Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Clinical and Economic Impact of Long-Term Inhaled Corticosteroid Withdrawal in Patients with Chronic Obstructive Pulmonary Disease Treated with Triple Therapy in Spain
Authors Neches García V , Vallejo-Aparicio LA, Ismaila AS , Sicras-Mainar A , Sicras-Navarro A, González C, Cuervo R, Shukla S , García-Peñuela M
Received 24 March 2022
Accepted for publication 17 August 2022
Published 7 September 2022 Volume 2022:17 Pages 2161—2174
DOI https://doi.org/10.2147/COPD.S367708
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Richard Russell
Victoria Neches García,1 Laura Amanda Vallejo-Aparicio,1 Afisi S Ismaila,2,3 Antoni Sicras-Mainar,4 Aram Sicras-Navarro,4 Cruz González,5 Rafael Cuervo,6 Soham Shukla,2 Marcos García-Peñuela6
1Market Access, GlaxoSmithKline, Madrid, Spain; 2Value Evidence and Outcomes, GlaxoSmithKline, Collegeville, PA, USA; 3Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, ON, Canada; 4Real Life Data, Atrys Health, Barcelona, Spain; 5Pneumology Unit, Hospital Clínico Universitario de Valencia, Valencia, Spain; 6Medical Affairs, GlaxoSmithKline, Madrid, Spain
Correspondence: Victoria Neches García, Market Access, GlaxoSmithKline, P.T.M Severo Ochoa, 2 28760 Tres Cantos, Madrid, Spain, Tel +34 677 50 37 57, Email [email protected]
Purpose: To determine the clinical and economic impact of inhaled corticosteroid (ICS) withdrawal in Spanish patients with COPD receiving triple therapy (TT) with ICS, long-acting β2-agonist (LABA), and long-acting muscarinic antagonist (LAMA).
Patients and Methods: This was an observational, retrospective study of BIG-PAC database medical records. Patients aged ≥ 40 years receiving TT from 2016 to 2018 were followed for 1 year. Two cohorts were identified: patients continuing TT (ICS+LABA+LAMA), and patients receiving TT with ICS withdrawn (LABA+LAMA). Variables included medication, exacerbations (moderate and severe), pneumonia, mortality, health resource use (HRU), and cost per patient/year. Cohorts were compared using propensity score matching (PSM). Multivariate statistical analysis using analysis of covariance and Cox proportional risks was conducted.
Results: Of 6541 patients included, 5740 (87.8%) continued TT and 801 (12.2%) had ICS withdrawn. Patients with ICS withdrawal were younger, had lower disease burden, higher ICS doses, and more exacerbations compared with those continuing ICS. PSM matched 795 patients in each cohort. Mean age was 68.5 years (SD: 11.2), 69.9% were male, and mean Charlson index was 2.0. Patients with ICS withdrawal had more total exacerbations in the 12 months following withdrawal compared with patients continuing TT (36.6% vs 31.4%; p=0.030). No significant differences were found for pneumonia (3.3% vs 3.6%; p=0.583) and mortality (9.9% vs 7.5%; p=0.092). Median time to first exacerbation was shorter in patients with ICS withdrawal compared with those continuing ICS (HR: 0.69, 95% CI: 0.57– 0.83; p< 0.001). Mean health cost per patient/year among patients with ICS withdrawal was higher than those continuing TT (€ 2993 vs € 2130; p< 0.001).
Conclusion: ICS withdrawal in patients with COPD receiving TT was associated with increased exacerbations, HRU, and costs compared with continuing TT, with health and economic impacts on patients and the Spanish National Healthcare System, respectively. Pneumonia and mortality rates were similar between groups.
