Back to Journals » Cancer Management and Research » Volume 12
Clinical Analysis and Prognostic Significance of Hepatitis B Virus Infections with Diffuse Large B-Cell Lymphoma
Authors Kang X , Bai L, Han C, Qi X
Received 31 December 2019
Accepted for publication 28 March 2020
Published 24 April 2020 Volume 2020:12 Pages 2839—2851
DOI https://doi.org/10.2147/CMAR.S244381
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Xindan Kang,1,2 Li Bai,1 Chun Han,1 Xiaoguang Qi1
1Department of Oncology, The First Medical Center, Chinese People’s Liberation Army General Hospital, Beijing 100853, People’s Republic of China; 2Department of Graduate Administration, Chinese PLA General Hospital/Medical School of Chinese PLA, Beijing, People’s Republic of China
Correspondence: Li Bai Email [email protected]
Background: China is a high endemic area for the hepatitis B virus (HBV). The studies established the epidemiology between HBV and diffuse large B-cell lymphoma (DLBCL), but further research is necessary to clarify the potential link between HBV and DLBCL.
Patients and Methods: A total of 319 patients diagnosed with DLBCL were recruited as cases at First Medical Centre of Chinese PLA General Hospital from September 2010 to December 2018. During the same time, two age- and sex-matched controls were selected for each case, and the control groups comprised of 319 patients with non-hematological malignancy and 319 subjects with non-malignant conditions. Relative risk of developing DLBCL among individuals tested positive for hepatitis B surface antigen was calculated. After that, we retrospectively analyzed clinical data from DLBCL patients with different HBV infection statuses.
Results: The HBV infection rate of patients with DLBCL (11.60%) was significantly higher than the other two control groups (5.02% and 4.08%), indicating the risk of DLBCL may increased in HBV infections. Meanwhile, this study demonstrated an independent association between HBV infection and poorer clinical outcomes.
Conclusion: Our study demonstrated that HBV infection may play an important role in the pathogenesis of DLBCL and show poor outcomes in HBV-endemic China.
Keywords: case–control study, diffuse large B-cell lymphoma, hepatitis B viral
Introduction
Diffuse large B-cell lymphoma (DLBCL), the most common pathological type of non-Hodgkin’s lymphoma (NHL), accounts for approximately 30%-40% of all non-Hodgkin’s disease and is slightly more common in men than women.1,2 The etiology of NHL has not been fully understood, however, multiple viral infections such as human immune deficiency virus (HIV), Epstein-Barr virus (EBV) or hepatitis B/C virus (HBV/HCV) can be recognized as the potential risk factors for developing NHL.3–7 Hermine found that treated with ribavirin in combination with peginterferon can result in complete or partial remissions for those who suffered HCV infected lymphoma of the spleen.6 Compared with HCV, the association of DLBCL with HBV has been studied much less intensively. There had no large amount of data to analyze the relationship between HBV infection and DLBCL. The risk of developing DLBCL appears to be high among patients in HBV-endemic areas suggesting that the risk could be driven by chronic antigenic stimulation by HBV in those highly endemic areas.
HBV has been discovered since 1966. With the chronic carriers at approximately 170 million, China is considered one of the leading countries for HBV prevalence.8,9 Previous studies have confirmed that the association between HBV infection and NLH. But very few literatures exclusively demonstrated the relevance of HBV infection and DLBCL in clinic characteristics and prognosis aspects in China. HBV infection caused not only the damage of the liver, but also the lymphotropic reaction, which might contribute to or promote the development of DLBCL.10,11 Several researches indicated the HBV infection individuals had an increased risk approximately 2–3 times of NHL compared with those patients without HBV infection. Further studies revealed that B-cell non-Hodgkin’s lymphoma and the HBV-endemic countries were much higher.12–17 Moreover, a few large-scale nationwide population-based researches in Taiwan and several investigations in Korean documented that HBV infection can increase the risk of NHL, especially for those with B-cell NHL.18,19
Among patients with DLBCL and co-existing HBV infection, the chemotherapy with or without combination of rituximab may cause HBV reactivation. The reason why HBV reactivation might be lymphoma therapy provides a milieu of immunosuppression. Whether HBV infection can affect prognosis of DLBCL or not is still pending. To further elucidate the difference of clinical features and potential prognostic factors between HBV related and non-HBV related DLBCL, we studied a case-control study. In this retrospective study, we try to find the association between HBV infection and DLBCL. And then, to study the clinical characteristics, pathogenesis and prognosis of DLBCL patients with or without HBV infection.
