Back to Journals » Clinical Ophthalmology » Volume 12
Clinical and genetic investigation of amantadine-associated corneal edema
Authors Hessen MM , Vahedi S, Khoo CT , Vakili G , Eghrari AO
Received 23 February 2018
Accepted for publication 3 May 2018
Published 6 August 2018 Volume 2018:12 Pages 1367—1371
DOI https://doi.org/10.2147/OPTH.S166384
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Michelle M Hessen, Sina Vahedi, Chloe T Khoo, Gelareh Vakili, Allen O Eghrari
Division of Cornea, Cataract, & External Diseases, The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA
Purpose: Amantadine use has been temporally associated with bilateral corneal edema in a series of cases; however, its pathophysiological mechanisms have yet to be elucidated. We sought to rule out subclinical Fuchs dystrophy as a contributor, characterize its pattern of corneal edema, and describe the long-term outcome of concurrent topical steroids while resuming amantadine.
Patient and methods: After a 44-year-old woman presented with new acute onset bilateral corneal edema, amantadine was discontinued, with clinical improvement. However, neurological decompensation required restarting amantadine, which she did concurrently with topical loteprednol. To determine whether subclinical Fuchs dystrophy might be present, triplet-primed polymerase chain reaction was conducted to measure copy number of the CTG18.1 trinucleotide repeat in TCF4. Specular microscopy and Scheimpflug imaging were conducted and followed for 32 months to assess for resolution and stability. Literature review was conducted to assess for consistency of the clinical phenotype.
Results: Corneal edema resolved clinically 4 weeks after discontinuation of amantadine. Serial Scheimpflug imaging demonstrated resolution of posterior and central corneal edema and specular microscopy revealed intracellular opacities with loss of endothelial cell density. Despite resuming amantadine, Scheimpflug imaging and specular microscopy measurements remained stable at 32 months. Triplet-primed PCR of CTG18.1 in TCF4 revealed no trinucleotide repeat expansion.
Conclusions: Amantadine-associated corneal edema is characteristically posterior and central and appears unlikely to represent early or subclinical decompensation of Fuchs dystrophy. We describe the unique outcome of continued corneal clearance after restarting amantadine concurrently with steroids, a pattern that has persisted over 32 months to date.
Keywords: amantadine, cornea, corneal edema, corneal endothelium, Fuchs dystrophy, Scheimpflug imaging
Introduction
Amantadine is an antiviral and antiparkinsonian drug associated with rare episodes of bilateral corneal edema, which often resolves upon cessation of the medication.1 Endothelial cell loss may require corneal transplantation even after discontinuation of amantadine.2 Although cases have been temporally associated with amantadine and its cessation, national data suggest a higher frequency of new diagnoses of Fuchs dystrophy among individuals starting amantadine therapy.3 To date, the causes of corneal edema are unclear; therefore, we sought to investigate the clinical and genetic features associated with amantadine-associated edema in a 44-year-old woman who presented with bilateral corneal edema.
Patient and methods
A 44-year-old female presented with decreased vision in both eyes over the course of 2 weeks, at which time she only utilized topical loteprednol 0.5% twice daily in both eyes for blepharitis. She also took amantadine 400 mg daily by mouth for over 3 years in the setting of ataxic cerebral palsy and associated neurological complications. Best corrected visual acuity at baseline was measured four months prior to presentation at 20/25 in both eyes (oculus uterque [OU]).
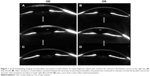
At the time of presentation, visual acuity was 20/125 in the right eye (oculus dextrus [OD]) and 20/60 in the left eye (oculus sinister [OS]). Pentacam Scheimpflug imaging (OCULUS Optikgeräte GmbH, Weztlar, Germany) was conducted which revealed pachymetry of 927 microns OD and 641 microns OS. Central corneal edema with Descemet folds, as visible in Figure 1, was exhibited without anterior chamber cell, in the right eye greater than the left. The patient had no known history of Fuchs dystrophy, viral prodrome or infection. Amantadine toxicity was considered and a discussion was held with her neurologist regarding discontinuation of the medication. However, given her need for amantadine, she was reluctant to discontinue the medication until an alternative treatment was identified. She returned 3 weeks later with worsening of vision to 20/125 OU, and pachymetry of 946 microns OD and 598 microns OS.
Given the disease progression, she discontinued amantadine and returned to the clinic 1 week later (Week 4 since presentation), with continued progression of edema. Visual acuity was 20/250 OD and 20/150 OS. She was monitored with serial visits, improving at Week 6 to 20/70 OD and 20/50 OS.
At 2 months (Week 8), her corneal edema had resolved and visual acuity returned to 20/30 OU, with pachymetry of 585 microns OD and 550 microns OS. Specular microscopy of both eyes with a machine unable to conduct flex-center analysis revealed severe endothelial cell loss, greater in the right eye, which subjectively appeared stable over time through subsequent analyses able to provide quantitative estimates (Figure 2).
