Back to Journals » International Journal of General Medicine » Volume 15
Clinical and Prognostic Significance of Baseline Serum Vitamin D Levels in Hospitalized Egyptian Covid-19 Patients
Authors Mostafa S, Mohammed SA, Elshennawy SI, Zakaria DM, Mahmoud SAK , Alsadek AM, Ahmad IH , Mohammed DS, Mohammed MA, Eltrawy HH
Received 19 August 2022
Accepted for publication 14 October 2022
Published 7 November 2022 Volume 2022:15 Pages 8063—8070
DOI https://doi.org/10.2147/IJGM.S386815
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Sadek Mostafa,1 Shaymaa A Mohammed,2 Salwa I Elshennawy,2 Doaa Mohammed Zakaria,1 Sammar Ahmed Kasim Mahmoud,1 Amira Mohammed Alsadek,3 Inass Hassan Ahmad,4 Doaa Sayed Mohammed,4 Marwa Abdelmonim Mohammed,1 Heba H Eltrawy3
1Internal Medicine Department, Al-Azhar University, Cairo, Egypt; 2Clinical Pathology Department, Al-Azhar University, Cairo, Egypt; 3Chest Diseases Department, Al-Azhar University, Cairo, Egypt; 4Endocrinology and Metabolism Department, Al-Azhar University, Cairo, Egypt
Correspondence: Heba H Eltrawy, Chest Diseases Department, Al-Azhar University, Cairo, Egypt, Tel +201006381297, Email [email protected]
Background and Aim: Vitamin D is a hormone with essential roles in both cellular metabolism and immunity. It controls calcium homeostasis and modulates innate and adaptive immune system responses. Many studies suggested an association between vitamin D deficiency and clinical outcomes of covid-19 infection, while others failed to document such a relation. The present study aimed to evaluate the clinical and prognostic significance of baseline vitamin D levels in hospitalized Egyptian covid-19 patients.
Patients and Methods: The present retrospective study included 300 hospitalized covid-19 patients. Patients were submitted to standard clinical, laboratory, and radiological assessment. According to vitamin D levels, patients were classified to have normal levels (≥ 30), insufficient levels (20– 29) or deficient levels (< 20).
Results: According to their vitamin D levels, patients were classified into those with normal vitamin D (n=135), others with vitamin D insufficiency (n=114), and a third group with vitamin D deficiency (n=51). Patients with normal vitamin D levels and vitamin D insufficiency are significantly younger [median (IQR): 49.0 (39.0– 57.0) versus 51.0 (40.0– 61.0) and 55.0 (43.0– 62.0) years, respectively, p=0.012] and had less frequency of severe disease (24.4% versus 40.4% and 51.0%, respectively) when compared with those with vitamin D deficiency. Moreover, they had significantly lower levels of D dimer [median (IQR): 1.5 (0.9– 2.5) versus 1.8 (0.9– 3.1) and 2.0 (1.0– 3.2)], CRP [median (IQR): 58.0 (30.0– 120.0) versus 76.0 (42.5– 160.0) and 105.0 (74.0– 208.0), respectively, p< 0.001], ferritin [median (IQR): 458.0 (240.0– 759.0) versus 606.0 (433.8– 897.8) and 820.0 (552.0– 1087.0), respectively, p< 0.001], and procalcitonin [median (IQR): 290.0 (152.0– 394.0) versus 372.5 (227.0– 530.5) and 443.0 (272.0– 575.0), respectively, p< 0.001]. Only lower vitamin D levels were significant predictors of mortality in multivariate analysis [OR (95% CI): 0.88 (0.84– 0.92), p< 0.001].
Conclusion: Low vitamin D levels are related to exaggerated inflammatory response, disease severity, and poor clinical outcome in hospitalized covid-19 patients.
Keywords: covid-19, vitamin D, vitamin D deficiency
Introduction
More than 30 months after the initial reports of covid-19 infections, the unprecedented threats created by the pandemic are still in the focus of global interest despite the significant achievements in the fields of diagnosis, prevention, and treatment. This is chiefly attributed to the fast-evolving nature of the virus.1
Until recently, five major variants (Alpha, Beta, Gamma, Delta, and Omicron) have been identified,2 and more are expected in the upcoming years. There is always tremendous need to identify clinical and biochemical factors related to covid-19 severity and prognosis.3 In the absence of definitive therapeutic options, supplementary and complementary treatments are also welcome.4
Vitamin D is a hormone with essential roles in both cellular metabolism and immunity. It controls calcium homeostasis and modulates innate and adaptive immune system responses.5 Vitamin D status remains a significant health issue worldwide. However, there has been no clear consensus on vitamin D deficiency and its measurement in serum, and the clinical practice of vitamin D deficiency treatment remains inconsistent.6
Many studies suggested an association between vitamin D deficiency and clinical outcomes of covid-19 infection, while others failed to document such a relation.7 Other studies investigated the role of vitamin D supplementation on infection severity and outcome.8,9
The present study aimed to evaluate the clinical and prognostic significance of baseline vitamin D levels in hospitalized Egyptian covid-19 patients.