Patients and Methods
Study Design and Setting
Patient Population
This case-control study enrolled 319 DLBCL patient with CD20+ called case group records retrospectively from September 2010 to December 2018. Compared to contemporaneous patients with non-hematologic malignancy called tumor control group 1 (319 cases), except primary liver cancer. And patients with non-malignant conditions called normal control group 2 (319 cases), receiving a routine check, who were free of malignant diseases by history, physical examination, screening laboratory tests. Informed consent was obtained from all patients prior to enrollment, in accordance with the ethical code. Both tumor control group 1 and normal control group 2 were matched to the DLBCL cases by age, sex, even more date of admission. Patients in the control groups were chosen randomly to facilitate matching and all patients were diagnosed at First Medical Centre of Chinese PLA General Hospital. Because this is a retrospective study, all recruited patients were informed and verbal informed consent was acceptable and approved by the review boards of First Medical Centre of Chinese PLA General Hospital.
The clinical and laboratory characteristics of patients were collected from medical records, including age, gender, date of admission, HBV markers, indicators of HCV/HIV infection, disease stage, pathologic subtypes, indicators of other international prognostic index (IPI), lactic dehydrogenase (LDH), Eastern Cooperative Oncology Group performance status (ECOG-PS), Ann Arbor stage and the number of extranidal involvements. Those indicates were collected for statistical analysis. Peripheral blood test results were collected from all of the patients before and after chemotherapy.
Follow-Up
The progression-free survival (PFS) was calculated from the time of diagnosis to the time of relapse including death; for the patients without relapse, the time was calculated to the end of the follow-up period. Overall survival (OS) was calculated as the time from diagnosis to date of last follow up or time of death regardless of cause. For the patients who missed a follow-up visit during the period of study, the PFS and OS were calculated to the day of the last follow-up visit. Follow up began at the time of diagnosis and ended on December 2019. The median follow-up time was 32.6 months (range: 1.8 to 112.2 months).
Inclusion and Exclusion Criteria
Inclusion Criteria
- The original histopathological diagnosed in the database were confirmed by cytology or pathology using definitions of the 2008 WHO classification of lymphoma.20
- An enzyme-linked immunosorbent assay (ELISA) was used to test serum samples. The hepatitis B virus serological indicator including the hepatitis B surface antigen (HBsAg), hepatitis B surface antibody (HBsAb), hepatitis B e antigen (HBeAg), hepatitis B e antigen (HBeAb), hepatitis B core antibody (HBcAg) were performed before they treated with therapy. In addition, all patients were tested for serum HIV/HCV antibody and syphilis antibody, with the negative results.
- Patients were diagnosed with diffuse large B-cell lymphoma who underwent CHOP (cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2 and prednisone100 mg/d, CHOP) or CHOP-like (EPOCH, CHOP plus etoposide) treatment with rituximab or not as the first line.
- Patients who were treated with more than 2 courses chemotherapy regimens and more than one therapeutic assessment.
Exclusion Criteria
- Patients with a diagnosis of HCV infection, or HIV infection, or syphilis antibody positive were excluded.
- Patients with follicular lymphoma transformed to DLBCL, Burkitt lymphoma, primary central nervous system diffuse large B-cell lymphoma and those people associated with other tumors were also excluded.
- Frail patients unable to receive treatment were excluded.
- A recorded history of malignant disease.
- Molecular subtype (GCB and non-GCG of DLBCL) is unclear.
Definitions
GCB and Non-GCB of DLBCL
Immunohistochemistry (IHC) was used to divide the diffuse large B-cell lymphoma into two categories in Figure 1: the germinal center B type (GCB) and non-germinal center B type (non-GCB). According to the expression of those three kinds of proteins, including CD10, bcl6 (B-cell lymphoma 6), and MUM-1 (multiple myeloma 1).21
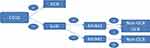 |
Figure 1 IHC to confirm the molecular classification of GCB and non-GCB. |
Inflammatory Indicators
- Neutrophil to lymphocyte ratio (NLR) = absolute count of neutrophils in peripheral blood (10^9/L)/absolute count of lymphocytes in peripheral blood (10^9/L);
- Platelet to lymphocyte ratio (PLR) = absolute count of peripheral blood platelets (10^9/L)/absolute count of peripheral blood lymphocytes (10^9/L);
- Prognostic nutritional index (PNI) = peripheral blood albumin level (g/dL) + 5* absolute value of peripheral lymphocytes (10^9/L);
The median of NLR, PLR and NLR was taken as the cut-off value. According to the cutoff value, the patients were divided into high and low groups.
