom and the proportion of patients with a CAT score ≥10 was very high (~90%), indicating that respiratory symptoms were heavy in these individuals. A large population-based study showed that more than one half of COPD individuals living at high altitude had at least one respiratory symptom and 87.2% had a CAT score ≥10,9 which was consistent with our study. Dyspnea was the most frequent symptom in this study, but the frequency was not as high as in our study.9 This may be due to different study designs. Asymptomatic mild COPD patients were less likely to visit the hospital. Additionally, in the regions below 1500 m sea level, only 39.8% of people with COPD self-reported typical symptoms of COPD.3 Hypoxemia worsening at very high altitude due to low barometric pressure and low partial oxygen pressure may explain this difference.
We also noticed that only one-third of our patients received regular pharmacological treatment. This proportion was much lower than that in relatively well-developed areas in China.25 At high altitude, only 1.1% of COPD patients were aware of their diagnosis previously.9 Unawareness of COPD, low access to healthcare, poor medication accessibility in rural or pastoral areas, low index of suspicion by local physicians may lead to inadequate diagnosis and treatment for COPD at high altitude. In addition, even in those individuals receiving regular pharmacological treatment, ICS plus LABA was the most commonly used medication. However, only 12.0% of the patients had a blood eosinophil count ≥300/μL, while more than 40% had signs of previous tuberculosis on CT scans, indicating that inhaled corticosteroids were not appropriate for some of these patients according to GOLD guidelines.1 LAMAs or LAMA plus LABA may be more suitable for reducing respiratory symptoms and exacerbations,30–32 but the optimal pharmacological therapy for COPD at high altitude still needs investigation.
In our study, the clinical features including COPD risk factors, respiratory symptoms, spirometry and GOLD classification were similar between those living at altitude 3000–4000 m and ≥4000 m. Atmospheric partial oxygen pressure decreases with altitude increasing, and respiratory symptoms are expected to be worse at higher altitude. But there may be some compensatory mechanisms. A larger study to assess the respiratory symptoms of COPD patients at different altitudes may help to answer this question.
Interestingly, we found that the degrees of emphysema on quantitative CT analysis in most of the patients were mild or moderate, although they had a heavy symptom burden. Furthermore, the severity of emphysema (LAA%) was greater in individuals living at 3000–4000 m than in those living at ≥4000 m above sea level. The emphysema-predominant phenotype of COPD was characterized by lower gas transfer coefficient and low arterial partial pressure of oxygen.21 This could partly explain the finding that clinical manifestations were not different between patients living at altitudes at 3000–4000 m and ≥4000 m. In other words, patients living at 3000–4000 m had a worse gas exchange function because of more severe emphysema, although where the atmospheric partial pressure of oxygen was relatively higher.
WA% and TDR are used for airway evaluation, especially for chronic airway diseases such as COPD and asthma. Compared with previous studies,21,33,34 the WA% in our study was relatively lower, although there is no normal reference for WA% at present. The WA% in healthy controls was around 55–60%,20,21 which was slightly higher than the mean WA% in our study, suggesting that airway remodeling may be less remarkable in COPD patients living at the Tibet Plateau.
Tuberculosis has been identified as an important risk factor for COPD.35 Previous pulmonary tuberculosis can cause permanent damage to lung structures and is associated with loss of lung function.36 In our study, 22.6% of the patients reported an ever-diagnosed tuberculosis history, and more patients with signs of previous tuberculosis on CT scans were identified in our cohort. Guo et al found that the prevalence of COPD in individuals with a tuberculosis history living at high altitude was 11.6% and tuberculosis was an independent risk factor for COPD. However, they did not report the prevalence of previous tuberculosis in those with spirometry-defined COPD.9 In a meta-analysis, the pooled prevalence of COPD in individuals with previous pulmonary tuberculosis was 21%.37 In the China Pulmonary Health (CPH) Study, the overall prevalence of tuberculosis history in COPD individuals was less than 5.0%,3 which was much lower than the prevalence in our study. However, a pooled analysis of individual data from the PREPOCOL-PLATINO-BOLD-EPI-SCAN studies showed that the prevalence of previous tuberculosis in individuals living at altitude >1500 m above sea level was significantly lower than those living at altitude <1500 m.38 Three studies included in this pooled analysis were from Latin American regions and this result was inconsistent with our study. High prevalence of previous tuberculosis may be another unique feature in patients with COPD living at the Tibet Plateau. Prevention and standard management of pulmonary tuberculosis for residents living at the Tibet Plateau may be helpful for reducing the risk of COPD.