Keywords: inhaled corticosteroid withdrawal, COPD, exacerbations, resource use, health costs
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable, treatable disease characterized by chronic and generally progressive airflow limitation, with little scope for reversion. The disease is associated with an abnormal inflammatory response of the lung to harmful particles or gases, especially tobacco, with significant systemic repercussions.1,2 The prevalence of COPD is high worldwide and there is a trend towards a continued increase.2,3 In Spain, the estimated prevalence of COPD is 11.8% (EPISCAN II) in people aged 40 or older.4 COPD is a public health problem; it is the third leading cause of death worldwide and the fourth leading cause of death in Spain, as well as one of the main causes of morbidity, occupational disability, and lost quality of life.4,5
The bases of the pharmacological treatment for COPD are long-acting bronchodilators (long-acting β2-agonists [LABA] and long-acting muscarinic antagonists [LAMA]) as first-line treatments, either alone or in combination. The combination of LABA and inhaled corticosteroids (ICS) is reserved for patients with moderate-to-severe COPD who experience frequent exacerbations and/or for patients with a mixed COPD-asthma phenotype, regardless of severity.6–9 The efficacy and convenience of triple therapy in patients with moderate-to-severe COPD has been shown by many studies.10,11 However, in clinical practice, ICS use in patients with COPD remains unclear due to the balance between efficacy and safety, and arguments both for and against their use.10–13
Few studies have addressed the subject of ICS use in usual clinical practice. A real-life study of patients with moderate COPD (OPTIMO)14 recruited from 43 Italian centers (mainly respiratory units and university hospitals) found that compared to continued treatment with ICS/bronchodilators, the risk of exacerbation did not increase significantly in the 6 months following ICS withdrawal. During this study, the decision to withdraw ICS was at the investigator’s discretion and the objective was to assess the impact of ICS withdrawal. In patients with mild-to-moderate COPD and no exacerbations, ICS may be safely withdrawn if regular treatment with long-acting bronchodilators is maintained.8 Additionally, the 2-year longitudinal, prospective, non-interventional DACCORD study15 of 6000 German patients with COPD, from 349 primary and secondary care centers, aimed to generate data on the course of COPD in routine clinical practice. A subgroup of patients in the DACCORD study16 were taking ICS prior to study entry and some continued to receive ICS during the 2-year follow-up, while in others the attending physician decided to withdraw ICS at study entry. This allowed assessment of the long-term effects of ICS withdrawal in a real-life environment with a longer follow-up than the OPTIMO study and found that ICS withdrawal was not associated with an increased risk of exacerbations or a deterioration in health status. However, it should be noted that a high proportion of patients who were categorized as Global Initiative for Chronic Obstructive Lung Disease (GOLD) group A or B were receiving ICS as the basis of their treatment. Although this should not be construed as an inappropriate prescription (the clinical characteristics of patients were unknown when ICS were initiated), it does suggest that a proportion of these patients were receiving drugs which were not recommended by clinical management guidelines. Initial exacerbations did not appear to correlate with ICS use; this may be due to patients being treated unnecessarily with ICS or due to ICS preventing exacerbations. However, these findings should be interpreted with caution as they are based on a retrospective study with a duration of 6 months.
Currently, there is still some controversy regarding ICS use in patients with COPD receiving triple therapy, especially when their symptoms appear to be controlled.17 There are arguments for and against ICS maintenance/withdrawal in patients with exacerbations (time period, withdrawal vs reintroduction, etc).18,19 GOLD guidelines recommend ICS is withdrawn if not prescribed correctly and when there is a lack of efficacy or repeated pneumonia.7 However, there are no official recommendations on ICS withdrawal in usual clinical practice. In addition, the results of ICS withdrawal studies17,18 have shown there may be a negative impact on lung function, an increase in exacerbations, and worsened quality of life when ICS is withdrawn. In Spain, evidence on the effects of ICS withdrawal in usual clinical practice is limited. The objective of this study was to determine the long-term clinical (exacerbations and mortality) and economic (health costs) impact of ICS withdrawal in patients receiving triple therapy for the treatment of COPD in routine clinical practice in Spain.
Material and Methods
Design and Study Population
This observational, retrospective study utilized electronic medical records obtained from the BIG-PAC20 administrative database (data source: secondary; proprietor: Atrys Health-RLD; population: 1.8 million patients [https://www.encepp.eu/encepp/viewResource.htm?id=29236]). Primary data were obtained from computerized medical records of seven publicly funded integrated health areas (primary care centers and hospitals) in seven Spanish autonomous communities. Prior to export to BIG-PAC,20 electronic medical records underwent rigorous anonymization in the centers/hospitals of origin, in compliance with Organic Law 3/2018, of December 5, on the Protection of Personal Data and guarantee of digital rights (https://www.boe.es/eli/es/lo/2018/12/05/3). Atrys Health-RLD has no access to primary data sources.
Patients receiving continuing triple therapy were defined as those with concurrent prescriptions of ICS, LABA, and LAMA in one, two, or three inhalers. Patients were identified between 1 January 2016 and 31 December 2018, and were classified into two study cohorts: a) continuing triple therapy: patients with continuing prescription of ICS, LABA, and LAMA, in one, two, or three inhalers during the follow-up; and b) ICS withdrawal: patients in whom ICS were discontinued or withdrawn and who were treated with LABA/LAMA (alone or in combination in one or two inhalers) during follow-up. Patients from the two cohorts were matched using propensity score matching (PSM) with a greedy nearest neighbor algorithm and a caliper of 0.20. Patients in whom ICS were withdrawn were cases, and patients continuing triple therapy were controls. The index date of patients in whom ICS were withdrawn was the date of the last available ICS prescription. The index date of patients on continuing triple therapy was the same as that of the matched case. Two study periods were established: a) 12 months prior to the index date (pre-index period), where the inclusion/exclusion criteria were confirmed and the variables required to compare the two study cohorts were obtained (previous exacerbations, demographic variables, comorbidities, treatments); and b) 12 months following the index date (follow-up period), in which health resource use, exacerbations, mortality, pneumonia, and treatment persistence were recorded. Follow-up was 12 months for both cases and controls.