At 6 months (Week 24), she returned for a follow-up appointment with worsening of her neurological state. Therefore, she resumed amantadine at a lower dose of 300 mg by mouth daily, divided into 200 mg in the morning and 100 mg in the evening. She maintained use of topical loteprednol 0.5% twice daily in both eyes. Her visual acuity remained stable subjectively and improved objectively to 20/25 OU. Pachymetry was stable in the right eye at 586 microns and continued to improve in the left eye at 523 microns.
At month 12 (Week 48), the patient returned for repeat specular microscopy, which revealed endothelial cell counts of 609 cells/mm2 OD and 1,387 cells/mm2 OS. In light of a guttate appearance to her cornea, she enrolled in a longitudinal study of Fuchs dystrophy for which she provided a blood sample and we sequenced the CTG18.1 trinucleotide repeat in TCF4 using methods we have previously described.1 She was found not to bear the implicated repeat expansion. She continued oral amantadine, and due to changes in insurance coverage, began topical fluorometholone 0.1% twice daily in place of topical loteprednol 0.5%.
At 18 months, the patient underwent repeat specular microscopy, which continued to be stable at 627 and 1,309 cells/mm2 in the right and left eyes, respectively, and 649 and 1,186 cells/mm2 at 2.7 years, despite continuing amantadine 300 mg daily. Images were analyzed with the flex center method. Visual acuity remained stable at 20/30 bilaterally, with no corneal edema present. She remains on topical fluorometholone twice daily in both eyes.
Table 1 delineates the progression and resolution of her corneal edema based on visual acuity, pachymetry and specular microscopy readings. Table 2 summarizes the patterns of corneal edema described in all known cases in the literature, revealing central corneal edema as a characteristic pattern present in a slight majority of cases at time of diagnosis. Written informed consent was provided by the patient to publish case details and accompanying images. The Institutional Research Board at Johns Hopkins University School of Medicine waived the need for approval for reporting a single case.
Discussion
Amantadine was first approved in 1966 for influenza prophylaxis and 3 years later, was discovered to address symptoms of Parkinson’s disease. It has weak N-methyl-D-aspartate (NMDA)-type glutamate receptor antagonist and anticholinergic properties, and both increase dopamine release and block dopamine reuptake. Its antiviral properties are related to an unrelated, separate effect on the M2 proton channel, a homotetramer located in viral envelopes of influenza viruses which modulates local pH levels to promote intracellular viral functions.
While its most common adverse effects are associated with central nervous system function, including anxiety, agitation, and modulation of pre-existing epileptic or psychiatric symptoms, corneal edema is uncommon, at 0.27% in a 2-year study among veterans;3 in a post-surveillance study of over 13,137 patients receiving amantadine over 2 years, 36 were newly diagnosed with either corneal edema or Fuchs dystrophy. It is notable that 73% of known cases (16 of 22) have occurred in females (Table 2), a predominance that is also seen in Fuchs dystrophy. It is unclear whether similar pathways may contribute to such disparity, as endothelial cell death appears to occur in both diseases.20 We demonstrate here that in this case, amantadine-associated corneal edema is independent of a common genetic change prevalent in approximately two-thirds of individuals diagnosed with Fuchs dystrophy. Moreover, the resolution of edema does not support a diagnosis of Fuchs dystrophy, which progressively worsens over time. Nevertheless, more cases and similar analyses will be needed to better elucidate an association or lack thereof.
The pathophysiological mechanisms of transient edema in such cases are poorly understood; however, based on specular microscopy and histopathologic findings, endothelial cell death appears to be induced or accelerated.8 In this study, we compared the temporal progression and resolution of corneal edema in two affected eyes with all reported instances of amantadine-associated corneal edema in the literature (Table 2) and identify characteristic patterns of edema. First, corneal edema often appears centrally and, in some cases, progresses to diffuse edema. Second, cases may be asymmetrically bilateral, a process captured with bilateral imaging.
Given the need in some cases for corneal transplantation,10 early identification and cessation of amantadine will be helpful to maximize the likelihood of achieving corneal clarity. However, in instances where amantadine cannot be discontinued, as no alternative therapy is available, we describe the possibility that resuming amantadine with steroids may have assisted to maintain corneal endothelial cell density. Our findings support prior reports that demonstrated partial responses to topical and intravenous corticosteroids.10 The relationship with long-term amantadine dosing is unclear; it may be that amantadine toxicity is not related to the cumulative dose, but rather potentially an alternative mechanism, such as rapid increase to a toxic level at a given point in time. In a nationwide cohort study in Taiwan of patients with Parkinson disease, a hazard ratio of 1.79 for corneal edema with amantadine use increased to 2.05 for those with a moderate dose (2,000–4,000 mg) and 2.84 for those with a high dose (>4,000 mg).21
Conclusion
Long-term outcomes of amantadine toxicity are unknown. We include here follow-up data for 32 months, the longest such interval to our knowledge. In contrast to patients whose edema returned following repeat challenge with amantadine,3 this case revealed that it may be possible to tolerate a dose if necessary without development of further corneal edema. However, a single case does not equate to a recommendation, and outcomes from additional cases will be beneficial to understanding the likelihood of such benefit.
