Back to Archived Journals » Journal of Vascular Diagnostics and Interventions » Volume 7
ClariVein®, mechanochemical endovenous ablation: patient selection and perspective
Authors Belramman A , Bootun R , Onida S, Davies AH, Lane TRA
Received 17 November 2018
Accepted for publication 31 July 2019
Published 2 September 2019 Volume 2019:7 Pages 1—8
DOI https://doi.org/10.2147/JVD.S167491
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Deipolyi
Amjad Belramman,1 Roshan Bootun,1,2 Sarah Onida,1,3 Alun H Davies,1,3 Tristan RA Lane1,3
1Academic Section of Vascular Surgery, Department of Surgery and Cancer, Imperial College London, London, UK; 2East of England Deanery Vascular Surgery Training Programme, Cambridge, UK; 3Imperial Vascular Unit, Imperial College Healthcare NHS Trust, London, UK
Correspondence: Tristan RA Lane
Section of Vascular Surgery, Department of Surgery and Cancer, Imperial College London, 4N12A, Charing Cross Hospital, Fulham Palace Road, London W6 8RF, UK
Email [email protected]
Abstract: The American Venous Forum and the National Institute for Health and Care Excellence recommend endothermal ablation (ETA) techniques as the first line treatment for superficial venous incompetence. However, these techniques require the use of tumescent anaesthesia prior to energy delivery, which may be a source of discomfort for the patient and can prolong procedure time. Recently, nonthermal, nontumescent (NTNTs) techniques such as mechanochemical ablation (MOCA) have been developed to address some of the negative aspects associated with ETA. This article reviews this technique from a patient selection and perspective point view.
Keywords: endovenous ablation, varicose veins, venous disease, mechanochemical ablation
Introduction
Varicose veins are a common condition affecting up to one-third of the population, with detrimental effects on quality of life (QoL).1,2 Forty thousand vein interventions are performed each year on the National Health Service (NHS) alone. Until the turn of the millennium, the traditional technique of ligation of the saphenofemoral (SFJ) or saphenopopliteal junction (SPJ) with or without vein stripping was the gold standard treatment for great and small saphenous incompetence. However, this has largely been replaced by endovenous (within the vein) thermal ablation (ETA) techniques. These endovenous techniques have dramatically changed the treatment of truncal venous reflux and are routinely performed as office-based local anaesthetic procedures. This has led to a reduction in morbidity3–5 compared to open surgery by reducing postoperative pain, providing faster recovery, improving QoL, and lowering complication rates.6,7 Long term trial follow-up has suggested that recurrence rates are lower in ETA compared to surgery.8 Therefore, both the American Venous Forum and the National Institute for Health and Care Excellence (NICE) have recommended ETA as the first line treatment for superficial venous incompetence since 20119 and 2013,10 respectively. However, these techniques require the use of tumescent anaesthesia prior to energy delivery, which can be a source of discomfort for the patient and prolongs procedure time.11 More recently, nonthermal, nontumescent (NTNTs) techniques have been developed to minimise these negative aspects associated with ETA. Mechanochemical ablation (MOCA) is one such method, with the brand name of ClariVein® (Merit Medical, Utah, USA) (Figure 1). It was developed in 2005 by Michael Tal and his colleagues and obtained a CE mark in 2010.12 In 2016, NICE issued interventional procedural guidance permitting the use of the MOCA for the treatment of varicose vein in the United Kingdom as a standard treatment.10
 |
Figure 1 The ClariVein® mechanochemical ablation catheter.Note: Photo courtesy of Merit Medical. |
The principle of this method has been reported before13 and combines mechanical abrasion of the venous wall using a rotating wire (3500 rpm) with simultaneous injection of liquid sclerosant (Figure 2). Since no heat is applied, the use of tumescent anaesthesia is not needed. The technique utilizes the standard endovenous ablation approach - modified Seldinger ultrasound guided vein puncture for cannulation, which can be with a micropuncture kit, use of an access sheath and then the MOCA catheter is passed up the vein using ultrasound guidance to ensure accurate placement of the catheter tip 2cm from the SFJ (Figures 3 and 4). The tip of the device is then unsheathed (and accurate placement confirmed) before treatment is commenced. This requires activation of the rotation motor with pullback of the catheter (at a rate of 7 seconds per cm) and instillation of sclerosant by hand using an attached syringe (Figure 5). To the patient it feels like a “buzzing”/“electric toothbrush” sensation.