Inclusion/Exclusion Criteria
Inclusion criteria were: a) age ≥40 years on the index date; b) confirmed diagnosis of COPD (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM], codes 491, 496; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes J41, J42, J44); c) patients active in the database for ≥12 months before study entry; d) inclusion in the medical prescription system (with recorded daily dose, time interval, and duration of each treatment administered; ≥2 prescriptions during the follow-up period); and e) ensured monitoring of patients (≥2 records in the electronic system). Exclusion criteria were: a) patients in whom ICS were withdrawn who restarted triple therapy; b) patients displaced or out-of-area; c) permanently institutionalized patients; and/or d) patients with terminal illness and/or on dialysis.
Demographic Variables and Comorbidity
Demographic and comorbidity variables were age (continuous and by range: 40 to 64 years, 65 to 74 years, and ≥75 years), sex, a history (ICD-9-CM) of high blood pressure, diabetes, dyslipidemia, obesity, smoking, ischemic heart disease, stroke, heart failure, kidney failure, malignancies, asthma, emphysema, and bronchiectasis. The Charlson comorbidity index was used as a summary variable of general comorbidity.21 These data were obtained on the index date of each patient. In addition, other variables were obtained: time from diagnosis (years), body mass index (BMI, kg/m2), forced expiratory volume in 1 second (FEV1; %), blood eosinophil levels (cells/µL), and functional severity of COPD (classification) in accordance with GOLD7 recommendations.
Definition of Chronic Obstructive Pulmonary Disease, Exacerbation, and Pneumonia
Records of patients with COPD were obtained using the ICD-9-CM codes 491, 492, and/or 496 for COPD and/or exacerbations, or their ICD-10-CM equivalents (codes: J41, J42, and J44). Diagnosis of COPD was at the discretion of the attending physician. The diagnosis was confirmed (spirometry/functional respiratory tests) by recording non-reversible airflow obstruction without significant reversibility following administration of bronchodilators, according to FEV1 values and a FEV1/forced vital capacity ratio of <70% in the stable phase. GOLD guidelines7 define exacerbations as events in the natural course of the disease, characterized by a change in dyspnea, cough, and/or baseline sputum which goes beyond daily variations, is of acute onset, and may require a change in the usual medication (sustained aggravation of the initial situation). Two types of exacerbations were considered: a) severe: requiring hospitalization; and b) moderate: requiring treatment with antibiotics and/or oral corticosteroids (≥1 prescription of antibiotics or oral corticosteroids, regardless of the time of administration). Patients with pneumonia (ICD-9-CM codes 482 to 486, or ICD-10-CM codes J12 to J18) were also recorded. Exacerbations were recorded during the pre-index period (12 months) and during follow-up (12 months). Rates of pneumonia and cardio-respiratory death were recorded during follow-up, as was the time from the index date to first exacerbation.
Treatment Description
Medicines (active substances) for the treatment of COPD were recorded according to the Anatomical Therapeutic Chemical Classification System.22 The information was obtained from the pharmacological dispensing from community pharmacies. The choice of drug for a patient was at the discretion of the attending physician. Patients who received chronic systemic corticosteroid treatment (≥6 months) were differentiated from those who solely received them for control of exacerbations. These drugs were recorded during the pre-index and follow-up periods. Treatment persistence (LABA/LAMA/ICS vs LABA/LAMA) was determined from the index date to discontinuation. Discontinuation was defined as: a) abandonment (>60 days without renewing medication); b) end of follow-up/death; and/or c) changes in initial medication from the index date. Dose modifications and changes in the type of inhaler device were not considered to constitute discontinuation. Patients were classified using the daily dose of ICS (high, medium, low) according to the Bassan criteria.23
Health Resource Use and Costs
Health costs (Table S1) related to care activity (primary care medical visits, specialist visits, days of hospital stay, hospital emergency room visits, diagnostic and therapeutic requests, and medication) due to respiratory causes were recorded. Costs were expressed as the mean cost per patient/year (mean/unit). Rates were obtained from hospital accounting, except for medication. Medical prescriptions were quantified according to the retail price per pack at the time of dispensing.24 Health resource use and costs were recorded throughout the 12-month follow-up period.
Ethical and Legal Aspects
The confidentiality of records (anonymous and dissociated) under the Law on the Protection of Personal Data was respected. The study was classified by the Spanish Medicines and Healthcare Products Agency as EPA-OD and subsequently approved by the Research Ethics Committee of the Hospital of Terrassa, Barcelona, Spain (code: 02–20-399-090).