Our study had several limitations. As a single-center, observational study, the sample size was relatively small. The whole area of the Tibet Autonomous Region is over 1.2 million square kilometers. Patients living in remote and mountainous areas were unlikely to visit our outpatients for respiratory symptoms. Therefore, generalizability needs to be assessed carefully. Secondly, the educational level in our patients was low and report bias may not be avoided. Thirdly, we did not include patients living at altitude <1500 m above sea level for comparison, which is expected to provide more information regarding the unique features of COPD at high altitude.
In conclusion, COPD patients living at the Tibet Plateau (≥3000 m above sea level) had a heavy respiratory symptom burden, but most of them may not receive adequate pharmacological treatment. Previous tuberculosis was prevalent in these patients and may be an important risk factor, in addition to biomass fuel, for development of COPD. Considering the small sample size and unavoidable bias in this study, larger scale or multi-center studies are needed to further explore the unique phenotypes and potential endotypes of COPD in this region.
Abbreviations
COPD, chronic obstructive pulmonary diseases; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CT, computed tomography; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; BMI, body mass index; BSA, body surface area; CAT, COPD Assessment Test; LAA, low attenuation area; WT, bronchial wall thickness; LA, luminal area (LA); WA, bronchial wall area; TDR, thickness-to-diameter ratio; LABA, long-acting β2 agonist; ICS, inhaled corticosteroids; LAMA, long-acting muscarinic antagonist; SABA, short–acting β-agonist; SAMA, short-acting muscarinic antagonist.
Data Sharing Statement
The data that supports the findings of this study will not be shared openly with other third parties due to contractual statements related to intellectual property, confidentiality, and proprietary rights.
Ethics Approval and Informed Consent
The study protocol was approved by the Independent Ethics Committee of the Peking University Third Hospital (IRB00006761-M2020430) and the Ethics Committee of Tibet Autonomous Region People’s Hospital (ME-TBHP-20-KJ-036). Written informed consents were obtained from the patients or their close relatives. This study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China [No. 81970041, 82090014, and 81700039] and Capital Health Development Research Project [No. 2020-2Z-40917].
Disclosure
The authors report no conflicts of interest for this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease, 2021 report. Available from: http://www.goldcopd.org/.
2. Soriano JB, Abajobir AA, Abate KH; GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. doi:10.1016/S2213-2600(17)30293-X
3. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
4. Khakban A, Sin DD, FitzGerald JM, et al. Ten-year trends in direct costs of COPD: a population-based study. Chest. 2015;148(3):640–646. doi:10.1378/chest.15-0721
5. Cohen JE, Small C. Hypsographic demography: the distribution of human population by altitude. Proc Natl Acad Sci USA. 1998;95(24):14009–14014. doi:10.1073/pnas.95.24.14009
6. Burtscher M. Effects of living at higher altitudes on mortality: a narrative review. Aging Dis. 2014;5(4):274–280. doi:10.14336/AD.2014.0500274
7. Ezzati M, Horwitz ME, Thomas DS, et al. Altitude, life expectancy and mortality from ischaemic heart disease, stroke, COPD and cancers: national population-based analysis of US counties. J Epidemiol Community Health. 2012;66(7):e17. doi:10.1136/jech.2010.112938
8. Pierson DJ. Pathophysiology and clinical effects of chronic hypoxia. Respir Care. 2000;45(1):
9. Guo Y, Xing Z, Shan G, et al. Prevalence and risk factors for COPD at high altitude: a large cross-sectional survey of subjects living between 2100–4700 m above sea level. Front Med (Lausanne). 2020;7:581763. doi:10.3389/fmed.2020.581763
10. van Gemert F, Kirenga B, Chavannes N, et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. Lancet Glob Health. 2015;3(1):e44–e51. doi:10.1016/S2214-109X(14)70337-7
11. Brakema EA, Tabyshova A, Kasteleyn MJ, et al. High COPD prevalence at high altitude: does household air pollution play a role. Eur Respir J. 2019;53(2):1801193. doi:10.1183/13993003.01193-2018
12. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
13. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
14. Wang Z, Gu S, Leader JK, et al. Optimal threshold in CT quantification of emphysema. Eur Radiol. 2013;23(4):975–984. doi:10.1007/s00330-012-2683-z