Patients and Methods
The present retrospective study was conducted at Al-Azhar University Hospitals. Patients included in the study were admitted during the period from November 2020 to December 2021. Access to patients’ data was approved by the ethical committee of Al-Azhar Faculty of Medicine in accordance with the Helsinki Declaration on clinical research involving human subjects. The study included 300 hospitalized covid-19 patients with positive reverse-transcriptase polymerase chain reaction (RT-PCR) of a nasopharyngeal swab and a median age of 51 years. Patients were excluded (n=37) if they had associated malignancy, chronic infection, or immunocompromised state. Also, patients with specific comorbidities or receiving medications that can affect vitamin D levels were excluded from the study. None of the included patients were vaccinated.
Included patients had severe covid-19 infection in the presence of at least one major or three minor criteria. Major criteria included: (1) septic shock with need for vasopressors, and (2) invasive mechanical ventilation. Minor criteria included: (1) respiratory rate ≥30 breaths/min, (2) PaO2/FiO2 ratio ≤250, (3) multilobar infiltrates, (4) confusion/disorientation, (5) uremia (BUN level ≥20 mg/dL), (6) leukopenia as a result of infection alone (WBC count <4000 cells/mL), (7) thrombocytopenia (platelets count <100,000/mL), (8) hypothermia (core temperature <36°C), (9) hypotension requiring aggressive fluid resuscitation).10
For laboratory assessment,
- 3 mL of venous blood was drawn on EDTA tube for CBC analysis on routine automated K X21Nhaematology cell counters (Sysmex, Kobe, Japan).
- 1 mL of venous blood was drawn on heparinized tube for D dimer analysis on Cobas h232 (Roche Diagnostics, Germany).
- 5 mL of venous blood was drawn on admission and allowed (within 10−20 minutes) to coagulate at room temperature then centrifuged for 20 minutes at 2000−3000 rpm to extract the serum that had been aliquoted, and stored at −80 °C. Serum was divided into three parts using suitable tubes: one used for assessment of urea, creatinine, ALT, AST, albumin, bilirubin, lipid profile tests, and CRP using the automated clinical analyzer Cobas Integra 400 plus (Roche Diagnostics, Germany). Second and third parts were stored at −80 °C to be used for procalcitonin analysis using Human Procalcitonin ELISA Kit (Cat. no. E0977Hu) and for vitamin D and ferritin assessment via a chemiluminescence-based immunoassay technique on Cobas 411 (Roche Diagnostics, Germany). According to vitamin D levels, patients were classified to have normal levels (≥30), insufficient levels (20–29), or deficient levels (<20).11
Data obtained from the present study were presented as number and percent for categorical data or median and interquartile range (IQR) for numerical data. Categorical data were compared using Fisher’s exact or chi-square tests as appropriate, while numerical data were compared using Mann–Whitney U-test. Correlation analysis was achieved using Spearman correlation coefficient. Receiver operating characteristic (ROC) curve analysis was used to identify sensitivity and specificity of investigated markers. Logistic regression analysis was utilized to identify predictors of disease severity or mortality in the studied patients. All statistical operations were processed using SPSS 25 (IBM, USA) with p value <0.05 considered statistically significant.