Statistical Analysis
The data were extracted using structured query language statements. Records were carefully reviewed through exploratory analysis and preparation by observing the frequency distributions and searching for possible recording or coding errors. PSM was used to match cohorts.25 The covariates included in the model were age, sex, Charlson index, time from diagnosis, high blood pressure, dyslipidemia, smoking, heart failure, COPD severity, eosinophil levels, ICS dose, and total moderate and severe exacerbations in the past year. Standardized coefficients (differences) of the quantitative and qualitative variables were recorded.
In the descriptive univariate analysis, qualitative data were described as absolute and relative frequencies, and quantitative data as the mean, SD, median, and 25th and 75th percentiles (interquartile range). The 95% confidence intervals were calculated to estimate parameters. Analysis of variance and the chi-squared test were used in the bivariate analysis. Multivariate models were used for specific endpoints: analysis of covariance (general linear model; procedure: estimate of marginal means; Bonferroni adjustment) was utilized to adjust health costs and a Cox proportional risk model was developed to examine the association between ICS withdrawal and exacerbations. In both cases, the covariates included were age, sex, time from diagnosis, and the Charlson index. The analysis used the SPSSWIN version 25 program. Statistical significance was established as p<0.05.
Results
Of an initial selection of 851,258 patients aged ≥40 years assigned to the centers, 17,990 had a confirmed diagnosis of COPD, and 6541 (36.4%) patients on continuing triple therapy met the inclusion criteria for our analyses (Figure S1). Of these, 5740 (87.8%) patients continued on triple therapy and 801 (12.2%) had ICS withdrawn. The overall mean age was 70.5 years (SD: 10.8), 70.9% were male, 64.7% had high blood pressure, 53.1% had dyslipidemia, and 24.3% had diabetes.
Demographic characteristics, comorbidities, COPD classification, and other parameters at index according to study cohorts are shown in Table 1. Patients receiving triple therapy in whom ICS were withdrawn had a lower mean age (68.2 vs 70.8 years), general comorbidity (Charlson index: 2.0 vs 2.2 points; especially heart failure and/or smokers), severity of COPD (moderate GOLD II: 64.4% vs 53.9%), time from diagnosis (16.4 vs 18.9 years), and blood eosinophil levels (≥300 cells/μL: 13.5% vs 18.4%).
 |
Table 1 Demographic Characteristics, Comorbidities, COPD Classification, and Other Variables in the 12-Month Pre-Index Period |
Patients in whom ICS were withdrawn also received fewer chronic oral corticosteroids (8.2% vs 10.6%; p=0.042), had fewer high doses of ICS (27.1% vs 33%; p=0.003), and had a lower percentage of total exacerbations (31.2% vs 35.2%; p=0.028), moderate exacerbations (30.7% vs 34.4%; p<0.001), and severe exacerbations (hospital admission: 8.2% vs 15.5%; p<0.001) during the previous year. The use of the active substances LAMA/LABA/ICS prior to ICS withdrawal was similar between the two study cohorts (Table 2).
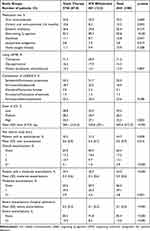 |
Table 2 Medications Administered and Exacerbation Rate During the 12-Month Pre-Index Period |
During PSM, 795 of the 801 patients (99.3%) in whom ICS were withdrawn were matched with patients on continuing triple therapy after analysis of 15 variables (clinically relevant and/or with significant differences between the two cohorts). Table 3 shows the demographic characteristics, comorbidities, COPD classification, and other variables at index (after PSM). Overall, the mean age was 68.4 years (SD: 11.2), 69.9% were male, 61.9% had high blood pressure, 45.6% had dyslipidemia, and 23.5% had diabetes. There was acceptable comparability between the two study cohorts as standardized differences were <0.10 for all variables analyzed, including the medicines administered and the exacerbation rates during the 12-month pre-study period (Table 4).
 |
Table 3 Demographic Characteristics, Comorbidities, COPD Classification, and Other Variables in the 12-Month Pre-Index Period (After Propensity Score Matching) |
 |
Table 4 Medications Administered and Exacerbation Rate During the 12-Month Pre-Index Period (After Propensity Score Matching) |
Table 5 shows medications administered and the main clinical outcome variables during the 12-month follow-up. Patients in whom ICS were withdrawn used slightly more oral corticosteroids (35.6% vs 30.9%; p=0.049), systemic antibiotics (31.9% vs 25.2%; p=0.003), and xanthines (11.4% vs 8.2%; p=0.028), and had a higher percentage of total exacerbations (36.6% vs 31.4%; p=0.030). Moderate exacerbations (34.1% vs 31.4%; p=0.251) and severe exacerbations (11.2% vs 8.4%; p=0.071) were also trending higher for patients with ICS withdrawal, although these did not reach significance. Pneumonia (3.3% vs 3.6%; p=0.583) and mortality (9.9% vs 7.5%; p=0.092) rates were similar between the two cohorts.
