Back to Journals » Infection and Drug Resistance » Volume 15
Clinical Analysis of Acute Suppurative Thyroiditis in 18 Children
Authors She X , Zhou YN, Guo J, Yi C
Received 3 June 2022
Accepted for publication 10 August 2022
Published 12 August 2022 Volume 2022:15 Pages 4471—4477
DOI https://doi.org/10.2147/IDR.S377279
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Xiang She, Yu-Neng Zhou, Jun Guo, Cong Yi
Department of Pediatrics, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, 621000, People’s Republic of China
Correspondence: Cong Yi, Department of Pediatrics, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, 621000, People’s Republic of China, Tel +8613980130553, Email [email protected]
Objective: To summarize our clinical experience with the diagnosis and treatment of children with acute suppurative thyroiditis (AST).
Methods: We retrospectively reviewed the clinical data of 18 children with AST treated at our hospital between January 2009 and May 2022.
Results: There were 8 boys and 10 girls, aged 7.8 ± 3.8 years at admission. The main clinical manifestations were fever (88.9%), neck pain (100%), and neck mass (100%). Blood and pus cultures were performed in 9 and 15 patients, respectively. All blood cultures were negative, while positive pus cultures were noted in eight cases (six Streptococcus spp., one Staphylococcus spp., and one Streptococcus spp. and Staphylococcus spp. mixed infection). Additionally, all patients received antibiotic treatment: three received antibiotics alone, seven received antibiotics and ultrasound (US)-guided needle aspiration, seven received antibiotics as well as surgical incision and drainage, and one received antibiotics, US-guided needle aspiration in addition to surgical incision and drainage. Consequently, the average length of hospital stay in patients who received antibiotics and US-guided needle aspiration was 9.1± 2.9 days compared to 14.0± 2.0 days in patients in the antibiotics alone group and 13.0 ± 2.2 days in patients in the antibiotics and surgical incision and drainage group. Follow-up was conducted in 15 of the 18 patients. Three patients relapsed, and the prognosis of the other patients was good.
Conclusion: AST has atypical clinical symptoms at the early stage. Regular monitoring of the thyroid gland using ultrasonography is strongly advised in unsure cases. Antibiotics combined with US-guided aspiration is a safe, effective, and minimally invasive treatment for AST in children and can reduce hospital stay. However, surgery may be necessary, particularly in the presence of complications. It is strongly recommended that patients with recurrence be examined for anatomical abnormalities and undergo radical treatment.
Keywords: children, acute suppurative thyroiditis, clinical manifestation, treatment
Introduction
Acute suppurative thyroiditis (AST) is usually caused by bacterial infection and is an extremely rare but potentially lethal endocrine emergency that occurs in 0.1–0.7% of all thyroid diseases.1–3 AST is easily overlooked or misdiagnosed as a result of its low incidence and atypical clinical symptoms. The onset of AST is sudden, and if not treated, the disease progresses rapidly, leading to a mortality of 12% or higher.4–6 To summarize the clinical experience and improve the understanding of this disease by pediatricians, the clinical data of 18 cases of children with AST admitted at our hospital from January 2009 to May 2022 were analyzed.
Method
This study was a retrospective investigation of 18 pediatric patients with AST in the Department of Pediatrics, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, China, from January 2009 to May 2022. AST was diagnosed based on clinical manifestations, laboratory examinations, ultrasonography, or contrast-enhanced computed tomography (CT). The electronic medical records of all included patients were manually reviewed, and relevant information was extracted and recorded in detail. Furthermore, patients were followed up by telephone.
This study was conducted in accordance with the Declaration of Helsinki. Owing to the retrospective nature of this study, the Mianyang Central Hospital Ethics Committee granted a waiver of informed consent (Waiver ID: S20220333-01). All participant data were strictly maintained with confidentiality.