Evaluation Index
The treatment efficacy was defined and classified as complete remission (CR, tumors disappeared completely maintaining for more than 4 weeks), partial remission (PR, the product of the largest tumor diameter and its vertical diameter was reduced by no less than 50% maintaining for more than 4 weeks), stable disease (SD, the product of two vertical diameters was reduced by less than 50% or increased by less than 25%, and no new lesions appeared) or progressive disease (PD, the product of two vertical diameters was increased by more than 25%, or new lesions appeared) based on the disease status at the oncology clinic visit immediately following the end of treatment and was assessed either from clinical and/or radiological findings.
Statistical Analysis
A Student’s t-test was used for continuous variables between the two groups. A χ2 test was used to assess the significance of differences in the distribution of characteristics between the cases group and control groups. Logistic regression analyses were performed to test the hypothesis that HBV infection may independently influence the occurrence of DLBCL, after controlling for confounders. The appropriate odds ratios and their 95% confidence intervals were calculated as estimates of the association between HBV infection and DLBCL. Progression-free survival (PFS) and overall survival (OS) were estimated by using the Kaplan-Meier. Univariate analysis by using Cox’s proportional hazards model was utilized to determine risk factors. Variables significantly identified in univariate analysis were tested in multivariate analysis by using Cox’s proportion hazard model. All statistical tests were two-sided, and P-values <0.05 were considered significant. Statistical analysis was performed using SPSS for Windows, version 25.
Result
Baseline Characteristics
In the study group, the cases consisted of 181 male patients (56.74%) and 138 female patients (43.26%), confirming DLBCL slightly more common in men than women. The control groups consisted of 319 patients who had other types of cancer except for primary liver cancer and 319 patients who had non-malignant conditions. In these three groups, their demographic characteristics are summarized in Table 1. There was no remarkable difference among these three groups of distribution according to sex and age. However, HBsAg seropositivity was in 37 of 319 patients (11.60%) in DLBCL compared with 16 of 319 patients (5.02%) with other types of tumors in tumor control group 1 and 13 of 319 patients (4.08%) in normal control group 2, showing a significant difference in HBsAg status. Our study results were slightly lower than Deng L’s whose findings indicate that 13.8% of DLBCL cases are HBV-associated in HBV-endemic China.22 In further study, we also identified that a higher risk of DLBCL was revealed in HBsAg-positive carriers than in the HBsAg-negative subjects. The corresponding odds ratios were 2.49 (95% CI 1.35–4.57, P=0.003) versus control group 1 and 3.09 (95% CI 1.61–5.93, P<0.001) versus control group 2 in Table 2.
 |
Table 1 Characteristics of DLBCL Cases Group and Their Age/Sex Matched Control Groups |
 |
Table 2 Odds Ratios for the Association Between HBsAg and DLBCL in Cases Group and Control Groups |
Relationship Between HBV Infection Status and DLBCL Clinical Characteristics
Classical HBV infection was defined as a serum HBsAg or HBeAg positive.23,24 Individuals with negative HBsAg assay are not completely free of the risk of reactivation of hepatitis since viral DNA and HBcAb may persist as an occult hepatitis B infection (OBI).25 That is to say, patients with HBsAg seronegativity who infected HBV previously indicates the clearance of natural HBV infection. But to a certain degree, virus replication may remain without HBsAg seropositivity. At the current time, there is no standard screening method for occult HBV infection.26 In addition, previous studies also indicated that both chronic HBV infection and acquired immunity by natural infection increased B-cell NHL.15,16 In our research, 132 of the patients were found HBV infected previously; 37 of those are HBsAg positive along with or not along with HBcAb positive patients. In other patients, there still have 95 patients with HBcAb positive. The proportion of HBsAg-positive patients was 11.60% (37/319), and the proportion of HBsAg-negative patients who were HBcAb positive was 33.69% (95/282). Before those people accepted the anti-tumor therapy, almost all of patients with positive HBsAg and/or HBcAb and some patients with only positive HBcAb were told to use antivirals (Entecavir, Tenofovir fumarate or Lamivudine, etc.).27 Only 8 in 132 patients not be treated with antivirals for various reasons, whose hepatitis B virus serological indicators were HBsAg-negative but HBcAb positive.