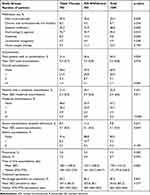 |
Table 5 Medication Administered, Exacerbations, and Other Variables During the 12-Month Follow-Up (After Propensity Score Matching) |
Patients in whom ICS were withdrawn had a shorter time from the index date to the first exacerbation (161 vs 186 days; p=0.002). The hazard ratio for continuing triple therapy was 0.69 (95% confidence interval: 0.57–0.83; p<0.001) (Figure S2). Treatment persistence (86.3% vs 84.2%; p=0.831) was similar between the two groups. All patients included in the study who had their ICS withdrawn maintained dual bronchodilation (LABA/LAMA).
Table 6 shows health resource use and costs. The total health cost of patients (N=1590) was €4.1 million. Patients in whom ICS were withdrawn used more health resources, specifically in primary care visits (10.4 vs 8.6; p<0.001), hospital care visits (1.1 vs 0.8; p=0.004), and days of hospital stay (1.9 vs 0.9; p<0.001), including hospital emergency visits (116.6 vs 74.8; p<0.001). The mean total health cost per patient/year was higher in patients in whom ICS were withdrawn after correction by covariates (€2993 vs €2130; p<0.001; difference: €863/patient). Figure S3 shows the health costs according to age ranges, the number of exacerbations, and COPD severity in the study cohorts.
 |
Table 6 Health Resource Use and Costs (Mean/Patient/Year) During the 12-Month Follow-Up (After Propensity Score Matching) |
Discussion
The results of this study show that, in routine clinical practice, ICS are withdrawn in a small percentage of patients receiving triple therapy for the treatment of COPD (12.2%), many of whom had exacerbations in the year prior to withdrawal. In addition, ICS withdrawal during follow-up was associated with more exacerbations and similar rates of pneumonia and mortality, which led to an increase in health resource use and costs for the Spanish National Health System.
The use of ICS for COPD is controversial.7,8,18,19,26,27 However, their use in combination with long-acting bronchodilators is a standard treatment for patients with frequent exacerbations or a mixed phenotype.7,8 The safety profile and risk-benefit ratio are clearly favorable if used according to current recommendations while avoiding high doses.7,8,26,27 Therefore, healthcare professionals may be cautious regarding their withdrawal, especially in patients with frequent exacerbations, reduced lung function, or a mixed phenotype (eosinophilia).7,8,28 In contrast, adding a LAMA to an ICS/LABA combination produces significant clinical benefits in patients with COPD (dual bronchodilation).6,7 Thus there are notable discrepancies between guideline recommendations and the therapy used in usual clinical practice.6
Studies of ICS withdrawal show heterogeneous results. While the WISDOM18 and OPTIMO14 studies found the risk of exacerbation in patients with COPD did not increase after ICS withdrawal, other studies did not confirm these results. In addition, in the WISP study,29 ICS withdrawal in primary care patients with COPD increased the risk of exacerbation and caused a worsening of symptoms. The COSMIC study30 showed that fluticasone propionate withdrawal was associated with increased exacerbations while the COPE study31 found that ICS discontinuation also led to an increased risk of exacerbations. Our results support the findings of these studies.
A meta-analysis of clinical trials by Nadeem et al32 concluded that there was no significant evidence that ICS withdrawal in usual clinical practice causes a significant worsening of patient outcomes. In our study we found that patients who withdrew ICS seemed to have stable symptoms and the clinician decided to step down their treatment. However, using real-life data, Ye et al33 found differing results. In two recent real-life studies, Magnussen et al34 found that discontinuation of ICS was not associated with an increased risk of exacerbation in primary care patients with very few exacerbations, but ICS should not be withdrawn in a subgroup of patients with frequent oral corticosteroid cycles and elevated blood eosinophil counts due to the risk of exacerbations. In a 6-month follow-up study after ICS withdrawal, Nielsen et al35 found that 60% of patients resumed ICS due to exacerbations. Our results show we were unable to predict the patients who could benefit from the withdrawal or continuation of ICS according to the rate of exacerbations or the eosinophil count. The methodological differences and populations analyzed in these studies make it difficult to compare the results, especially as in many cases the number and severity of exacerbations was dependent on the study’s own definition.