Data analysis was performed using the IBM SPSS Statistics 22.0. In addition, quantitative data were presented as mean and standard deviation. One-way analysis of variance (one-way ANOVA) was performed to detect statistical significance between the groups. Enumeration data were expressed as number (percentage), and a P value of less than 0.05 was considered statistically significant.
Results
General Information
Between January 2009 and May 2022, eighteen pediatric patients were diagnosed with AST. Of the 18 children, eight were boys, and ten were girls. The average age of the patients was 7.8 ± 3.8 years (Table 1).
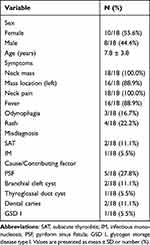 |
Table 1 General Conditions and Clinical Characteristics |
Clinical Manifestations
The main clinical symptoms in the patients are shown in Table 1. The most common clinical features were fever (16 cases, 88.9%), neck pain (18 cases, 100%), and neck mass (18 cases, 100%). Sixteen of the 18 patients (88.9%) had a neck mass on the left side. Three cases (16.7%) of AST were misdiagnosed during early disease stages. Among these three, two were misdiagnosed as subacute thyroiditis (SAT), and one was misdiagnosed as infectious mononucleosis. There were five children with congenital pyriform sinus fistula (PSF), two children with dental caries, two children with a branchial cleft cyst, one child with a thyroglossal duct cyst, and one child with glycogen storage disease type I (GSD I) (Table 1).
Laboratory Examination Findings
Routine blood test for the 18 patients revealed high white blood cell counts in 16 (88.9%) patients. Five of the 18 patients underwent erythrocyte sedimentation rate (ESR) examination, ten underwent C-reactive protein (CRP) testing, and eight underwent procalcitonin (PCT) examination. The values of the inflammatory markers (ESR, CRP, and PCT) were higher than normal. Among the 13 patients who completed thyroid function tests, two were found to have transient thyrotoxicosis, while the other 11 had normal thyroid function. Blood and pus cultures were performed in 9 and 15 patients, respectively. The blood cultures were all negative; on the contrary, the pus cultures were positive in eight cases (six cases of Streptococcus spp., one case of Staphylococcus spp., and one case of both Streptococcus spp. and Staphylococcus spp.). All patients underwent neck ultrasound (US) examinations, wherein seven patients underwent neck contrast-enhanced CT examinations. All radiographic findings were abnormal (Table 2).
 |
Table 2 Laboratory Examination and Imaging Examination |
Treatment and Outcomes
After admission, all patients were empirically treated with antibiotics, which were mainly active against Gram-positive cocci. In cases where the antibiotic was ineffective, other antibiotics were used according to experience or drug sensitivity results. Second-generation cephalosporin and semisynthetic penicillin/β-lactamase inhibitors were used in most of these cases, particularly in 10 (55.6%) and 7 (38.9%) cases, respectively. Only one patient was treated with a trisubstituted cephalosporin. Furthermore, during the treatment, only one patient was switched from second-generation cephalosporin to imipenem-cilastatin. At discharge, all patients were cured. Among them, three (16.7%) patients were treated with antibiotics alone, seven (38.9%) with antibiotics and US-guided needle aspiration, and seven (38.9%) with antibiotics and surgical incision and drainage. In one (5.5%) case, treatment with antibiotics and US-guided needle aspiration failed; hence, surgical incision and drainage was required. Furthermore, compared to patients who received other treatment types, the length of hospital stay was shorter in patients who received antibiotics and US-guided needle aspiration (Table 3). Notably, owing to the misdiagnosis of AST as SAT at another hospital, two children were initially treated with glucocorticoids, leading to aggravation of the condition. In one case, there were symptoms of airway compression.