 |
Figure 2 (A) Device inside the vein. (B) Mechanism action of action (Wire rotating and sclerosant injection). |
 |
Figure 3 Introduction of the catheter via sheath. |
 |
Figure 4 Ultrasound images showing tip of MOCA catheter. |
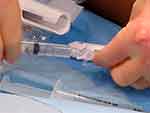 |
Figure 5 Picture showing connection of syringe containing sclerosant to treatment device. Notes: Initially a three-way tap, this has now been replaced by a built in non-return valve. |
The push-pull (push syringe and pull catheter) whilst pressing the motor button does have a learning curve but after this is reproducible and reliable.13
Recently, two animal and ex vivo studies have shown that both the mechanical and chemical components are necessary to obtain optimum treatment results.14,15
Patient selection
General considerations
The device is designed to treat refluxing truncal veins as with other endovenous ablation devices. When treating patients with the MOCA device, it is vital to assess the length of vein appropriately. As the device is 2 2/3 Fr in calibre and is compatible with an 18-gauge cannula (3.8Fr), it is tempting to puncture as distally as possible. However, whilst the device is available in 45/65/85 cm lengths, the working length is 5 cm shorter due to the taper at the handle. A 4Fr vessel is only 1.33 mm in diameter and therefore may be difficult to puncture appropriately.
Dosage and concentration of sclerosant utilised during the treatment have limited evidence. A dose finding study16 currently being undertaken has shown that 2% and 3% polidocanol liquid sclerosant have equivalent efficacy but that 1% polidocanol foam is inferior when used with MOCA. Most studies utilise either 1.5% or 2% sodium tetradecyl sulphate (STS) sclerosant or polidocanol (POL). Robust evidence on optimum concentration is lacking. The instructions for use (IFU) provide a nomogram for dosing which suggests a maximum dosage of 10 mL of sclerosant. There is no evidence on toxicity in the MOCA context and this dosage is extrapolated from sclerotherapy guidelines.
The procedure is reportedly less painful than radio-frequency ablation11,17,18 and, so, is often better tolerated than tumescent injections. However, the patient should always be warned of the unusual nature of the sensation produced by the device (the authors liken it to an electric toothbrush) as when the first segment is treated without warning, patients may flinch or move, displacing the catheter tip. Similarly, a complete lack of feedback indicates incorrect positioning and need for review.
For assessment of the treated segment patience is key, as the chemical process invoked takes time. Rapid assessment may lead to overtreatment of the proximal segment and subsequent under-treatment of distal segments due to sclerosant volume limitations.
Recurrent or phlebitic veins
Initial experience with MOCA suggested that patients with fibrotic or previously treated truncal veins were not suitable for treatment with this technique. This is due to the fact that the webs and synechiae that arise inside the vein can catch the rotating tip of the device. However, with increasing experience, it is evident that this seldom leads to significant pain or problems. If the rotating tip catches in a web, or a sclerotic valve, usually identified by a slowing of the motor and increased motor noise, this can be managed by stopping the motor then unwinding slightly in an anticlockwise direction, which frees the tip in most cases. Alternatively, a small tug (with or without a local anaesthetic injection) will release the device. In the worst cases, a small incision and dissection to the vein will allow direct surgical release. If the catheter tip does catch in the vein this should not be treated as a failure – the intimal damage causing the catching will allow ingress of sclerosant and appropriate treatment.
Superficial or small veins
Superficial and small veins need consideration as the vibration sensation can be extremely intense and can be painful. These types of veins benefit from a slower motor speed to allow comfortable treatment.
Superficial veins treated with MOCA appear to have an excellent result with a reduced risk of skin pigmentation or burn compared to endothermal techniques.
Anticoagulation
Anticoagulation is not a barrier to treatment, similar to other endovenous techniques. It does, however, reduce the risk of postoperative thrombosis. There is no evidence to suggest that MOCA has a different efficacy in anticoagulated patients.
Active ulceration and advanced disease
MOCA offers the opportunity of treating below the ulcer via a retrograde approach.19 Due to the nature of treatment, without thermal energy but with sclerosant dispersal, the sub-ulcer plexus is treated without the risk of nerve injury.