There is debate surrounding the side effects of long-term ICS use, such as pneumonia and systemic adverse effects. Some reports suggest that the safety profile of ICS depends on the dose administered. This can have practical effects, highlighting the value of low doses of ICS in the treatment of COPD.8 Cheng et al36 concluded that high doses of ICS have greater clinical effectiveness in patients with COPD, without a higher incidence of pneumonia, and suggested that high-dose ICS therapy may be appropriate in patients with COPD. Our results seem to support these recommendations, and demonstrate the complexity of COPD treatment, especially in severe patients. Although areas of uncertainty persist, our results suggest that in patients with moderate airflow limitation and very few exacerbations, the ICS dose could be reduced without increasing the risk of exacerbations, provided adequate bronchodilator treatment is maintained. These results are similar to some available evidence, although controlled studies are required to improve decision making on the use of ICS in clinical practice.7,8,37,38
The corrected mean health cost per patient/year of ICS withdrawal compared with continuing triple therapy was higher (€2993 vs €2130). These results are directly related to the proportion of exacerbations, particularly severe exacerbations requiring hospitalization. Reviews by Press et al39 and Ehteshami-Afsha et al40 found wide methodological differences in the studies analyzed and concluded that the reports consistently showed that better disease control reduces COPD costs. They also noted a growing need for economic studies based on the latest guideline recommendations. Our results are difficult to compare as we found no similar studies in the literature. Most studies have concluded that COPD severity, hospital exacerbations, emergency visits, and medication are the most prominent cost components, and better health education could reduce COPD costs. In addition, patients with COPD with eosinophil counts of ≥220 cells/µL are more likely to have had acute or severe exacerbations, resulting in higher health costs.7,41 Given the methodological limitations, our results seem to be in line with the available literature.
The study has some limitations, mainly those inherent to retrospective studies, which are related to the categorization of COPD severity, the possible classification bias of patients, and limitations in the measurement of variables attributable to the information system. In this sense, the possible inaccuracy of diagnostic coding of COPD and other comorbidities, the definition of exacerbation, and the absence of variables that could influence the results (ie, socioeconomic level, environmental/occupational exposure, the evolution of the prescribed pharmacological dose, verification of the inhalation technique, therapeutic adherence, and/or differentiation of phenotypes) should be considered limitations. In addition, we could not discriminate between the form of ICS withdrawal (brusque, sequential). The index date matching method in the two study cohorts may also have caused some temporary bias that could not be accounted for. However, the groups were correctly balanced. It should be noted that it was not feasible to determine the exact date of last ICS intake, therefore the index date for the cohort withdrawing ICS was defined as the date of last available ICS prescription. Other unmeasured factors that may have influenced the results may include: a) the duration of ICS use in triple therapy; b) the pharmacological characteristics of the drugs; c) the combination of drugs in each device (ICS/LAMA/LABA, ICS/LABA+LAMA, LABA/LAMA+ICS); d) dispensing of a prescription does not guarantee that a patient has taken the drug; and/or e) characteristics after ICS withdrawal (combination of drugs after step down and possible modification to fewer devices). In addition, dependent upon the demand for medical care, non-disease-related factors such as access to health resources, comorbidities, and patient-specific characteristics that could lead to worsening of episodes may not have been reported by patients and were thus not considered, which may have influenced the outcomes. However, the main limitation is possible selection bias by the attending physician when withdrawing ICS. The effect of pneumococcal vaccines, the severity of COPD-associated asthma, and clinical evaluations (COPD assessment test or other questionnaires) may also not be considered due to the lack of data on these variables. Another possible limitation was due to the fact that clinical practice guidelines may not have recommended ICS be withdrawn in patients with asthma, with blood eosinophils ≥300 cells/uL and who use oral corticosteroids. However, our intention was to create comparable and homogeneous study cohorts via PSM. In addition, the proportions of these variables separately were low, and representative of patients seen in routine clinical practice. In our opinion, this should not influence the final results of the study (exacerbations and/or health costs). Other possible limitations include a lack of differentiation between the number and type of devices (pressurized cartridge inhalers, dry powder, or nebulizers) which could have influenced the results. Modifications to concomitant respiratory treatment were also not considered due to time restraints that prevented their quantification in the database. A more detailed analysis by molecules was not possible due to the small sample size. However, this study reflects usual clinical practice in which the degree of adherence to clinical practice guidelines is quite low. In fact, in clinical practice it is common for more than 50% of patients to be taking a dose three times higher than the high dose recommended by GOLD guidelines.7 It could be interesting for a future analysis to include patients who withdrew and then reinitiated triple therapy.
Conclusion
ICS withdrawal in patients on triple therapy was associated with a higher percentage of exacerbations and similar rates of pneumonia and mortality when compared with those continuing ICS, which could have an impact on health resource use and costs for the Spanish National Health System. Further real-life studies will be needed to reinforce the consistency of our results.
Abbreviations
ANCOVA, analysis of covariance; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification; ICS, inhaled corticosteroids, LABA, long-acting β2-agonists; LAMA, long-acting muscarinic antagonists; P, percentiles; PSM, propensity score matching; SD, standard deviation; TT, triple therapy.