A total of 319 patients with biopsy proven DLBCL were identified (median age, 49 years; range, 14–65 years; 56.7% male). Patients clinical characteristics are presented in Table 3. About 45.5% of patients were found to have B symptoms and 59.2% of patients were diagnosed with advanced stage III–IV. In addition, a performance status (PS) >2 was observed in 4.1% of patients, elevated lactate dehydrogenase (LDH) levels occurred in 41.7%, high-intermediate to high-risk age adjusted international prognostic index (aaIPI) was noted in 42% of patients and extra-nodal sites involvement ≥2 were reported in 32%.
 |
Table 3 Patient Characteristics at First Presentation with DLBCL |
Inflammation often already exists in the tumor microenvironment before tumor occurrence and can be considered as proxy variables for poor performance status. Recently, using blood-based biomarkers such as high level of neutrophil-to-lymphocyte ratio (NLR), high level of platelet-to-lymphocyte ratio (PLR) and low level of prognostic nutritional index (PNI) have been recognized as poor prognostic indicators in various tumors. NLR, PLR and PNI within peripheral blood have been shown to be correlated with prognosis in various solid tumors including lung cancer, gastric cancer, and hepatocellular carcinoma.28–31 Due to the unspecific nature of these inflammatory biomarkers, they have not made their way into the clinic recently. In the present cohort, there were no detectable differences between the two groups in prognostic factors for DLBCL outcome in Table 3 (gender, incidence of B symptoms, performance status, Ann Arbor stage, LDH levels, extra-nodal sites, aaIPI score and inflammation biomarkers).
Cell of Origin and KI-67 Level in HBV-Positive and HBV-Negative of DLBCL Patients
DLBCL patients with different cells of origin have distinct outcomes. Therefore, it can be speculated that HBsAg and/or HBcAb positive may more frequent in non-GCB. And so it proved, 93 cases (93/132, 70.5%) in HBV-positive group compared with 110 (110/187, 58.8%) in HBV-negative group were classified as non-GCB, respectively (P=0.033). However, there was no significant difference between the two groups in ki67 proliferation index.
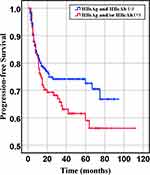 |
Figure 2 Progression-free survival of HBsAg- and HBcAb- and HBsAg or HBcAb+ groups (P= 0.086). |
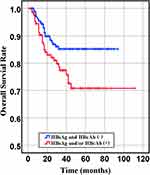 |
Figure 3 Overall survival of HBsAg- and HBcAb- and HBsAg or HBcAb+ groups (P= 0.018). |
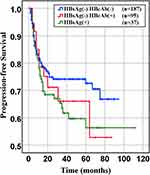 |
Figure 4 Progression-free survival of HBsAg- HBcAb-, HBsAg-HBcAb+, HBsAg+ DLBCL groups (HBsAg- HBcAb- vs HBsAg- HBcAb+, P= 0.77. HBsAg- HBcAb+ vs HBsAg+, P= 0.674). |
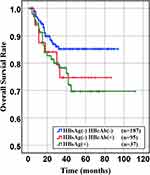 |
Figure 5 Overall survival of HBsAg- HBcAb-, HBsAg- HBcAb+, HBsAg+ DLBCL groups (HBsAg- HBcAb- vs HBsAg- HBcAb+, P= 0.022. HBsAg- HBcAb+ vs HBsAg+, P= 0.863). |
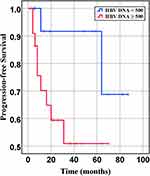 |
Figure 6 Progression-free survival of DLBCL patients with different quantities of HBV-DNA at baseline (P= 0.07). |
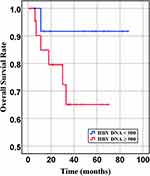 |
Figure 7 Overall survival of DLBCL patients with different quantities of HBV-DNA at baseline (P= 0.166). |
Chemotherapy Efficacy and Survival Analysis Associated with HBV Positive and HBV Negative of DLBCL Patients
In our retrospective study, there was no significant difference in the overall response rate either HBV positive or HBV-negative serology. In the first-line chemotherapeutic regimen, rituximab combined with chop-(like) in HBV-negative DLBCL patients was more frequent than those in HBV positive (97.9% vs 87.1%, P <0.001). Response rates are presented in Table 3. The median PFS and median OS for the whole group likewise not reached (Figure 2 and Figure 3). However, there was significant difference in OS rates (P =0.018; Figure 3) between HBsAg and/or HBcAb positive patients and HBsAg and HBcAb negative patients. In order to further understand the association between patients with HBV infection status and outcomes, analyses were stratified according to HBsAg-negative patients who were HBcAb positive and HBsAg-positive patients (Figure 4 and Figure 5). After that, we continue to investigate the correlation between the serum HBV-DNA quantitation at baseline and prognosis of DLBCL (Figure 6 and Figure 7).