MOCA also allows treatment without tumescent injections so veins under fragile skin can be treated safely – potentially with the use of antegrade and retrograde access.
Tortuous truncal veins
Tortuous truncal veins have always been a significant hurdle; however, the steerable nature of the angled catheter tip allows traversal of most truncal veins.
Large veins
Many surgeons initially feared that endovenous thermal ablation would not be able to treat large veins effectively. However, extensive experience has shown that this apprehension is misplaced. The same appears to apply to MOCA. The catheter tip rotation diameter will easily treat diameters of 20–24 mm, especially when treated in the Trendelenburg position. Patients should be able to feel the device working – a lack of feedback indicates a need for treatment adjustment. Due to the nature of MOCA, partial ablation leads to a narrowed vein, which may be sufficient for symptomatic improvement, and as is seen in other modalities, technical success does not always indicate clinical success.
Clinical data
Prospective studies
In 2012, Elias et al published the first-in-man clinical trial on the safety and efficacy of using the ClariVein device to treat the great saphenous vein (GSV). Twenty-nine patients (30 GSVs) were recruited with an average age of 54 years. The majority of patients were Clinical-Etiological-Anatomical-Pathophysiological (CEAP) Class 2 and the average diameter of the treated vein was 8.1 mm (5.5–13 mm). 1.5% liquid STS was used as a sclerosant solution. During the procedures, all patients were free of pain and no analgesia was required. At 6 months follow-up, there was a 97.6% occlusion rate.13 Twenty-four patients attended 2-year follow-up and all treated veins were successfully occluded.20 No major adverse complications, including deep vein thromboses (DVTs), skin or nerve injury, were reported. However, it is to be noted that 77% of patients did not have advanced disease. Three patients had ecchymosis and the authors presumed that this was due to the rotating wire getting caught on the vein wall or valve cusp. The authors deemed MOCA to be safe and efficacious in the treatment of saphenous vein reflux.
A second clinical study was conducted in the Netherlands to assess the clinical efficacy of this device with 30 GSVs in 25 patients using POL (Aethoxysklerol, Kreussler Pharma, Wiesbaden, Germany) at two hospitals. The initial technical success rate was 100% but 26 of 30 GSV (87%) remained closed at 6 weeks. Three veins showed partial recanalization, one vein developed complete recanalization and nine patients had localised ecchymosis at the puncture site. There was also transient superficial phlebitis in 4 limbs. No other major adverse event was noted. Median peri-procedural maximal pain score was 4 (IQR 3–6) and the mean maximum pain on the first postoperative day was 9 (0–100 mm visual analogue scale [VAS]); patient satisfaction was high (median 8.5 [IQR 8–9] on a 10-point scale). Compared to baseline, the median venous clinical severity score (VCSS) improved significantly from 3.0 to 1.0 (p<0.001) 6 weeks after treatment.21 This study, therefore, demonstrated that MOCA, using POL, was again safe and feasible in the treatment of venous reflux. However, this study only included patients with GSV incompetence.
In order to investigate the effectiveness of the MOCA on small saphenous vein insufficiency. Boersma et al22 conducted a prospective, non-controlled, observational study in which 55 SSV reflux patients were treated using ClariVein with POL and followed up for 12 months, which was the longest follow-up in the literature at that time. Occlusion rates at 6 months and 1-year follow-up were 100% and 94%, respectively, and no major complications, including nerve injury, were noted. The median VAS peri-procedural pain score was 20 mm (IQR 20–40 mm) and the median patient satisfaction was 8 (IQR 8–9) at 6 weeks. Clinical disease severity, measured via VCSS, was significantly decreased from 3 at baseline to 1 (IQR 1–3, p<0.001) at 6 weeks. At 1-year follow up, VCSS scores were also 1 (IQR 1–2, p<0.001).