15. Occhipinti M, Paoletti M, Bartholmai BJ, et al. Spirometric assessment of emphysema presence and severity as measured by quantitative CT and CT-based radiomics in COPD. Respir Res. 2019;20(1):101. doi:10.1186/s12931-019-1049-3
16. Occhipinti M, Paoletti M, Crapo JD, et al. Validation of a method to assess emphysema severity by spirometry in the COPDGene study. Respir Res. 2020;21(1):103. doi:10.1186/s12931-020-01366-4
17. Gu S, Fuhrman C, Meng X, et al. Computerized identification of airway wall in CT examinations using a 3D active surface evolution approach. Med Image Anal. 2013;17(3):283–296. doi:10.1016/j.media.2012.11.003
18. Han MK, Kazerooni EA, Lynch DA, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274–282. doi:10.1148/radiol.11110173
19. Bai Y, Wang X, Lin F, Liao Y, Tao L, Chen Y. [Quantitative study on HRCT phenotype of the GOLD COPD assessment staging system]. Zhonghua Jie He He Hu Xi Za Zhi. 2015;38(5):356–360. Chinese. doi:10.3760/cma.j.issn.1001-0939.2015.05.009
20. Niimi A, Matsumoto H, Amitani R, et al. Airway wall thickness in asthma assessed by computed tomography. Relation to clinical indices. Am J Respir Crit Care Med. 2000;162(4):1518–1523. doi:10.1164/ajrccm.162.4.9909044
21. Subramanian DR, Gupta S, Burggraf D, et al. Emphysema- and airway-dominant COPD phenotypes defined by standardised quantitative computed tomography. Eur Respir J. 2016;48(1):92–103. doi:10.1183/13993003.01878-2015
22. Jin J, Yu W, Li S, Lu L, Liu X, Sun Y. Factors associated with bronchiectasis in patients with moderate-severe chronic obstructive pulmonary disease. Medicine (Baltimore). 2016;95(29):e4219. doi:10.1097/MD.0000000000004219
23. Liang Y, Chen Y, Wu R, et al. Chronic bronchitis is associated with severe exacerbation and prolonged recovery period in Chinese patients with COPD: a multicenter cross-sectional study. J Thorac Dis. 2017;9(12):5120–5130. doi:10.21037/jtd.2017.11.54
24. Jin J, Li S, Yu W, Liu X, Sun Y. Emphysema and bronchiectasis in COPD patients with previous pulmonary tuberculosis: computed tomography features and clinical implications. Int J Chron Obstruct Pulmon Dis. 2018;13:375–384. doi:10.2147/COPD.S152447
25. Liang Y, Chang C, Chen Y, Dong F, Zhang L, Sun Y. Symptoms, management and healthcare utilization of COPD patients during the COVID-19 epidemic in Beijing. Int J Chron Obstruct Pulmon Dis. 2020;15:2487–2494. doi:10.2147/COPD.S270448
26. Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176(8):753–760. doi:10.1164/rccm.200612-1749OC
27. Hu G, Zhou Y, Tian J, et al. Risk of COPD from exposure to biomass smoke: a meta analysis. Chest. 2010;138(1):20–31. doi:10.1378/chest.08-2114
28. Nicolaou L, Checkley W. Differences between cigarette smoking and biomass smoke exposure: an in silico comparative assessment of particulate deposition in the lungs. Environ Res. 2021;197:111116. doi:10.1016/j.envres.2021.111116
29. Zhou Y, Zou Y, Li X, et al. Lung function and incidence of chronic obstructive pulmonary disease after improved cooking fuels and kitchen ventilation: a 9-year prospective cohort study. PLoS Med. 2014;11(3):e1001621. doi:10.1371/journal.pmed.1001621
30. Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–1103. doi:10.1056/NEJMoa1008378
31. Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1(7):524–533. doi:10.1016/S2213-2600(13)70158-9
32. Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(7):CD009285. doi:10.1002/14651858.CD009285.pub3
33. Orlandi I, Moroni C, Camiciottoli G, et al. Chronic obstructive pulmonary disease: thin-section CT measurement of airway wall thickness and lung attenuation. Radiology. 2005;234(2):604–610. doi:10.1148/radiol.2342040013
34. Lim JU, Kim EK, Lim SY, et al. Mixed phenotype of emphysema and airway wall thickening is associated with frequent exacerbation in chronic obstructive pulmonary disease patients. Int J Chron Obstruct Pulmon Dis. 2019;14:3035–3042. doi:10.2147/COPD.S227377
35. Byrne AL, Marais BJ, Mitnick CD, Lecca L, Marks GB. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis. 2015;32:138–146. doi:10.1016/j.ijid.2014.12.016
36. Ravimohan S, Kornfeld H, Weissman D, Bisson GP. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev. 2018;27:147. doi:10.1183/16000617.0077-2017
37. Fan H, Wu F, Liu J, et al. Pulmonary tuberculosis as a risk factor for chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ann Transl Med. 2021;9(5):390. doi:10.21037/atm-20-4576
38. Horner A, Soriano JB, Puhan MA, et al. Altitude and COPD prevalence: analysis of the PREPOCOL-PLATINO-BOLD-EPI-SCAN study. Respir Res. 2017;18(1):162. doi:10.1186/s12931-017-0643-5
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