 |
Table 3 Length of Hospital Stay |
After discharge, during the quiescent period, two patients were diagnosed with PSF by CT with meglumine diatrizoate and laryngoscopy, respectively. They were then treated via complete excision of the fistulous tract through hemithyroidectomy or endoscopic CO2-laser cauterization of the internal opening of the fistula at the pyriform sinus. These patients were followed up for 6.5 years and 6 months, respectively, and no relapse was observed. Three out of 18 patients were lost to follow-up, while the remaining 15 were followed up for one month to 11 years, and three patients relapsed. One patient who was diagnosed with PSF relapsed two years later. After recovery, treatment for PSF was recommended, but the patient refused due to financial reasons. And this patient was lost to follow-up. Additionally, one patient with a history of neck abscess was treated with fistulectomy and lumpectomy during the quiescent period. Histopathological examination confirmed the presence of a branchial cleft cyst. This patient relapsed twice during follow-up. Furthermore, another patient relapsed two years after disease cure. During the quiescent period, the patient underwent a surgical fistulectomy. Histopathological examination confirmed the presence of a thyroglossal duct cyst. The patient was followed up for eight years, and no relapse was observed. The other two patients diagnosed with PSF, one patient with a branchial cleft cyst, and the remaining seven patients received no additional treatment but had no recurrence. Furthermore, there was no recurrence in the patient with a history of GSD I; however, the patient had neutropenia, ulcerative stomatitis, and colitis during follow-up.
Discussion
AST is an urgent condition in children that may result in abscess formation, descending necrotizing mediastinitis, tracheal and esophageal perforation, or even tracheal compression, leading to asphyxia and death.7,8 The thyroid is relatively resistant to most infections owing to its tough capsule, high iodine concentration, extensive lymphatic drainage, and rich blood supply.9 Similar to previous studies, in our study, we observed that AST occurs in a slightly higher percentage of females.10
Most children with AST have anatomical abnormalities, such as cysts and PSF.11,12 Branchial cleft cyst anomalies are more susceptible to thyroid infections and abscess formation.13 PSF originates from a third or fourth branchial cleft cyst anomaly and forms a tract from the pyriform sinus to the left lobe of the thyroid almost exclusively.14 Therefore, most cases of AST in children occur in the left lobe of the thyroid, which is consistent with our results. AST also occasionally occurs in immunocompromised patients, such as those with HIV, systemic lupus erythematosus, pre-existing thyroid disease, or metabolic diseases.15,16 GSD Ib is a complex disorder of glucose metabolism that causes severe chronic neutropenia,17 which may lead to a chronic immunosuppressive state in patients and a predisposition to recurrent infection.
The primary clinical manifestations of AST include neck pain, fever, dysphagia, dysphonia, and erythema.10 Laboratory examinations may show leukocytosis and elevated ESR and CRP levels in some children without specific serum markers.18 Thyroid function tests are usually normal.6 Nevertheless, AST could contribute to thyrotoxicosis by destroying thyroid follicles and releasing thyroid hormones, which is a common finding in SAT.19 Due to the overlapping features of AST and the limited experience of most physicians investigating changes in AST, it can be difficult to clinically distinguish AST from SAT.
SAT is a relatively benign, self-limiting, inflammatory thyroid disease that may require treatment with glucocorticoids. However, AST does not respond to glucocorticoids, rather administering glucocorticoids to a patient with AST may lead to the spread of infection and aggravation of the condition, as highlighted by two of our cases. Thus, when patients with SAT fail to respond to glucocorticoids, they should be reassessed for AST. Imaging can help distinguish between AST and SAT. US imaging is the preferred diagnostic method for thyroid disorders. Generally, US reveals unifocal hypoechoic lesions in AST; however, SAT is usually associated with multiple and bilateral hypoechoic lesions.20 Unless US findings are inconclusive, CT or magnetic resonance imaging is usually not needed. In the earliest stages of AST, when no abscess is evident, CT and US findings are ineffective in distinguishing AST from SAT.20 When AST is in the acute inflammation stage, US and CT scans facilitate the structural assessment of the abscess and its surroundings.20 The process of abscess development is dynamic. Therefore, repeat US imaging is considered an objective diagnostic indicator.