In univariate analysis, clinical characteristics such as B symptom, stage, aaIPI, serum level of LDH, extranodal involvement and inflammatory indicators (NLR, PLR, PNI) were factors significantly affected the PFS. However, in multivariate analysis, extranodal involvement were the only independent factors that affected the PFS (HR = 1.821; 95% CI: 1.151–2.879; P= 0.010) in Table 4. For OS, clinical factors such as B symptom, stage, aaIPI, serum level of LDH, extranodal involvement, PNI and HBV infection were also found to be significant on univariate analysis. On multivariate analysis, the HBV infection status was the only factor independently affected the OS (HR =1.813, 95% CI: 1.043–3.153, P=0.035) in Table 5. This result was similar to Huang’s results.27
 |
Table 4 Univariate and Multivariate Cox Regression Analyses of Progression-Free Survival in Relation to HBV Infection |
 |
Table 5 Univariate and Multivariate Cox Regression Analyses of Overall Survival in Relation to HBV Infection |
Discussion
In the first phase of our study, 957 patients have been collected. The HBsAg-positive infection rate was 11.60% (37/219) higher than the other control groups. It is also higher than the reported HBsAg-positive rate in the general Chinese population (7.2%).32 Meanwhile, we confirmed that patients had a significant higher rate of seropositivity for HBsAg in DLBCL cases compared with the other control groups (OR 2.49, 95% CI 1.35–4.57 and OR 3.09, 95% CI 1.61–5.03). In Xi Zhou’s study, they indicated an increased OR of DLBCL with HBsAg-positive patients (OR 2.69, 95% CI: 1.88–3.86, P<0.01) in high HBV prevalence countries.16 Also, Zhou Xiang revealed that aggressive B-NHL like DLBCL exhibited significantly higher HBV prevalence than indolent B-NHL like follicular lymphoma.33 Previous studies have reported that the HBV infected individuals’ risk of developing NHL is around 2–3 folds greater than the risk of general population, far more in DLBCL.7,34 Our findings are similar to theirs. The HBV infection rate in DLBCL is significantly higher than that in patients with other tumor disease and in the general population, suggesting the impact of a higher HBV infection rate in patients with DLBCL cannot be overlooked. Whether this means a screening of retroperitoneal masses and swollen lymph nodes in chronic HBV infected population might help an early diagnosis of DLBCL.
Looking at the mechanism through the HBV infection individuals had an increased risk of DLBCL compared with those patients without HBV infection, we try to find the possible mechanism for HBV infection-causing DLBCL. Has following several aspects specifically: 1) The HBV infection belongs to systemic diseases, not just liver disease. In response to sustained viral antigen stimulation, lymphomagenesis occurs when inflammatory responses initiate signaling for B cells proliferate and induce oxidative damage to the cell.35 This hypothesis has been confirmed by perihepatic lymph node enlargement in patients with chronic hepatitis B.36 The low level of HDL-cholesterol induced reactive oxygen species overexpression in DLBCL patients, the investigators found. However, the HDL-cholesterol plays an anti-inflammatory role by inhibiting oxidative damage.35,37 Our study also supported this mechanism by elevated inflammation biomarkers. 2) HBV-specific nucleic acid sequences have been detected in peripheral blood mononuclear cells and in the hematopoietic tumor cells,38 which may also result in chronic stimulation of B cells. Our study demonstrated that the HBsAg-negative group was associated with an earlier onset age (50±10.9 years for HBsAg and/or HBcAb positive vs 47±13.6 years for HBsAg and HBcAb negative, P=0.008), which is in contrast with the result of an earlier relevant study.39 Most likely because a strong emphasis on hepatitis B vaccination after the 90s, HBV infection might mainly occur in elderly patients. It causes HBsAg and/or HBcAb positive patients’ age distribution older than HBsAg and HBcAb negative patients’ age. It may have obscured the truth of patients with HBV infection still have an earlier onset age. For the further improvement, a subgroup analysis based on age has been carried out. Unfortunately, due to the small sample size, there were no statistically significant differences that HBsAg-positive DLBCL patients group has an earlier age of disease onset (42±10.9 years for HBsAg-positive vs 47±13.6 years for HBsAg and HBcAb negative, P=0.956). On the other hand, HBsAg positivity and high level of HBV-DNA seem to be the cause of poor prognosis, although the differences were not statistically significant.