In the following year, van Eekrren23 and his colleagues evaluated 92 patients (106 limbs) undergoing MOCA for GSV insufficiency. Sixty-seven of the patients were females with a mean age of 52 years. Two concentration of POL (1.5% to 10 proximal segments and 2% for the remaining segment) were used. The median post-procedural pain score during the first 14 days after treatment was 7.5 mm (IQR 0.0–10.0 mm) on a 100 mm visual analogue scale. The median time to return to normal activities was noted as 1.0 day (IQR, 0.0–1.0 day), and the time to return to work for employees was 1.0 day (IQR, 1.0–4.0 days). Superficial thrombophlebitis (3%), induration (12%), localized haematoma (9%), and mild hyperpigmentation at the puncture site (5%) were observed. However, no major adverse events such as DVT, saphenous nerve neuralgia, and skin necrosis were noted. Technical success (defined as performing the procedure without technical problem) was achieved in 99% (105/106). A total of 93.2% of the treated veins remained obliterated at six months. Eight patients developed recanalization (4 complete, 8 partial) giving a primary closure rate of 88.2% at 1-year follow-up. Both clinical disease severity and disease-specific QoL improved significantly at the 6-month and 12-month follow-up (p<0.001). At 3-year follow-up, the clinical success and anatomical success were 83.1% and 86.5%, respectively. Although patient-reported health status remained significantly improved up to 36 months follow up, a significant deterioration in the venous clinical severity score (VCSS) was observed. The authors attributed this to the recurrent nature of varicose veins.24
Tumescent-based versus tumescent-less
Data from a non-randomised study of MOCA compared to radiofrequency ablation (RFA) in sixty-eight patients with GSV incompetence demonstrated significantly less postoperative pain, faster recovery, and earlier work resumption in the MOCA group. At 6 weeks, both groups had a significant improvement in health status and disease-specific QoL. Limitations of the study include its non-randomized design, the absence of criteria used for patient selection and non-inclusion of the occlusion rate. Finally, the procedural pain was not significantly different between the two groups and the authors suggested that this was because of the small sample size in the study.17
Lower pain scores with MOCA were confirmed in a randomised controlled trial (RCT) of MOCA (Clarivein) versus RFA (Venefit). One hundred and seventy patients with primary truncal venous incompetence were recruited and randomised to receive either MOCA or RFA. The primary study end point was pain level during the procedure. This was evaluated by a VAS, which demonstrated that MOCA was significantly less painful (median 15 mm [IQR: 7–36 mm]) than RFA (median 34 mm [IQR: 16–53]) (p=0.003). Patients undergoing MOCA also reported less pain on a 0 to 10 number scale (median 3 [IQR: 1–5]) than RFA (4 [IQR: 3–6.5]; p=0.002). Both the MOCA and RFA groups had similar improvement in clinical severity scores, disease-specific or generic QoL scores, and time to resume normal activities. Occlusion rates were also comparable. One case of DVT was reported in each group.11,18
Tumescent-less versus tumescent-less
Interrogation of four international registries reveals only one trial aiming to compare an endovenous tumescentless method with another. This study, the MOCCA randomised controlled trial25 (mechanochemical ablation versus cyanoacrylate adhesive in the treatment of truncal saphenous incompetence) was designed to compare the degree of pain that patients experience while receiving MOCA or CAE ablation. So far, the trial has recruited 120 patients of the target 180, and the final results of this trial are expected towards the end of 2020.
The published studies about MOCA of superficial veins are summarized in Tables 1 and 2.
 |
Table 1 Published prospective studies of mechanochemical ablation for saphenous reflux |
 |
Table 2 Published comparative studies of MOCA of superficial veins |
Complication profile
Complications after endovenous ablation are rare, and MOCA offers a different profile. Nerve injury is extremely rare due to the non-thermal nature, and very few venous thromboembolism events have been recorded in the literature. Phlebitis has been reported in equal rates to endothermal ablation. Recurrence appears to be similar to other endovenous techniques. Despite the use of sclerosant, there have been no reports of neurological events.
Advantages and disadvantages
MOCA has its own unique advantages and disadvantages in the management of superficial venous incompetence as shown in Table 3 below.
 |
Table 3 MOCA advantages and disadvantages |
Conclusion
Currently available data support the use of MOCA to ablate insufficient saphenous veins. The technique has been shown to be effective with an improved pain profile compared to radio-frequency ablation. Return to normal activity and QoL improvement are similar when compared to the gold standard of thermal ablation. Two further advantages of this technique are safety, with the apparent elimination of the risk of nerve damage when treating any below the knee segment; and that it can be used in a retrograde fashion for management of more advanced disease where it is difficult to place tumescence in an area of ulceration. High-quality randomised controlled trials with longer follow-up are required to confirm the results observed thus far.