Ethics Approval and Informed Consent
The study was classified by the Spanish Medicines and Healthcare Products Agency as EPA-OD and subsequently approved by the Research Ethics Committee of the Hospital of Terrassa, Barcelona, Spain (code: 02-20-399-090). Patient consent was not required as anonymized patient-level data were used in this analysis.
Acknowledgments
Editorial support (in the form of writing assistance, grammatical editing, and referencing) was provided by Kathryn Wardle of Aura, a division of Spirit Medical Communications Group Limited (Manchester, UK) and was funded by GlaxoSmithKline.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was funded by GlaxoSmithKline (study number: 213389). The sponsor was involved in study conception and design, data interpretation, and the decision to submit the article for publication. The sponsor was also given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Disclosure
VNG, LAV-A, ASI, RC, SS, and MG-P are employees of GlaxoSmithKline; LAV-A and ASI also hold stocks in GlaxoSmithKline. AS-M and AS-N are employees of Real Life Data. Real Life Data received payments from GlaxoSmithKline to perform the study, but not for manuscript development. CG received fees from GlaxoSmithKline for her involvement in the study, but not for manuscript development. The authors report no other conflicts of interest in this work.
References
1. Reyes-García A, Torre-Bouscoulet L, Pérez-Padilla R. Controversies and limitations in the diagnosis of chronic obstructive pulmonary disease. Rev Invest Clin. 2019;71(1):28–35. doi:10.24875/RIC.18002626
2. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
3. Blanco I, Diego I, Bueno P, et al. Geographical distribution of COPD prevalence in Europe, estimated by an inverse distance weighting interpolation technique. Int J Chron Obstruct Pulmon Dis. 2017;13:57–67. doi:10.2147/COPD.S150853
4. Soriano JB, Alfageme I, Miravitlles M, et al. Prevalence and determinants of COPD in Spain: EPISCAN II. Arch Bronconeumol. 2021;57(1):61–69. doi:10.1016/j.arbres.2020.07.024
5. Criner RN, Han MK. COPD care in the 21st Century: a public health priority. Respir Care. 2018;63(5):591–600. doi:10.4187/respcare.06276
6. Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol. 2017;53(6):324–335. doi:10.1016/j.arbres.2017.03.018
7. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease 2021 report. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf.
8. Izquierdo JL, Cosio BG. The dose of inhaled corticosteroids in patients with COPD: when less is better. Int J Chron Obstruct Pulmon Dis. 2018;13:3539–3547. doi:10.2147/COPD.S175047
9. Vogelmeier CF, Román-Rodríguez M, Singh D, Han MK, Rodríguez-Roisin R, Ferguson GT. Goals of COPD treatment: focus on symptoms and exacerbations. Respir Med. 2020;166:105938. doi:10.1016/j.rmed.2020.105938
10. López-Campos JL, Carrasco-Hernández L, Rodríguez LR, Quintana-Gallego E, Bernal CC, Navarrete BA. The clinical implications of triple therapy in fixed-dose combination in COPD: from the trial to the patient. Arch Bronconeumol. 2020;56(4):242–248. doi:10.1016/j.arbr.2020.02.002
11. Vanfleteren L, Fabbri LM, Papi A, Petruzzelli S, Celli B. Triple therapy (ICS/LABA/LAMA) in COPD: time for a reappraisal. Int J Chron Obstruct Pulmon Dis. 2018;13:3971–3981. doi:10.2147/COPD.S185975
12. Rossi A, van der Molen T, del Olmo R, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548–1556. doi:10.1183/09031936.00126814
13. Langham S, Lewis J, Pooley N, et al. Single-inhaler triple therapy in patients with chronic obstructive pulmonary disease: a systematic review. Respir Res. 2019;20(1):242. doi:10.1186/s12931-019-1213-9
14. Rossi A, Guerriero M, Corrado A; OPTIMO/AIPO Study Group. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res. 2014;15(1):77. doi:10.1186/1465-9921-15-77
15. Worth H, Buhl R, Criée C-P, Kardos P, Mailänder C,Vogelmeier C. The ‘real-life’ COPD patient in Germany: the DACCORD study. Respir Med. 2016;111:64–71. doi:10.1016/j.rmed.2015.12.010
16. Vogelmeier C, Worth H, Buhl R, et al. “Real-life” inhaled corticosteroid withdrawal in COPD: a subgroup analysis of DACCORD. Int J Chron Obstruct Pulmon Dis. 2017;12:487–494. doi:10.2147/COPD.S125616
17. Chapman KR, Hurst JR, Frent S-M, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329–339. doi:10.1164/rccm.201803-0405OC
18. Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. doi:10.1056/NEJMoa1407154
19. Watz H, Tetzlaff K, Wouters EFM, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4(5):390–398. doi:10.1016/S2213-2600(16)00100-4
20. Sicras-Mainar A, Enriquez JL, Hernández I, Sicras-Navarro A, Aymerich T, Leon M. PMU146 validation and representativeness of the Spanish BIG-PAC database: integrated computerized medical records for research into epidemiology, medicines and health resource use (real word evidence). Value Health. 2019;22(Supplement 3):S734. doi:10.1016/j.jval.2019.09.1764
21. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
22. World Health Organization. Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD). Available from: https://www.whocc.no/atc_ddd_index/.
