Back to Journals » Cancer Management and Research » Volume 13
Clinical Analysis of the Short-Term Outcome of Papillary Thyroid Micro Carcinoma After 131I Treatment
Authors Cao J , Yun C, Zhu X, Li X, Sun Y, Zhang W
Received 25 February 2021
Accepted for publication 29 May 2021
Published 14 June 2021 Volume 2021:13 Pages 4691—4698
DOI https://doi.org/10.2147/CMAR.S308012
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Jingjia Cao, Canhua Yun, Xiaolu Zhu, Xiao Li, Yaru Sun, Wei Zhang
Department of Nuclear Medicine, The Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, 250033, People’s Republic of China
Correspondence: Wei Zhang
Department of Nuclear Medicine, The Second Hospital, Cheeloo College of Medicine, Shandong University, No. 247 Road, Tianqiao District, Jinan, 250033, People’s Republic of China
Tel +86 17660086070
Email [email protected]
Purpose: To explore the factors that influence the short-term clinical outcome after the first 131I treatment of papillary thyroid micro carcinoma (PTMC).
Patients and Methods: From October 2015 to June 2018, patients who were diagnosed with PTMC with lymph node metastasis were analyzed retrospectively, excluding patients with incomplete clinical data, distant metastasis, positive TGAb, TSH< 30 mIU/L. The baseline data of sex, age, time from last surgery to first 131I treatment, tumor pathology information, and biochemical information were collected before admission. All patients included had radioactive iodine (RAI) with 3.70 GBq. The treatment response of patients was evaluated 6– 8 months after discharge. By means of univariate and multivariate analysis, including excellent response (ER) and non-excellent response (NER) groups of clinical data, we assessed the impact of 131I on patients’ outcome. A nomogram model was established based on the above independent risk factors.
Results: A total of 206 patients (59 males and 147 females, mean age 43.4 ± 10.6 years) were included in the study. The median follow-up time was 169.4 ± 10.5 days, including 139 patients in ER group (67.4%) and 67 patients in NER group (32.5%). Four factors including combining Hashimoto’s thyroiditis, pre-ablative Tg levels, UIE levels, and lateral lymph node numbers were statistically different between ER group and NER group with significance at P < 0.05. Further multivariate analysis showed that Hashimoto’s thyroiditis and Ps-Tg levels could be used as independent factors. The model verification showed that the C-index of the modeling set was 0.822, indicating that the nomogram model had a good predicted accuracy.
Conclusion: Our data suggest that coexisting Hashimoto’s thyroiditis and elevated Ps-Tg levels are predictive factors for short-term outcome of thyroid micro papillary carcinoma after 131I treatment. Also, the nomogram model had a good predicted accuracy.
Keywords: micro papillary carcinoma, curative effect, 131I treatment, outcome
Introduction
In recent years, the incidence rate of papillary thyroid carcinoma (PTC) is increasing rapidly, in which the proportion of papillary thyroid micro carcinoma (PTMC) has gradually risen. PTMC refers to a papillary thyroid carcinoma with the largest diameter of less than 10 mm.1 The definition of PTMC seems to emphasize more on the role of tumor size in prognosis, while ignoring the impact of tumor pathological characteristics, molecular phenotypes and other invasive characteristics on prognosis. This leads to clinical controversy on the choice of PTMC operation and postoperative 131I treatment.1–3 At present, studies generally believe that “tiny” does not represent a low-risk tumor. Its biological behavior and that of a papillary carcinoma larger than 10 mm in diameter are similar. They are also characterized by cervical lymph node metastasis, extraglandular invasion, and systemic metastasis.2 Radioactive iodine (RAI) therapy is one of the important adjuvant treatments for PTC, similarly, which can significantly reduce the recurrence rate and death risk of patients.3 The 2015 version of the American Thyroid Association (ATA) guidelines first classified patients as having an excellent response (ER), indeterminate response (IDR), biochemical incomplete response (BIR), and structural incomplete response (SIR) after 131I therapy response.3 Research showed that patients with clinical outcome reaching ER had a 10-year recurrence rate of 1–4% and a mortality risk of less than 1% compared with non-excellent response (NER), while those with SIR had a mortality risk of 11–50% and a recurrence rate of 50–85%.3
At present, there are few analyses at home and abroad on factors affecting 131I treatment response after PTMC surgery. This paper aimed to investigate factors influencing short-term clinical outcome after first 131I therapy for PTMC.