Acknowledgements
The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health. SO is supported by an NIHR Clinical Lectureship and acknowledges support from the NIHR Imperial Biomedical Research Centre.
Disclosure
Mr Roshan Bootun reports grants from Graham-Dixon Charitable Trust and Kreussler, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Bootun R, Onida S, Lane TRA, Davies AH. Varicose veins and their management. Surg (United Kingdom). 2016. doi:10.1016/j.mpsur.2016.02.002
2. Lurie F, Creton D, Eklof B, et al. Prospective randomized study of endovenous radiofrequency obliteration (closure procedure) versus ligation and stripping in a selected patient population (EVOLVeS study). J Vasc Surg. 2003;38(2):207–214. doi:10.1016/S0741-5214%2803%2900228-3
3. Kugler NW, Brown KR. An update on the currently available nonthermal ablative options in the management of superficial venous disease. J Vasc Surg Venous Lymphat Disord. 2017;5(3):422–429. doi:10.1016/j.jvsv.2017.01.014
4. Braithwaite B, Hnatek L, Zierau U, et al. Radiofrequency-induced thermal therapy: results of a European multicentre study of resistive ablation of incompetent truncal varicose veins. Phlebology. 2013. doi:10.1258/phleb.2012.012013
5. Yang L, Wang XP, Su WJ, Zhang Y, Wang Y. Randomized clinical trial of endovenous microwave ablation combined with high ligation versus conventional surgery for varicose veins. Eur J Vasc Endovasc Surg. 2013. doi:10.1016/j.ejvs.2013.07.004
6. Subwongcharoen S, Praditphol N, Chitwiset S. Endovenous microwave ablation of varicose veins: in vitro, live swine model, and clinical study. Surg Laparosc Endosc Percutan Tech. 2009. doi:10.1097/SLE.0b013e3181987549
7. Van Den Bos RR, Milleret R, Neumann M, Nijsten T. Proof-of-principle study of steam ablation as novel thermal therapy for saphenous varicose veins. J Vasc Surg. 2011. doi:10.1016/j.jvs.2010.06.171
8. Wallace T, El-Sheikha J, Nandhra S, et al. Long-term outcomes of endovenous laser ablation and conventional surgery for great saphenous varicose veins. Br J Surg. 2018. doi:10.1002/bjs.10961
9. Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the society for vascular surgery and the American venous forum. J Vasc Surg. 2011;53(5SUPPL):2S–48S. doi:10.1016/j.jvs.2011.01.079
10. Belramman A, Bootun R, Lane TRA, Davies AH. Endovenous management of varicose veins. Angiology. 2018:70(5):388–396. 000331971878004. doi:10.1177/0003319718780049
11. Lane T, Bootun R, Dharmarajah B, et al. A multi-centre randomised controlled trial comparing radiofrequency and mechanical occlusion chemically assisted ablation of varicose veins - final results of the venefit versus clarivein for varicose veins trial. Phlebology. 2017;32(2):89–98. doi:10.1177/0268355516651026
12. Tal MG, Dos Santos SJ, Marano JP, Whiteley MS. Histologic findings after mechanochemical ablation in a caprine model with use of clariVein. J Vasc Surg Venous Lymphat Disord. 2015;3(1):81–85. doi:10.1016/j.jvsv.2014.07.002
13. Elias S, Raines JK. Mechanochemical tumescentless endovenous ablation: final results of the initial clinical trial. Phlebology. 2012;27(2):67–72. doi:10.1258/phleb.2011.010100
14. Boersma D, van Haelst STW, van Eekeren RRJP, et al. Macroscopic and histologic analysis of vessel wall reaction after mechanochemical endovenous ablation using the clariVein OC device in an animal model. Eur J Vasc Endovasc Surg. 2017;53(2):290–298. doi:10.1016/j.ejvs.2016.11.024
15. Whiteley MS, Dos Santos SJ, Lee CT, Li JM. Mechanochemical ablation causes endothelial and medial damage to the vein wall resulting in deeper penetration of sclerosant compared with sclerotherapy alone in extrafascial great saphenous vein using an ex vivo model. J Vasc Surg Venous Lymphat Disord. 2017;5(3):370–377. doi:10.1016/j.jvsv.2016.12.009