Acknowledgments
Financial support was received from: NIH K12 EY015025-10 (AOE), NIH L30 EY024746 (AOE).
Disclosure
The authors report no conflicts of interest in this work.
References
Beran M, Okyere B, Vova J. Amantadine-induced corneal edema in a pediatric neuro-oncology patient: a case report. PM R. 2018;S1934–S1482(18):30126–30126. | ||
Hood CT, Langston RH, Schoenfield LR, et al. Amantadine-associated corneal edema treated with descemet’s stripping automated endothelial keratoplasty. Ophthalm Surg Lasers Imaging. 2010;41 Online:1–4. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21158374. Accessed July 10, 2018. | ||
French DD, Margo CE. Postmarketing surveillance of corneal edema, Fuchs dystrophy, and amantadine use in the Veterans Health Administration. Cornea. 2007;26:1087–1089. | ||
Deogaonkar M, Wilson K, Vitek J. Amantadine induced reversible corneal edema. J Clin Neurosci. 2011;18:298–299. | ||
Dubow JS, Rezak M, Berman AA. Reversible corneal edema associated with amantadine use: an unrecognized problem. Move Disord. 2008;23:2096–2097. | ||
Esquenazi S. Bilateral reversible corneal edema associated with amantadine use. J Ocul Pharmacol Therapeut. 2009;25:567–570. | ||
Ghaffariyeh A, Honarpisheh N. Amantadine-associated corneal edema. Parkinsonism Relat Disord. 2010;16:427. | ||
Hotehama A, Mimura T, Usui T, et al. Sudden onset of amantadine-induced reversible bilateral corneal edema in an elderly patient: case report and literature review. Japan J Ophthalmol. 2011;55:71–74. Available from: https://link.springer.com/article/10.1007%2Fs10384-010-0888-8. Accessed July 10, 2018. | ||
Hughes B, Feiz V, Flynn SB, Brodsky MC. Reversible amantadine-induced corneal edema in an adolescent. Cornea. 2004;23:823–824. Available from: https://journals.lww.com/corneajrnl/Fulltext/2004/11000/Reversible_Amantadine_Induced_Corneal_Edema_in_an.12.aspx. Accessed July 10, 2018. | ||
Jeng BH, Galor A, Lee MS, et al. Amantadine-associated corneal edema potentially irreversible even after cessation of the medication. Ophthalmology. 2008;115:1540–1544. | ||
Kim YE, Yun JY, Yang HJ, et al. Amantadine induced corneal edema in a patient with primary progressive freezing of gait. J Mov Disord. 2013;6:34–36. | ||
Koenig SB, McDermott ML, Simons KB. Nonimmunologic graft failure after Descemet’s Stripping Automated Endothelial Keratoplasty (DSAEK) for presumed amantadine-induced corneal edema. Eye Contact Lens. 2009;35:209–211. | ||
Kubo S, Iwatake A, Ebihara N, Murakami A, Hattori N. Visual impairment in Parkinson’s disease treated with amantadine: case report and review of the literature. Parkinsonism Rel Disord. 2008;14:166–169. | ||
Park CY, Chuck RS. Sudden bilateral corneal oedema in a patient with Parkinson’s disease. Acta Ophthalmolog. 2011;89:198–199. | ||
Pond A, Lee MS, Hardten DR, Harrison AR, Krachmer JH. Toxic corneal oedema associated with amantadine use. Br J Ophthalmol. 2009;93:413. | ||
Santiago-Caban LA, Rivera E, Lopez-Beauchamp V. Bilateral corneal edema secondary to amantadine in the pediatric population: a case report. Bol Asoc Med Puerto Rico. 2012;104:69–76. | ||
Yang Y, Teja S, Baig K. Bilateral corneal edema associated with amantadine. CMAJ. 2015;187:1155–1158. | ||
Avendano-Cantos EM, Celis-Sanchez J, Mesa-Varona D, Gálvez-Martínez J, López-Arroquia E, González del Valle F. Toxicidad corneal por amantadina [Corneal toxicity due to amantadine]. Arch Sociedad Espanola Oftalmol. 2012;87:290–293. Spanish. | ||
Chang KC, Kim MK, Wee WR, Lee JH. Corneal endothelial dysfunction associated with amantadine toxicity. Cornea. 2008;27:1182–1185. | ||
Jurkunas UV, Bitar MS, Funaki T, Azizi B. Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy. Am J Pathol. 2010;177:2278–2289. | ||
Lee PY, Tu HP, Lin CP, et al. Amantadine use as a risk factor for corneal edema: a nationwide cohort study in Taiwan. Am J Ophthalmol. 2016;171:122–129. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.