Materials and Methods
Study Subjects
Patient data extracted from the clinical electronic medical record system were de-identified so that all private information of patients was not included. The Institutional Review Board of the Second Hospital, Cheeloo College of Medicine, Shandong University approved the use of medical records and allowed us to obtain written consent from each patient (KYLL-2018[LW]013). All procedures complied with the Declaration of Helsinki for research involving human subjects. All the patients signed informed consent before RAI.
From October 2015 to June 2018, a total of 810 patients treated with 131I for the first time after total thyroidectomy surgery and neck lymph node dissection were retrospectively reviewed. The extent of resection was described in detail in the surgical record, no residual gland was seen as confirmed by neck ultrasound. Patients were excluded due to at least one of the following reasons: (1) with pathologically confirmed not PTMC; (2) no cervical lymph node metastases and belonging to the low-risk group; (3) incomplete clinical data; (4) inadequate information of follow-up; (5) distant metastases found before initial 131I therapy; (6) interfering antithyroglobulin antibody (TgAb) (above upper reference range); (7) pre-ablative stimulated thyroid stimulating hormone(Ps-TSH) <30 mIU/L. Finally, a total of 206 patients were included in the study.
Grouping and Methods
Baseline data before patients’ admission were collected. The main outcomes included gender, age, time from last surgery to first 131I treatment (TT), and tumor pathology information, such as tumor maximum diameter (TMD), multifocality, extracapsular spread, Hashimoto’s thyroiditis (HT), BRAFV600E mutation status, lymph node metastasis. The biochemical information of the patients after admission was collected, mainly including urinary iodine level, Ps-TSH level, pre-ablative stimulated thyroglobulin (ps-Tg) level, and other data.
Before admission, L-Thyroxine 4 (LT4) treatment was stopped for 1 month. Patients were asked to follow a low-iodine diet for a month prior to 131I treatment. All patients included had RAI with 3.70 GBq (100 mCi) 131I administered orally. A whole-body post-treatment scan (Rx-131I-WBS) was obtained 72 h after the administration of iodine. Patients were subsequently discharged on a TSH-suppressive dose of LT4, 100–200 μg orally per day. 6–8 months after discharge, patients were monitored for serological indicators (Tg, TgAb, TSH, free triiodothyronine, free thyroxine, triiodothyronine, and thyroxine), neck ultrasonography, and diagnostic whole body 131I imaging (Dx-131I-WBS).
TSH was determined by chemiluminescence immunoassay (provided by Beckman Coulter Inc., Brea, California) with a functional sensitivity of 0.015 mIU/L. Tg and TgAb levels were determined using electro-chemiluminescence immunoassay (provided by Beckman Coulter Inc., Brea, California) with a measuring range of 0.100–500 ng/mL and 0.900–2500 IU/mL, respectively. Urinary iodine was determined by arsenic-cerium catalytic spectrophotometry with mild acid digestion.
Evaluation Criteria
Based on imaging findings and stimulated/suppressed Tg levels of the initial follow-up examination, patients were classified into four response-to-therapy categories recommended by the ATA guidelines as shown in Table 1. According to ATA guidelines, considering follow-up Tg level, Dx-WBS examination, neck ultrasonography, and other imaging examinations, patients were divided into two groups: ER group and NER group. IDR, BIR, and SIR were classified into NER group. Three nuclear medicine physicians independently assessed the outcome of follow-up examinations after treatment and reached agreement through consultation when discordance appeared.