23. Bassam M, Mayank V. Steroids in asthma: friend or foe. In: Qian X, editor. Glucocorticoids - New Recognition of Our Familiar Friend. IntechOpen; 2012.
24. General Council of Official Colleges of Pharmacists of Spain. Health knowledge database. Available from: https://botplusweb.portalfarma.com/.
25. Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53(6):1112–1117. doi:10.1093/ejcts/ezy167
26. Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388(10048):963–973. doi:10.1016/S0140-6736(16)31354-X
27. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
28. Micheletto C, Braido F, Contoli M, Di Marco F, Santus P. A framework for step down or therapeutic re-organization for withdrawal of inhaled corticosteroids in selected patients with COPD: a proposal for COPD management. Int J Chron Obstruct Pulmon Dis. 2019;14:2185–2193. doi:10.2147/COPD.S216059
29. Choudhury AB, Dawson CM, Kilvington HE, et al. Withdrawal of inhaled corticosteroids in people with COPD in primary care: a randomised controlled trial. Respir Res. 2007;8(1):93. doi:10.1186/1465-9921-8-93
30. Wouters EFM, Postma DS, Fokkens B, et al. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial. Thorax. 2005;60(6):480–487. doi:10.1136/thx.2004.034280
31. van der Valk P, Monninkhof E, van der Palen J, Zielhuis G, van Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. Am J Respir Crit Care Med. 2002;166(10):1358–1363. doi:10.1164/rccm.200206-512OC
32. Nadeem NJ, Taylor SJC, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD—a systematic review and comment on trial methodology. Respir Res. 2011;12(1):107. doi:10.1186/1465-9921-12-107
33. Ye W, Guo X, Yang T, Han F. Systematic review of inhaled corticosteroid withdrawal effects in chronic obstructive pulmonary disease, and comparison with two “real-life” studies. J Thorac Dis. 2018;10(7):4565–4573. doi:10.21037/jtd.2018.06.151
34. Magnussen H, Lucas S, Lapperre T, et al. Withdrawal of inhaled corticosteroids versus continuation of triple therapy in patients with COPD in real life: observational comparative effectiveness study. Respir Res. 2021;22(1):25. doi:10.1186/s12931-021-01615-0
35. Nielsen AO, Hilberg O, Jensen JUS, et al. Withdrawal of inhaled corticosteroids in patients with COPD - a prospective observational study. Int J Chron Obstruct Pulmon Dis. 2021;16:807–815. doi:10.2147/COPD.S294217
36. Cheng S-L, Su K-C, Wang H-C, Perng D-W, Yang P-C. Chronic obstructive pulmonary disease treated with inhaled medium- or high-dose corticosteroids: a prospective and randomized study focusing on clinical efficacy and the risk of pneumonia. Drug Des Devel Ther. 2014;8:601–607. doi:10.2147/DDDT.S63100
37. Sicras-Mainar A, de Abajo FJ, Izquierdo-Alonso JL. Clinical and economic consequences of inhaled corticosteroid doses and particle size in triple inhalation therapy for COPD: real-life study. Int J Chron Obstruct Pulmon Dis. 2020;15:3291–3302. doi:10.2147/COPD.S281333
38. Chalmers JD, Laska IF, Franssen FME, et al. Withdrawal of inhaled corticosteroids in COPD: a European Respiratory Society guideline. Eur Respir J. 2020;55(6):2000351. doi:10.1183/13993003.00351-2020
39. Press VG, Konetzka RT, White SR. Insights about the economic impact of chronic obstructive pulmonary disease readmissions post implementation of the hospital readmission reduction program. Curr Opin Pulm Med. 2018;24:138–146. doi:10.1097/MCP.0000000000000454
40. Ehteshami-Afshar S, FitzGerald JM, Doyle-Waters MM, Sadatsafavi M. The global economic burden of asthma and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2016;20(1):11–23. doi:10.5588/ijtld.15.0472
41. Iheanacho I, Zhang S, King D, Rizzo M, Ismaila AS. Economic burden of chronic obstructive pulmonary disease (COPD): a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2020;15:439–460. doi:10.2147/COPD.S234942
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