16. Lam YL, Toonder IM, Wittens CHA. ClariVein® mechano-chemical ablation an interim analysis of a randomized controlled trial dose-finding study. Phlebology. 2016;31(3):170–176. doi:10.1177/0268355515599692
17. Van Eekeren RRJP, Boersma D, Konijn V, JPPM DV, Reijnen MMJP. Postoperative pain and early quality of life after radiofrequency ablation and mechanochemical endovenous ablation of incompetent great saphenous veins. J Vasc Surg. 2013;57(2):445–450. doi:10.1016/j.jvs.2012.07.049
18. Bootun R, Lane TRA, Dharmarajah B, et al. Intra-procedural pain score in a randomised controlled trial comparing mechanochemical ablation to radiofrequency ablation: the multicentre venefitTM versus ClariVein® for varicose veins trial. Phlebology. 2016;31(1):61–65. doi:10.1177/0268355514551085
19. Moore HM, Lane TR, Franklin IJ, Davies AH. Retrograde mechanochemical ablation of the small saphenous vein for the treatment of a venous ulcer. Vascular. 2014;22(5):375–377. doi:10.1177/1708538113516320
20. Elias S
21. van Eekeren RRJP, Boersma D, Elias S, et al. Endovenous mechanochemical ablation of great saphenous vein incompetence using the ClariVein device: a safety study. J Endovasc Ther. 2011;18(3):328–334. doi:10.1583/11-3394.1
22. Boersma D, Van Eekeren RRJP, Werson DAB, Van Der Waal RIF, Reijnen MMJP, De Vries JPPM. Mechanochemical endovenous ablation of small saphenous vein insufficiency using the clariVein®device: one-year results of a prospective series. Eur J Vasc Endovasc Surg. 2013;45(3):299–303. doi:10.1016/j.ejvs.2012.12.004
23. Ramon RJP, van Eekeren D, Boersma S, et al. MD, PhD a A and N, Netherland T. Mechanochemical endovenous ablation for the treatment of great saphenous vein insufficiency. Ann Acad Med Singapore. 2014;33(2):204–208. doi:10.1016/j.jvsv.2014.01.001
24. Van EekerWitte ME, Holewijn S, Van Eekeren RR, De Vries JP, Zeebregts CJ, Reijnen MMPJ. Midterm outcome of mechanochemical endovenous ablation for the treatment of great saphenous vein insufficiency. J Endovasc Ther. 2017;24(1):149–155. doi:10.1177/1526602816674455
25. Belramman A, Bootun R, Tang TY, Lane TRA, Davies AH. Mechanochemical ablation versus cyanoacrylate adhesive for the treatment of varicose veins: study protocol for a randomised controlled trial. Trials. 2018;19(1):1–8. doi:10.1186/s13063-018-2807-0
26. Bishawi M, Bernstein R, Boter M, et al. Mechanochemical ablation in patients with chronic venous disease: a prospective multicenter report. Phlebology. 2014;29(6):397–400. doi:10.1177/0268355513495830
27. Deijen CL, Schreve MA, Bosma J, et al. ClariVein mechanochemical ablation of the great and small saphenous vein: early treatment outcomes of two hospitals. Phlebology. 2016;31(3):192–197. doi:10.1177/0268355515600573
28. Kim PS, Bishawi M, Draughn D, et al. Mechanochemical ablation for symptomatic great saphenous vein reflux: a two-year follow-up. Phlebology. 2017;32(1):43–48. doi:10.1177/0268355515627260
29. Tang TY, Kam JW, Gaunt ME. ClariVein® – early results from a large single-centre series of mechanochemical endovenous ablation for varicose veins. Phlebology. 2017;32(1):6–12. doi:10.1177/0268355516630154
30. Leung C, Carradice D, Mohamed A, Hull IC, Hospitals EY. A Randomized controlled trial comparing endovenous laser ablation vs mechanochemical ablation in the treatment of super fi cial venous incompetence : the LAMA trialJ Vasc Surg. 2017;65(6):35S. doi:10.1016/j.jvs.2017.03.070
31. Khor SN, Lei J, Kam JW, Kum S, Tan YK, Tang TY. ClariVeinTM – one year results of mechano-chemical ablation for varicose veins in a multi-ethnic Asian population from Singapore. Phlebology. 2018;33(10):687–694. doi:10.1177/0268355518771225
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