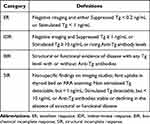 |
Table 1 Clinical Implications of Response to Therapy Reclassification in Patients with Differentiated Thyroid Cancer Treated with Total Thyroidectomy and Radioiodine Remnant Ablation |
Statistical Analysis
Data were analyzed by SPSS 20.0, and the counting data were described as the number of cases or percentage, and the measurement data conformed to the normal distribution with results expressed as means ± standard deviation (SD). Continuous data with skewed distribution were expressed as median (quartile). χ2 test, independent sample t-test, and Mann–Whitney U-test were used to compare the differences between two groups of patients. The binary logistic regression model was established, and the factors with P <0.10 in univariate analysis were included in the model to further explore the independent factors affecting the prognosis. P <0.05 was considered statistically significant. The software package R (version 3.5.3) was used for the nomogram prediction model. Bootstrap method with the caret package was also applied for internal validation.
Results
General Information
A total of 206 patients were included in this study (147 females and 59 males; mean age, 43.4 ± 10.6), with a median follow-up time of 169.4 ± 10.5 days. ER was observed in 139 (67.4%) patients. IDR was observed in 30 (14.5%), 22 (10.6%) patients had BIR, 15 (7.2%) patients had SIR, and three of them were classified into the group of NER (n = 67, 32.5%).
Factors Influencing Excellent Response
Only four factors including combining Hashimoto’s thyroiditis, pre-ablative Tg levels, UIE levels, and lateral lymph node numbers were statistically different between ER group and NER group with significance at P < 0.05. Details are shown in Table 2 and Figure 1. Compared with central node dissection, lateral node dissection was a risk factor for ER groups, however, which was not associated with multivariate analysis, OR = 1.078 (95% CI: 0.932–1.247, P = 0.312). Moreover, it was found that there was a correlation between UIE levels and ER or NER groups tested by χ2 test and Mann–Whitney U-test (P = 0.047, P = 0.042), which was not included in the final multivariate model. Details are shown in Table 3.
 |
Table 2 Comparison of General Data Between ER Group and NER Group |
 |
Table 3 Multivariate Analysis for Achieving Optimal Treatment (ER) Response |
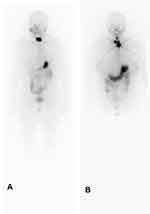 |
Figure 1 (A) indicates RX-WBC residual thyroid only; (B) indicates residual thyroid with lymph node metastasis. |
With multivariate analysis, the study found that combining Hashimoto’s thyroiditis, pre-ablative Tg levels were independent predictors of response to ER (OR = 0.231, OR = 1.14).
Development of a Predictive Nomogram Risk Model
A nomogram model to predict the short-term outcome of papillary thyroid micro carcinoma after 131I treatment was developed based on logistic regression analysis (Figure 2). The model was validated by modeling set self sampling (bootstrap method). The results showed that the C- indices of the modeling set was 0.822, and the calibration curves of both sets were close to the ideal curves (diagonal) (Figure 3).
 |
Figure 2 The nomogram risk model for predicting the short-term outcome of papillary thyroid micro carcinoma after 131I treatment. |
 |
Figure 3 Calibration curve verification of the nomogram model. |
Discussion
At present, there are many studies on the diagnosis, treatment and prognostic factors of differentiated thyroid cancer, but there are still disputes on the treatment of PTMC, such as surgical methods, 131I treatment, TSH inhibition level and so on.4,5 Patients without lymph node metastasis, according to the guidelines, are not recommended to receive radioiodine remnant ablation, but to actively carry out TSH suppression therapy.5 Although patients with fewer than 3 lymph node metastases belong to the low-risk population, we also carried out RAI to facilitate the follow-up monitoring of Tg. Chinese experts have pointed out that it can be implemented according to each patient’s wishes.1,2
The choice of operation (total or near total thyroidectomy) is an important prerequisite for 131I, that is, only PTMC patients after total thyroidectomy are recommended for 131I treatment. At the same time, 131I therapy can assist the adjuvant treatment after lymph node dissection. It is controversial whether lateral neck dissection should be performed in patients with BRAFV600E mutation.3 Blind lateral neck lymph node dissection is not only time-consuming, but also has a high risk of injury to important tissue structures such as the vagus nerve.6,7 In this paper, we also included the patients with BRAFV600E mutation and wild type to observe the curative effect after central lymph node dissection. It is found that it is risky to do lymph node dissection in the central region only, however, this part is not fully shown in the data of the article. Therefore, 131I therapy is significant for PTMC patients with lymph node metastasis.