AST in children is mostly caused by bacteria. The most common bacteria are Gram-positive cocci, such as Staphylococcus and Streptococcus species, accounting for 40% of all cases.21 In the present study, the positivity rate was significantly higher for pus cultures than for blood cultures. The causative pathogens of AST were identified as Staphylococcus or Streptococcus species in the pus cultures of eight patients. Treatment modalities for AST include antibiotics alone or in combination with needle aspiration and/or surgical incision and drainage.
Overall, treatment choice is determined by the stage of AST. Due to the potentially high mortality rate associated with AST, it is imperative to begin empiric antibiotic therapy as soon as possible after clinical suspicion of AST. In cases of small abscesses or abscess formation, antibiotic treatment alone can be effective.6 Antibiotics, such as second-generation cephalosporins and semisynthetic penicillin/β-lactamase inhibitors, were chosen based on availability and activity against Gram-positive cocci. If available, the final choice was determined based on blood or pus culture results. Surgical incision and drainage is the traditional treatment for AST when an abscess has formed. Surgical complications include damage to tissues in the area, such as the parathyroid glands and the recurrent laryngeal nerve. Previous studies state that needle aspiration under US guidance is an effective and safe alternative to surgical therapy for AST.11,22,23 However, the available studies were mostly case reports. In this study, we found that the length of hospitalization with antibiotics and US-guided needle aspiration treatment was the shortest among all treatments. Shorter hospital stay results in reduced complications and costs. However, surgical drainage is necessary in severe cases of AST or in cases of deterioration despite antimicrobial therapy and/or needle aspiration, as was highlighted in one of our cases.
AST is prone to recurrence if anatomical anomalies are left untreated.24–26 Thus, after controlling the acute infection, patients with no apparent cause of AST should be examined for anatomical anomalies. PSF is the major route of infection in AST. During the early or acute inflammatory phase, swelling of the mucosa and adjacent tissue can cause partial or complete obstruction of the fistula tract, leading to a low rate of PSF diagnosis using CT or US.20,26 While laryngoscopy or esophagography are more sensitive after controlling the acute infection.6,20,26 In the present study, fistula formation was not detected in two cases during the acute inflammatory stage; however, a fistula was detected upon re-examination during the quiescent stage. In a study of 12 cases of AST in children with PSF who were treated with surgical excision, the recurrence rate was 25%.27 The reported success rate of endoscopic management ranges from 78% to 100%.28–30 Furthermore, in the present study, one of the three patients treated with surgery recurred during follow-up. The patient who was treated with endoscopic obliteration had no recurrence. These results suggest that inadequate excision may increase the risk of recurrence after surgery. Notably, in the current study, three cases of recurrence were diagnosed with anatomical anomalies. However, the other two patients diagnosed with PSF, one patient with a branchial cleft cyst, and the remaining seven patients with unspecified anatomic anomalies received no additional treatment and yet had no recurrence. A study suggested that some patients with a very fine fistula may undergo spontaneous cure.25 Therefore, it is recommended that anatomic anomalies be identified by esophagography or laryngoscopy in patients with recurrence during the quiescent stage. Once anatomic anomalies are identified, surgical fistulectomy or nonsurgical obliteration of the fistula should be performed to prevent recurrence.
This study had some limitations. It was a retrospective analysis of a single-center study based on a small sample size, which may have caused some deviations in the results. Therefore, a multicenter study on pediatric AST should be conducted in the future. In addition, the present study did not include long-term follow-up for some patients, which is necessary for confirmation of our proposed management strategy for AST.