Results
The present study included 300 patients with covid-19 infection. They included 172 men and 128 women. According to their vitamin D levels, patients were classified into those with normal vitamin D (n=135), others with vitamin D insufficiency (n=114), and a third group with vitamin D deficiency (n=51). Comparison between the three groups regarding clinical and laboratory data revealed that patients with normal vitamin D levels and vitamin D insufficiency are significantly younger [median (IQR): 49.0 (39.0–57.0) versus 51.0 (40.0–61.0) and 55.0 (43.0–62.0) years, respectively, p=0.012] and had less frequency of severe disease (24.4% versus 40.4% and 51.0%, respectively) when compared with those with vitamin D deficiency. Moreover, they had significantly lower levels of D dimer [median (IQR): 1.5 (0.9–2.5) versus 1.8 (0.9–3.1) and 2.0 (1.0–3.2)], CRP [median (IQR): 58.0 (30.0–120.0) versus 76.0 (42.5–160.0) and 105.0 (74.0–208.0), respectively, p<0.001], ferritin [median (IQR): 458.0 (240.0–759.0) versus 606.0 (433.8–897.8) and 820.0 (552.0–1087.0), respectively, p<0.001], and procalcitonin [median (IQR): 290.0 (152.0–394.0) versus 372.5 (227.0–530.5) and 443.0 (272.0–575.0), respectively, p<0.001] when compared with the other two groups. Also, patients with normal vitamin D levels had significantly lower rates of ICU admission (6.7% versus 22.0% and 70.6%, respectively, p<0.001), MV (3.0% versus 16.3% and 58.8%, respectively, p<0.001), and mortality (3.0% versus 11.4% and 41.2%, respectively, p<0.001) (Table 1).
 |
Table 1 Clinical, Laboratory, and Outcome Parameters in the Studied Patients (n=300) |
Correlation analysis showed significant inverse correlation between vitamin D levels and age (r=−0.23, p<0.001), D dimer (r=−0.20, p<0.001), CRP (r=−0.31, p<0.001), ferritin (r=−0.28, p<0.001), procalcitonin (r=−0.29, p<0.001), MV days (r=−0.17, p=0.012), and ICU stay (r=−0.2, p=0.007) (Table 2). Multivariate logistic regression analysis identified age [OR (95% CI): 1.9 (1.06–1.12), p<0.001], male sex [OR (95% CI): 2.71 (1.35–5.41), p=0.005], D dimer levels [OR (95% CI): 1.43 (1.16–1.77), p=0.001], CRP [OR (95% CI): 1.006 (1.003–1.010), p=0.001], ferritin [OR (95% CI): 1.002 (1.001–1.003), p=0.004], procalcitonin [OR (95% CI): 1.002 (1.001–1.003), p=0.004], and vitamin D levels [OR (95% CI): 0.96 (0.92–0.99), p=0.043] as significant predictors of severe disease (Table 3). Only lower vitamin D levels were significant predictors of mortality in multivariate analysis [OR (95% CI): 0.88 (0.84–0.92), p<0.001] (Table 4).
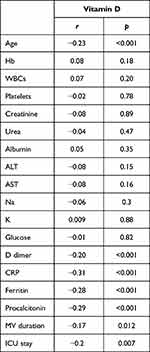 |
Table 2 Correlation Between Vitamin D Levels and Clinical and Laboratory Data |
 |
Table 3 Predictors of Disease Severity in the Studied Patients |
 |
Table 4 Predictors of Mortality in Patients with Severe Disease |
Discussion
The present study recognized a significant association between low vitamin D levels and covid-19 severity in this cohort of hospitalized covid-19 patients. In addition, we found a relation between vitamin D levels and mortality in patients with severe covid-19 infection. Moreover, we could identify an inverse correlation between vitamin D levels and patients' age, inflammatory marker levels, MV days, and ICU stay.
Our conclusions are in harmony with the results of multiple reports. In the study of Takase et al12 the authors found that low serum vitamin D levels are an independent risk factor for severe covid-19. Also, Hafez et al13 documented an association between vitamin D deficiency and poor clinical outcome parameters including ICU admission and mortality. In addition, Nguyen et al14 found that patients with vitamin D deficiency had increased risk-adjusted odds of in-hospital mortality while those with insufficient levels had significantly increased risk for mechanical ventilation during hospitalization. Moreover, Gholi et al15 noted that, in critically ill covid-19 patients, vitamin D levels are determinants of in-hospital mortality. Similar findings were reported by the other studies.16,17 The inverse correlation between vitamin D levels and longer ICU stay was reported by the study of Herrera-Quintana et al,18 while Notz et al19 recognized an association between lower vitamin D levels and longer duration of MV.
In the present study, significant inverse correlations were found between levels of vitamin D and D-dimer and other proinflammatory mediators including CRP, ferritin, and procalcitonin in accordance with previous works. In one meta-analysis of 22 observational studies comprising 7771 patients, the authors concluded that patients that were vitamin D sufficient had lower levels of IL-6, CRP, ferritin, LDH, fibrinogen, and D-dimer compared to vitamin D deficient counterparts.20 Likewise, an association was found between vitamin D levels and anti-SARS-CoV-2 IgG levels as shown by one study.21
Importantly, the study of Povaliaeva et al22 found that severely ill covid-19 patients do not have only low levels of vitamin D but they also have profound abnormalities in the metabolism of vitamin D regardless of the clinical course of the disease. Also, the experimental study of Arora et al23 provided evidence of the protective role of vitamin D against pulmonary viral infection.