In this study, 67.5% of PTMC patients reached ER status. According to Liu, 63.2% of the patients who received 131I treatment for the first time after PTC reached ER status, which is a consistent finding.8 The male to female ratio of patients included in this study was 1:2.49, which indicated that PTMC was more likely to occur in female patients. It is suggested that men are associated with poor prognosis of PTC. Age (≥55 years old) was an independent risk factor for postoperative staging of differentiated thyroid cancer (DTC); there was no significant difference in treatment response (P >0.05). A meta-analysis of 3523 patients with PTMC showed that age and gender were not associated with PTMC recurrence.9 In this study, it is also considered that gender and age do not affect the ER status of patients. The time between surgery and the first 131I treatment is considered to be one of the factors influencing the treatment response. This is quite different from the previous literature.10,11 Generally, the deadline is 3 months. Li et al. considered that delayed initial RAI therapy (≥3 months after thyroidectomy) was related to incomplete response in low- to intermediate-risk DTC. Multivariate analysis demonstrated that the time interval was an independent risk factor for NER (P = 0.008).10 Wang et al. suggested that RAI therapy within 6 months after total thyroidectomy may have no significant effect on outcomes.11 The median time from last surgery to first 131I treatment (TT) in this study in the ER and NER groups were 81 and 78.5 days, respectively. There was no statistical difference between the two groups (Z = −1.224, P = 0.221). Taking 3 months as the cut-off point, there was no statistical difference between the two groups (χ2 = 1.053, P = 0.305). This shows that the cut-off point of TT time is about 3 months, which has no effect on the short-term clinical outcome.
The pathological characteristics of tumor are often mentioned in the previous literature as the influencing factors of 131I treatment. Liu and colleagues suggested that Ps-Tg (with a cut-off value of 9.05 ng/mL) could predict the ER in this cohort, and its combination with tumor size might better predict the non-ER response to initial treatment; 1.05 cm of tumor diameter was set as the cut-off value with relatively low sensitivity and specificity of 53.5% and 72.0%, respectively.8 However, Du suggested that the maximum diameter of tumor did not influence the efficacy in the treatment of PTMC.12 In this study, multifocality, capsular invasion, and maximum tumor diameter did not affect the efficacy treatment of PTMC, which suggested that PTMC, as a type of PTC, had different influencing factors on therapeutic efficacy than non PTMC patients.
Study reports suggested that papillary thyroid cancers are more frequently associated with Hashimoto’s thyroiditis (HT). HT may be a precancerous condition.13 In this study, the ER status of patients with HT was significantly lower than that of patients without HT (P < 0.001), which was similar to the conclusion of Kwon. The author reports that coexisting Hashimoto’s thyroiditis and elevated sTg are negative predictive factors for successful low-dose radioactive-iodine remnant ablation treatment.14 It is possible that the lower level of sodium iodine symporter (NIS) expression in the basement membrane of residual inflammatory thyroid follicular epithelial cells compared with normal thyroid tissue analyzed in this study affected 131I uptake by residual thyroid tissue. Further multivariate analysis revealed that concomitant Hashimoto’s thyroiditis (OR = 0.231, P = 0.001) could be an independent risk factor for treatment response in patients.