As a result of hepatitis B vaccination, it might become an effective measure to control HBV infection after the 90s.40 In China, HBsAg prevalence has been consistently low. Even though there was a slight difference about HBV-positive rates in DLBCL patients reported by different literatures, these results all showed that patients with DLBCL had a higher HBV-positive rate. Consequently, increasing vaccination against HBV not only reduce the risk of liver cancer but also DLBCL. For those DLBCL patients infected with HBV, HBV reactivation is a serious complication for lymphoma patients who are being treated with rituximab containing regimen. The risk is higher for patients who are HBsAg-positive at presentation, and ranges from 26% to 53%, while the risk is approximately 2% to 20% for HBcAb positive patients. In our study, there are three of HVB positive patients (all come from HBsAg-positive) experience HBV reactivation with a 10-folds or more increase in the HBV-DNA when compared to their lowest level. This finding is relatively consistent with the result reported in the study of Al-Mansour M M.41 During continuous antiviral therapy, all of them were under control through reducing dose and extend interval of chemotherapy. So far, one of them was alive still and no recurrent are observed after received eight cycles of chemotherapy. The other two patients have poor outcomes who died a few months (7 months and 11.3 months, respectively) after anti-tumor therapy.
This study demonstrated that clinician combined rituximab with chemotherapy as the first-line chemotherapeutic regimen more cautiously in HBV-positive DLBCL patients (87.1% vs.97.9%, P<0.001). Consequently, those patients had poorer PFS and OS in DLBCL patients with HBV patients. One of the reason may be the percentage of patients receiving rituximab in the HBV-negative group was higher compared with that in HBV-positive group. The other reason is worse pathology patterns. Literature states that DLBCL patients with non-GCB subtype have an inferior prognosis.21 We identified 70.5% in HBV-positive group and 58.8% in both HBV-negative group as non-GCB subtype. In addition, almost all of patients with HBsAg serum positive or HBcAb serum positive were treated with anti-viral therapy during accepted anti-tumor therapy. But it still leads a worse prognosis than one without HBV negative (Figure 2 and Figure 3).15 One possible explanation might be that the anti-viral treatment can effectively reduce the HBV DNA but not on HBsAg. It means HBV infection leads to a poorer prognosis and poorer response to standard chemotherapy, especially when HBsAg positivity and HBV reconstructed active level index cause more severe outcomes. Therefore, to improve the chemotherapy efficacy and the patients’ prognosis, it is necessary for DLBCL patients with a history of hepatitis to monitor the HBV status, HBV-DNA copy number and accept anti-virus treatment during diagnosis and treatment.
Conclusion
This study indicates that the risk of DLBCL is increased in HBV infections, which may be associated with many unfavorable clinical characteristics. It is also an unfavorable prognostic factor for PFS and OS in DLBCL patients, especially among the HBsAg seropositivity and high HBV-DNA copy number populations. We should pay more attention on those patients. It is highly recommended that patients be treated with antivirals through the entire chemotherapy and more intensive therapies might overcome the unfavorable outcome of these patients.
Abbreviations
HBV, hepatitis B virus; DLBCL, diffuse large B-cell lymphoma; NHL, non-Hodgkin lymphoma; HIV, human immunodeficiency virus; EBV, Epstein-Barr virus; ELISA, an enzyme-linked immunosorbent assay; IHC, immunohistochemistry; IPI, international prognostic index; aaIPI, age-adjusted international prognostic index; LDH, lactic dehydrogenase; ECOG-PS, Eastern Cooperative Oncology Group performance status; GCB, the germinal center B type; Non-GCB, non-germinal center B type; PFS, progression-free survival; OS, overall survival; HBsAg, hepatitis B surface antigen; HBsAb, hepatitis B surface antibody; HBcAb, hepatitis B core antibody; HBeAg, hepatitis B e antigen; HBeAb, hepatitis B e antibody; PCR, polymerase chain reaction; OR, odds ratio; OBI, occult hepatitis B infection; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Anderson J, Armitage J, Berger F, et al. A clinical evaluation of the International lymphoma study group classification of non-hodgkin’s lymphoma: a report of the non-hodgkin’s lymphoma classification project. Blood. 1997;89(11):3909–3918.