On the other hand, Ozturk et al24 found no significant relation between vitamin D levels and covid-19 severity nor with the other inflammatory markers. Of note, the study of Huțanu et al25 concluded that low vitamin D levels are related to more severe forms of the disease but not with inflammatory markers or mortality. Also, the study of Bogliolo et al26 failed to document a relation between vitamin D levels and mortality.
Noteworthily, the large study of Lin et al27 that used UK Biobank data found no evidence of an association between historical vitamin D status and hospitalization or mortality due to covid-19. However, this study only used historical but not recent vitamin D levels. Interestingly, another large UK-based study found an association between covid-19 infection and mortality and percentage of households with access to total open space. They linked covid-19 incidence and mortality across London with environmental variables linked to vitamin D status.28
In conclusion, low vitamin D levels are related to exaggerated inflammatory response, disease severity, and poor clinical outcomes in hospitalized covid-19 patients.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Consent for Publication
All authors reviewed the manuscript and approved its submission.
Ethics Approval and Consent to Participate
This article was approved by the ethical committee of Al-Azhar Faculty of Medicine in accordance with the Helsinki Declaration on clinical research involving human subjects. A written informed consent was obtained from all patients.
Informed Consent
Informed consent was obtained from all patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Huang P, Zuo Q, Li Y, et al. A vicious cycle: in severe and critically Ill COVID-19 patients. Front Immunol. 2022;13:930673. PMID: 35784318; PMCID: PMC9240200. doi:10.3389/fimmu.2022.930673
2. Jacobs JL, Haidar G, Mellors JW. COVID-19: challenges of viral variants. Annu Rev Med. 2022. PMID: 35850493. doi:10.1146/annurev-med-042921-020956
3. Schneider M. The role of biomarkers in hospitalized COVID-19 patients with systemic manifestations. Biomark Insights. 2022;17:11772719221108909. PMID: 35783222; PMCID: PMC9243490. doi:10.1177/11772719221108909
4. Bhattacharya J, Booy R, Casadevall A, et al. A practical treatment for COVID-19 and the next pandemic. Pharmacol Res Perspect. 2022;10(4):e00988. doi:10.1002/prp2.988 PMID: 35837790; PMCID: PMC9284194.
5. Carlberg C. Nutrigenomics of Vitamin D. Nutrients. 2019;11(3):676. PMID: 30901909; PMCID: PMC6470874. doi:10.3390/nu11030676
6. DeLuca HF. Vitamin D: historical Overview. Vitam Horm. 2016;100:1–20. PMID: 26827946. doi:10.1016/bs.vh.2015.11.001
7. Chiang WF, Hsiao PJ, Chan JS. Vitamin D for Recovery of COVID-19 in patients with chronic kidney disease. Front Nutr. 2022;9:930176. PMID: 35782942; PMCID: PMC9240470. doi:10.3389/fnut.2022.930176
8. Subramanian S, Griffin G, Hewison M, et al. Vitamin D and COVID-19-Revisited. J Intern Med. 2022;292:604–626. PMID: 35798564. doi:10.1111/joim.13536
9. Quesada-Gomez JM, Lopez-Miranda J, Entrenas-Castillo M, et al. Vitamin D endocrine system and COVID-19: treatment with Calcifediol. Nutrients. 2022;14(13):2716. doi:10.3390/nu14132716 PMID: 35807895; PMCID: PMC9268645.
10. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi:10.1164/rccm.201908-1581ST
11. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi:10.1210/jc.2011-0385
12. Takase T, Tsugawa N, Sugiyama T, et al. Association between 25-hydroxyvitamin D levels and COVID-19 severity. Clin Nutr ESPEN. 2022;49:256–263. PMID: 35623823; PMCID: PMC8994250. doi:10.1016/j.clnesp.2022.04.003
13. Hafez W, Saleh H, Arya A, et al. Vitamin D status in relation to the clinical outcome of hospitalized COVID-19 patients. Front Med. 2022;9:843737. PMID: 35425774; PMCID: PMC9004341. doi:10.3389/fmed.2022.843737
14. Nguyen NN, Raju MNP, da Graca B, et al. 25-hydroxyvitamin D is a predictor of COVID-19 severity of hospitalized patients. PLoS One. 2022;17(5):e0268038. doi:10.1371/journal.pone.0268038 PMID: 35503795; PMCID: PMC9064100.