In conclusion, AST is a rare but potentially life-threatening bacterial infection of the thyroid gland that requires immediate medical attention. AST should be suspected when patients show signs and symptoms of AST. Therefore, regular thyroid monitoring by ultrasonography is strongly advised in inconclusive cases. In children with AST, when abscesses have formed, US-guided needle aspiration performed as early as possible, with empirical antibiotic therapy, is considered safe, effective, and minimally invasive; however, surgery may be necessary, particularly in the presence of complications. Streptococcus spp. and Staphylococcus spp. are the most common pathogens responsible for AST. Empirical antibiotics should cover both pathogenic bacteria. The prognosis of most AST cases is good, with recurrence occurring in a few cases. It is strongly recommended that patients with recurrence be examined for anatomical abnormalities, with the initiation of radical treatment.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This study was supported by a research project from Mianyang Central Hospital (No.2021FH003).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Al-Dajani N, Wootton SH. Cervical lymphadenitis, suppurative parotitis, thyroiditis, and infected cysts. Infect Dis Clin North Am. 2007;21(2):523–viii. doi:10.1016/j.idc.2007.03.004
2. Sen S, Ramakant P, Paul MJ, Jennifer A. Acute suppurative thyroiditis secondary to urinary tract infection by E. coli: a rare clinical scenario. BMJ Case Rep. 2016;2016:bcr2015213231. doi:10.1136/bcr-2015-213231
3. Singh G, Jaiswal R, Gulati N, Campbell Granieri E. A case of idiopathic thyroid abscess caused by Escherichia coli. J Community Hosp Intern Med Perspect. 2019;9(2):159–161. doi:10.1080/20009666.2019.1586277
4. Yu EH, Ko WC, Chuang YC, Wu TJ. Suppurative Acinetobacter baumanii thyroiditis with bacteremic pneumonia: case report and review. Clin Infect Dis. 1998;27(5):1286–1290. doi:10.1086/514998
5. Halenka M. Non-surgical management of thyroid abscess with ultrasound-guided fine-needle application of an antibiotic followed by sclerotization with absolute alcohol. Minerva Endocrinol. 2013;38(3):281–287.
6. Paes JE, Burman KD, Cohen J, et al. Acute bacterial suppurative thyroiditis: a clinical review and expert opinion. Thyroid. 2010;20(3):247–255. doi:10.1089/thy.2008.0146
7. Pereira O, Prasad DS, Bal AM, McAteer D, Abraham P. Fatal descending necrotizing mediastinitis secondary to acute suppurative thyroiditis developing in an apparently healthy woman. Thyroid. 2010;20(5):571–572. doi:10.1089/thy.2009.0145
8. Brown J, Nguyen HH, Cohen SH. A pain in the neck: thyroid abscess. Am J Med. 2014;127(3):e5–e6. doi:10.1016/j.amjmed.2013.09.036
9. Har-el G, Sasaki CT, Prager D, Krespi YP. Acute suppurative thyroiditis and the branchial apparatus. Am J Otolaryngol. 1991;12(1):6–11. doi:10.1016/0196-0709(91)90067-p
10. Lafontaine N, Learoyd D, Farrel S, Wong R. Suppurative thyroiditis: systematic review and clinical guidance. Clin Endocrinol. 2021;95(2):253–264. doi:10.1111/cen.14440
11. Yang H, Li D, Ye X, et al. Aspiration with or without lavage in the treatment of acute suppurative thyroiditis secondary to pyriform sinus fistula. Arch Endocrinol Metab. 2020;64(2):128–137. doi:10.20945/2359-3997000000207
12. Kawanaka M, Sugimoto Y, Suehiro M, Fukuchi M. Thyroid imaging in a typical case of acute suppurative thyroiditis with abscess formation due to infection from a persistent thyroglossal duct. Ann Nucl Med. 1994;8(2):159–162. doi:10.1007/BF03165022