2. Mörth C, Valachis A, Sabaa AA, et al. Autoimmune disease in patients with diffuse large B-cell lymphoma: occurrence and impact on outcome. Acta Oncol (Madr). 2019;1–8.
3. Dalia S, Chavez J, Castillo JJ, et al. Hepatitis B infection increases the risk of non-Hodgkin lymphoma: a meta-analysis of observational studies. Leukres. 2013;37(9):1107–1115. doi:10.1016/j
4. He H, Zhai M, Zhang D, et al. Clinical significance of detecting serum label of hepatitis B virus in NHL patients. J Chinese Clin Med. 2003;4:8–10.
5. Kuniyoshi M, Nakamuta M, Sakai H, et al. Prevalence of hepatitis B or C virus infections in patients with non-Hodgkin’s lymphoma. J Gastroenterol Hepatol. 2001;16:215–219. doi:10.1046/j.1440-1746.2001.02406.x
6. Hermine O, Lefrère F, Bronowicki JP, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of Hepatitis C virus infection. N Engl J Med. 2002;347(2):89–94. doi:10.1056/NEJMoa013376
7.. Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011;117(6):1792–1798.
8.. Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. doi:10.1056/NEJM199712113372406
9.. Li YH, He YF, Jiang WQ, et al. Lamivudine prophylaxis reduces the incidence and severity of hepatitis in hepatitis B virus carriers who receive chemotherapy for lymphoma. Cancer. 2006;106:1320–1325. doi:10.1002/cncr.21701
10. Kwok RM, Tran TT. Hepatitis B and risk of non–hepatocellular carcinoma malignancy. Clin Liver Dis. 2016;S1089326116300502.
11. Coluccio C, Begini P, Marzano A, et al. Hepatitis B in patients with hematological diseases: an update. World J Hepatol. 2017;25:8–18.
12. Qi Z, Wang H, Gao G. Association of risk of non-Hodgkin’s lymphoma with hepatitis B virus infection: a meta-analysis.. Int J Clin Exp Med. 2015;8(12):22167.
13. Tung‐Hung SU, Liu C-J, Tseng T-C, et al. Chronic hepatitis B is associated with an increased risk of B‐cell non‐Hodgkin’s lymphoma and multiple myeloma. Aliment Pharmacol Ther. 2019;49(5):589–598. doi:10.1111/apt.15132
14. Kim JH, Bang YJ, Park BJ, et al. Hepatitis B virus infection and B-cell non-hodgkin’s lymphoma in a Hepatitis B endemic area: a case-control study. Cancer Sci. 2002;93(5):471–477.
15. Zhou X, Wuchter P, Egerer G, et al. Role of virological serum markers in patients with both hepatitis B virus infection and diffuse large B‐cell lymphoma. Eur J Haematol. 2019;103(4):410–416. doi:10.1111/ejh.13300
16. Zhou X, Pan H, Yang P, et al. Both chronic HBV infection and naturally acquired HBV immunity confer increased risks of B-cell non-Hodgkin lymphoma[J]. BMC Cancer. 2019;19:1. doi:10.1186/s12885-019-5718-x
17. Taborelli M, Polesel J, Maurizio M. Hepatitis B and C viruses and risk of non-Hodgkin lymphoma: a case-control study in Italy. Infect Agent Cancer. 2016;11:1. doi:10.1186/s13027-016-0073-x
18. Fwu CW, Chien YC, You SL, et al. Hepatitis B virus infection and risk of intrahepatic cholangiocarcinoma and non-Hodgkin lymphoma: a cohort study of parous women in Taiwan. Hepatology. 2011;53(4):1217–1225. doi:10.1002/hep.24150
19. Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol. 2010;11(9):827–834. doi:10.1016/S1470-2045(10)70167-4
20. Sabattini E, Bacci F, Sagramoso C, et al. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102(3):83–87.
21. Hans CP. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi:10.1182/blood-2003-05-1545
22. Deng L, Song Y, Young KH, et al. Hepatitis B virus-associated diffuse large B-cell lymphoma: unique clinical features, poor outcome, and hepatitis B surface antigen-driven origin. Oncotarget. 2015;6(28):28. doi:10.18632/oncotarget.4677
23. Wu XJ, Xu CG. Analysis of HBV, HCV and EBV infections in diffuse large B cell lymphoma. Zhong Huaxue Ye Xue Za Zhi. 2016;37(9):813–816.