15. Gholi Z, Yadegarynia D, Eini-Zinab H, Vahdat Shariatpanahi Z. Vitamin D deficiency is associated with increased risk of delirium and mortality among critically Ill, elderly covid-19 patients. Complement Ther Med. 2022;70:102855. PMID: 35868492; PMCID: PMC9293788. doi:10.1016/j.ctim.2022.102855
16. Neves FF, Pott-Junior H, de Sousa Santos S, et al. Vitamin D deficiency predicts 30-day hospital mortality of adults with COVID-19. Clin Nutr ESPEN. 2022;50:322–325. PMID: 35871942; PMCID: PMC9195455. doi:10.1016/j.clnesp.2022.05.027
17. Fairfield KM, Murray KA, Anzalone AJ, et al. Association of vitamin D prescribing and clinical outcomes in adults hospitalized with COVID-19. Nutrients. 2022;14(15):3073. doi:10.3390/nu14153073 PMID: 35893927; PMCID: PMC9332080.
18. Herrera-Quintana L, Gamarra-Morales Y, Vázquez-Lorente H, et al. Bad prognosis in critical Ill patients with COVID-19 during short-term ICU stay regarding vitamin D Levels. Nutrients. 2021;13(6):1988. doi:10.3390/nu13061988 PMID: 34207873; PMCID: PMC8229686.
19. Notz Q, Herrmann J, Schlesinger T, et al. Vitamin D deficiency in critically ill COVID-19 ARDS patients. Clin Nutr. 2021;S0261–5614(21):135–137. doi:10.1016/j.clnu.2021.03.001 PMID: 33745749; PMCID: PMC7937427.
20. Hopefl R, Ben-Eltriki M, Deb S. Association between vitamin D levels and inflammatory markers in COVID-19 patients: a meta-analysis of observational studies. J Pharm Pharm Sci. 2022;25:124–136. doi:10.18433/jpps32518
21. Latifi-Pupovci H, Namani S, Pajaziti A, et al. Relationship of anti-SARS-CoV-2 IgG antibodies with Vitamin D and inflammatory markers in COVID-19 patients. Sci Rep. 2022;12(1):5699. doi:10.1038/s41598-022-09785-7 PMID: 35383273; PMCID: PMC8982653.
22. Povaliaeva A, Bogdanov V, Pigarova E, et al. Impaired vitamin D metabolism in hospitalized COVID-19 patients. Pharmaceuticals. 2022;15(8):906. doi:10.3390/ph15080906 PMID: 35893730; PMCID: PMC9330123.
23. Arora J, Patel DR, Nicol MJ, et al. Vitamin D and the ability to produce 1,25(OH)2D are critical for protection from viral infection of the lungs. Nutrients. 2022;14(15):3061. doi:10.3390/nu14153061 PMID: 35893921; PMCID: PMC9332570.
24. Ozturk G, Eraslan BZ, Akpinar P, Karamanlioglu Silte D, Ozkan Unlu F, Aktas I. Is there a relationship between vitamin D levels, inflammatory parameters, and clinical severity of COVID-19 infection? Bratisl Lek Listy. 2022;123(6):421–427. PMID: 35576543. doi:10.4149/BLL_2022_065
25. Huțanu A, Georgescu AM, Voidăzan S, Andrejkovits AV, Negrea V, Dobreanu M. Low serum vitamin D in COVID-19 patients is not related to inflammatory markers and patients’ outcomes-a single-center experience and a brief review of the literature. Nutrients. 2022;14(10):1998. PMID: 35631138; PMCID: PMC9146893. doi:10.3390/nu14101998
26. Bogliolo L, Cereda E, Klersy C, et al. NUTRI-COVID19 collaborative working group. Vitamin D 25OH deficiency and mortality in moderate to severe COVID-19: a multi-center prospective observational study. Front Nutr. 2022;9:934258. PMID: 35866079; PMCID: PMC9296047. doi:10.3389/fnut.2022.934258
27. Lin LY, Mulick A, Mathur R, Smeeth L, Warren-Gash C, Langan SM. The association between vitamin D status and COVID-19 in England: a cohort study using UK Biobank. PLoS One. 2022;17(6):e0269064. PMID: 35666716; PMCID: PMC9170112. doi:10.1371/journal.pone.0269064
28. Borna M, Woloshynowych M, Schiano-Phan R, Volpi EV, Usman M. A correlational analysis of COVID-19 incidence and mortality and urban determinants of vitamin D status across the London boroughs. Sci Rep. 2022;12(1):11741. PMID: 35817805; PMCID: PMC9272647. doi:10.1038/s41598-022-15664-y
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