13. Jasmi AY, Rohaizak Meah FA, Sulaiman BT. Typhoid thyroiditis. Med J Malaysia. 1998;53(1):109–111.
14. Har-el G. Persistent third branchial apparatus. J Pediatr Surg. 1993;28(11):1525–1526. doi:10.1016/0022-3468(93)90495-7
15. McLaughlin SA, Smith SL, Meek SE. Acute suppurative thyroiditis caused by Pasteurella multocida and associated with thyrotoxicosis. Thyroid. 2006;16(3):307–310. doi:10.1089/thy.2006.16.307
16. Siddiqui N, Deletic N, Raal F, Mohamed F. Acute suppurative thyroiditis secondary to Escherichia coli infection. Eur J Case Rep Intern Med. 2021;8(11):003009. doi:10.12890/2021_003009
17. Dale DC, Bolyard AA, Marrero T, et al. Neutropenia in glycogen storage disease Ib: outcomes for patients treated with granulocyte colony-stimulating factor. Curr Opin Hematol. 2019;26(1):16–21. doi:10.1097/MOH.0000000000000474
18. AlYousef MK, Al-Sayed AA, Al Afif A, Alamoudi U, LeBlanc JM, LeBlanc R. A pain in the neck: salmonella spp. as an unusual cause of a thyroid abscess. A case report and review of the literature. BMC Infect Dis. 2020;20(1):436. doi:10.1186/s12879-020-05161-w
19. Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab. 2003;88(5):2100–2105. doi:10.1210/jc.2002-021799
20. Masuoka H, Miyauchi A, Tomoda C, et al. Imaging studies in sixty patients with acute suppurative thyroiditis. Thyroid. 2011;21(10):1075–1080. doi:10.1089/thy.2010.0366
21. Yedla N, Pirela D, Manzano A, Tuda C, Lo Presti S. Thyroid abscess: challenges in diagnosis and management. J Investig Med High Impact Case Rep. 2018;6:2324709618778709. doi:10.1177/2324709618778709
22. Ilyin A, Zhelonkina N, Severskaya N, Romanko S. Nonsurgical management of thyroid abscess with sonographically guided fine needle aspiration. J Clin Ultrasound. 2007;35(6):333–337. doi:10.1002/jcu.20288
23. Yildar M, Demirpolat G, Aydin M. Acute suppurative thyroiditis accompanied by thyrotoxicosis after fine-needle aspiration: treatment with catheter drainage. J Clin Diagn Res. 2014;8(11):ND12–ND14. doi:10.7860/JCDR/2014/9550.5186
24. Taylor WE, Myer CM, Hays LL, Cotton RT. Acute suppurative thyroiditis in children. Laryngoscope. 1982;92(11):1269–1273. doi:10.1288/00005537-198211000-00009
25. Miyauchi A, Matsuzuka F, Kuma K, Takai S. Piriform sinus fistula: an underlying abnormality common in patients with acute suppurative thyroiditis. World J Surg. 1990;14(3):400–405. doi:10.1007/BF01658538
26. Sheng Q, Lv Z, Xiao X, et al. Diagnosis and management of pyriform sinus fistula: experience in 48 cases. J Pediatr Surg. 2014;49(3):455–459. doi:10.1016/j.jpedsurg.2013.07.008
27. Sai Prasad TR, Chong CL, Mani A, et al. Acute suppurative thyroiditis in children secondary to pyriform sinus fistula. Pediatr Surg Int. 2007;23(8):779–783. doi:10.1007/s00383-007-1939-1
28. Parida PK, Gopalakrishnan S, Saxena SK. Pediatric recurrent acute suppurative thyroiditis of third branchial arch origin–our experience in 17 cases. Int J Pediatr Otorhinolaryngol. 2014;78(11):1953–1957. doi:10.1016/j.ijporl.2014.08.034
29. Chen EY, Inglis AF, Ou H, et al. Endoscopic electrocauterization of pyriform fossa sinus tracts as definitive treatment. Int J Pediatr Otorhinolaryngol. 2009;73(8):1151–1156. doi:10.1016/j.ijporl.2009.04.019
30. Sun JY, Berg EE, McClay JE. Endoscopic cauterization of congenital pyriform fossa sinus tracts: an 18-year experience. JAMA Otolaryngol Head Neck Surg. 2014;140(2):112–117. doi:10.1001/jamaoto.2013.6105
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