24. Wang F, Xu RH, Han B, et al. High incidence of hepatitis B virus infection in B-cell subtype non-Hodgkin lymphoma compared with other cancers. Cancer. 2007;109(7):1360–1364. doi:10.1002/cncr.22549
25. Elbedewy TA, Elashtokhy HEA, Rabee ES, Kheder GE. Prevalence and chemotherapy-induced reactivation of occult hepatitis B virus among hepatitis B surface antigen negative patients with diffuse large B-cell lymphoma: significance of hepatitis B core antibodies screening. J Egypt Natl Canc Inst. 2015;27(1):11–18. doi:10.1016/j.jnci.2015.01.004
26. Said Ahmed ZN. An overview of occult hepatitis B virus infection. World J Gastroenterol. 2011;17(15):1927.
27. Huang H-H, Hsiao FY, Chen HM, et al. Anti-viral prophylaxis for Hepatitis B virus is associated with improved outcome in patients with diffuse large B cell lymphoma in Taiwan-a population-based study between 2011 and 2015. 2019;4765.
28. Xiao-Bin G, Tian T, Tian X-J, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci Rep. 2015;5:12493. doi:10.1038/srep12493
29. Jingxu S, Xiaowan C, Peng G, et al. Can the neutrophil to lymphocyte ratio be used to determine gastric cancer treatment outcomes? A systematic review and meta-analysis. Dis Markers. 2016;1–10.
30. Xiao W-K, Chen D, Li S-Q, et al. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2014;14(1):117. doi:10.1186/1471-2407-14-117
31. Melchardt T, Troppan K, Weiss L, et al. Independent prognostic value of serum markers in diffuse large B-cell lymphoma in the era of the NCCN-IPI. J Natl Compreh Cancer Net. 2015;13(12):1501–1508. doi:10.6004/jnccn.2015.0178
32. Liang X, Bi S, Yang W, et al. Reprint of: epidemiological serosurvey of Hepatitis B in China—declining HBV prevalence due to Hepatitis B vaccination. Vaccine. 2013;31:J21–J28.
33. Zhou X, Wuchter P, Egerer G, et al. Serological hepatitis B virus (HBV) activity in patients with HBV infection and B‐cell non‐Hodgkin’s lymphoma. Eur J Haematol. 2020. doi:10.1111/ejh.13388
34. Marcucci F, Spada E, Mele A, et al. The association of hepatitis B virus infection with B-cell non-Hodgkin lymphoma – a review. Am J Blood Res. 2012;2(1):18–28.
35. Gaman MA, Epingeac ME, Gaman AM. The evaluation of oxidative stress and high-density lipoprotein cholesterol levels in diffuse large B-cell lymphoma. Rev Chim. 2019;70:977–980. doi:10.37358/RC.19.3.7043
36. Ko Y-L, Sun C-S, Chung K-M, et al. Manifestations of perihepatic lymph nodes in acute flare of chronic Hepatitis B: association with HBeAg status and with HBeAg seroconversion. PLoS One. 2015;10(2):e0117590. doi:10.1371/journal.pone.0117590
37. Epingeac ME, Gaman MA, Diaconu CC, Gad M, Gaman AM. The evaluation of oxidative stress levels in obesity. Revista De Chimie-Bucharest Orig Ed. 2019;70:2241–2244.
38. Galun E, Ilan Y, Livni N, et al. Hepatitis B virus infection associated with hematopoietic tumors. Am J Pathol. 1994;145(5):1001–1007.
39. Wanzhuo X, Zhou DE, Keyue HU, et al. Clinical analysis and prognostic significance of hepatitis B virus infections for diffuse large B-cell lymphoma with or without rituximab therapy. Exp Ther Med. 2013;6(1):109–114. doi:10.3892/etm.2013.1079
40. Wei XL, Qiu MZ, Jin Y, et al. Hepatitis B virus infection is associated with gastric cancer in China: an endemic area of both diseases. Br J Cancer. 2015;112(7):1283–1290. doi:10.1038/bjc.2014.406
41. Al-Mansour MM, Alghamdi SA, Alsubaie MA, et al. Negative effect of hepatitis in overall and progression-free survival among patients with diffuse large B-cell lymphoma. Infect Agent Cancer. 2018;13(1):18. doi:10.1186/s13027-018-0190-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