Central regional lymph nodes were the most common metastatic site for PTMC, occurring in approximately 24.1–65.0% of patients with central regional lymph node metastases and 3.1–55.0% of patients with lateral cervical lymph node metastases.15
The metastatic sites and the number of metastases to lymph nodes were included in this study and found not to be statistically different. The median number of lateral neck region lymph node metastases was 0 and 2 in the ER and NER groups, respectively (Z = −3.227, P = 0.001). Moreover, the number of lateral neck lymph node metastases was included in multivariate analysis. It was found to be rejected in multivariate analysis, which illustrates that the number of lymph node metastases does not act as an independent risk factor for predicting treatment response. RX-WBS results (Figure 1) were not statistically different between ER and NER groups, suggesting that RX-WBS after first dose 131I does not imply a reduced likelihood of achieving an optimal therapeutic response, even if lymph node metastases are found. This suggests that the finding of lymph node metastases by RX-WBS after the first 131I treatment does not imply a reduced likelihood of attaining ER status.
Guidelines now include BRAFV600E mutations as an indication for intermediate risk recurrence risk.1–5 A large meta-analysis definitively demonstrates that BRAFV600E-mutation-positive PTMC are more likely to manifest with aggressive clinicopathological characteristics.16 However, the BRAFV600E mutation was observed in 72 patients (71.3%). There was no statistically significant correlation in age, gender, multifocality, extrathyroidal extension, presence of Hashimoto’s thyroiditis, and lymph node metastasis between the BRAFV600E mutant group and wild group.17 We also believe that the BRAFV600E mutation is not significantly associated with prognostic factors in PTMC.
Biochemicisty levels prior to 131I treatment were also included in the analysis as a contributing factor to efficacy. In clinical practice, as recommended by ATA guidelines, DTC patients who are scheduled for RAI must achieve pre-ablation TSH levels > 25–30 mIU/L.3 Recent data suggest that this TSH level is not actually required for proper 131I ablation.18 The median TSH levels in the ER and NER groups were 100.0 mIU/L, respectively, which were not statistically different (P = 0.35). We further divided the TSH levels into three groups, which were not statistically different. We confirmed that higher TSH levels before RAI were not associated with a better outcome. The median UIE levels in the ER and NER groups were 88.0 ug/L and 15.0 ug/L, respectively, which were statistically different (P = 0.047). The findings of this study are similar to the conclusions of Sohn et al. However, it has not become an independent predictor, which requires a large number of prospective studies to determine whether UIE level can be an indicator of short-term clinical efficacy.19 There is a consensus in the literature that the level of Ps-Tg affects the response to treatment. Kim et al. suggested that postoperative Ps-Tg levels in PTC can effectively predict 131I treatment efficacy, and elevated Ps-Tg levels may predict treatment failure.20 The results of the present study are compatible with this. Also, it was found that the lower the Ps-Tg level before treatment, the easier the best therapeutic response was achieved after the first 131I treatment.
Conclusion
Our data suggest that coexisting Hashimoto’s thyroiditis and elevated Ps-Tg levels are predictive factors for short-term outcome of papillary thyroid micro carcinoma after 131I treatment, and the nomogram model had a good predicted accuracy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chinese Association of Thyroid Oncology. Chinese expert consensus on diagnosis and treatment of papillary thyroid microcarcinoma. Chin J Clin Oncol. 2016;43(10):405–411. doi:10.3969/j.issn.1000-8179.2016.10.001
2. Chinese Society of Nuclear Medicine. Clinical guidelines for 131I therapy of differentiated thyroid cancer (2021). China J Nucl Med Mol Imaging. 2021;41(4):218–241. doi:10.3760/cma.j.cn321828-20201113-00412
3. Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi:10.1089/thy.2015.0020
4. Thyroid cancer committee of Chinese Society of Clinical Oncology. Expert consensus on preoperative assessment of 131I after operation for differentiated thyroid cancer. China Oncol. 2019;29(10):832–840. doi:10.19401/j.cnki.1007-3639.2019.10.011
5. Xin Z, Yansong L. Progress of 131I therapy for non distant metastatic differentiated thyroid cancer: interpretation of “ESMO clinical practice guidelines: diagnosis, treatment and follow-up of thyroid cancer” in 2019. China J Nucl Med Mol Imaging. 2020;40(06):343–350. doi:10.3760/cma.j.cn321828-20200220-00056
6. Calò PG, Medas F, Conzo G, et al. Intraoperative neuromonitoring in thyroid surgery: is the two-staged thyroidectomy justified? Int J Surg. 2017;41(Suppl 1):S13–S20. doi:10.1016/j.ijsu.2017.02.001
7. Conzo G, Docimo G, Mauriello C, et al. The current status of lymph node dissection in the treatment of papillary thyroid cancer. A literature review. Clin Ter. 2013;164(4):e343–6. PMID: 24045534. doi:10.7417/CT.2013.1599
8. Jierui L, Jun L, Yansong L. Relationship between preablative stimulated thyroglobulin and the excellent response in differentiated thyroid carcinoma. China Oncol. 2019;29(2):125–130. doi:10.19401/j.cnki.1007-3639.2019.02.005
9. Mehanna H, Al-Maqbili T, Carter B, et al. Differences in the recurrence and mortality outcomes rates of incidental and nonincidental papillary thyroid microcarcinoma: a systematic review and meta-analysis of 21 329 person-years of follow-up. J Clin Endocrinol Metab. 2014;99(8):2834–2843. doi:10.1210/jc.2013-2118
10. Li H, Zhang Y-Q, Wang C, et al. Delayed initial radioiodine therapy related to incomplete response in low- to intermediate-risk differentiated thyroid cancer. Clin Endocrinol (Oxf). 2018;88(4):601–606. doi:10.1111/cen.13551
11. Wang X, Song Q, Dong X. Effects of timing of initial postoperative radioactive iodine therapy on the outcome of patients with differentiated thyroid cancer. J China Med Univ. 2019;48(4):359–369. doi:10.12007/j.issn.0258-4646.2019.04.016
12. Du F, Hu S, Wu C, et al. Analysis of the factors affecting the efficacy of 131I remnant ablation in patients after thyroidectomy for papillary thyroid microcarcinoma. Chin J Oncol. 2018;40(8):610–613. doi:10.3760/cma.j.issn.0253-3766.2018.08.009
13. Konturek A, Barczyński M, Nowak W, et al. Risk of lymph node metastases in multifocal papillary thyroid cancer associated with Hashimoto’s thyroiditis. Langenbecks Arch Surg. 2014;399(2):229–236. doi:10.1007/s00423-013-1158-2
14. Kwon H, Choi JY, Moon JH, et al. Effect of Hashimoto thyroiditison low-dose radioactive-iodine remnant ablation. Head Neck. 2016;38(Suppl l):E730–735. doi:10.1002/hed.24080
15. Danyang S, Wei Z, Jian T. Clinical features of lymph node metastasis in papillary thyroid microcarcinoma with 131I treatment. Chin J Endocrinol Metab. 2016;32(11):900–905. doi:10.3760/cma.j.issn.1000-6699.2016.11.003
16. Li F, Chen G, Sheng C. BRAFV600E mutation in papillary thyroid microcarcinoma: a meta-analysis. Endocr Relat Cancer. 2015;22(2):159–168. doi:10.1530/ERC-14-0531
17. Choi SY, Park H, Kang MK, et al. The relationship between the BRAF(V600E) mutation in papillary thyroid microcarcinoma and clinicopathologic factors. World J Surg Oncol. 2013;11:291. doi:10.1186/1477-7819-11-291
18. Vrachimis A, Riemann B, Mäder U, et al. Endogenous TSH levels at the time of (131)I ablation do not influence ablation success, recurrence-free survival or differentiated thyroid cancer-related mortality. Eur J Nucl Med Mol Imaging. 2016;43:224–231. doi:10.1007/s00259-015-3223-2
19. Sohn SY, Choi JY, Jang HW, et al. Association between excessive urinary iodine excretion and failure of radioactive iodine thyroid ablation in patients with papillary thyroid cancer. Thyroid. 2013;23(6):741–747. doi:10.1089/thy.2012.0136
20. Kim H, Kim SJ, Kim IJ, et al. Limited clinical value of periablative changes of serum markers in the prediction of biochemical remission in patients with papillary thyroid cancer. Nucl Med Mol Imaging. 2013;47(4):268–272. doi:10.1007/s13139-013-0220-x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
