Back to Journals » Cancer Management and Research » Volume 14
CirRNA F-circEA-2a Suppresses the Role of miR-3613-3p in Colorectal Cancer by Direct Sponging and Predicts Poor Survival
Received 27 November 2021
Accepted for publication 27 April 2022
Published 25 May 2022 Volume 2022:14 Pages 1825—1833
DOI https://doi.org/10.2147/CMAR.S351518
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xueqiong Zhu
Fu Xiang, Xuedong Xu
Department of General Surgery, The First Affiliated Hospital of Dalian Medical University, Dalian City, Liaoning Province, People’s Republic of China
Correspondence: Xuedong Xu, Department of General Surgery, The First Affiliated Hospital of Dalian Medical University, No. 5 Longbin Road, Dalian City, Liaoning Province, 116000, People’s Republic of China, Tel +86-83635963-7098, Email [email protected]
Purpose: CirRNA F-circEA-2a and miR-3613-3p are two recently identified novel cancer-related RNAs. To date, their participation in colorectal cancer (CRC) is unknown. This research was therefore conducted to analyze their participation in CRC.
Patients and Methods: Plasma and paired CRC and non-tumor tissues from CRC patients (n=64) and plasma samples from healthy controls (HCs, n=64) were collected. F-circEA-2a and miR-3613-3p levels in these samples were analyzed using RT-qPCR. The 64 CRC patients were followed up for five years to analyze the prognostic value of plasma F-circEA-2a for CRC. The direct interaction between wild type F-circEA-2a (F-circEA-2a-wt) or mutant F-circEA-2a (F-circEA-2a-mut) and miR-3613-3p was analyzed through RNA-RNA pulldown assay. The role of F-circEA-2a and miR-3613-3p in regulating each other’s expression was analyzed through overexpression assay. Their roles in cell proliferation were analyzed using BrdU assay. The role of F-circEA-2a in regulating EZH2 expression was analyzed by RT-qPCR and Western blot.
Results: CircEA-2a was overexpressed in CRC, while miR-3613-3p was under-expressed in CRC. Most patients who died during the follow-up had high F-circEA-2a levels. F-circEA-2a-wt, but not F-circEA-2a-mut, directly interacted with miR-3613-3p. F-circEA-2a and miR-3613-3p showed no role in regulating each other’s expression. F-circEA-2a reduced the inhibitory effects of miR-3613-3p on cell proliferation. F-circEA-2a upregulated EZH2 at both mRNA and protein levels.
Conclusion: F-circEA-2a may suppress the role of miR-3613-3p in CRC by direct sponging and predicts poor survival.
Keywords: F-circEA-2a, miR-3613-3p, colorectal cancer, prognosis
Introduction
Originating from the cells of the inner wall of the colon or rectum, colorectal cancer (CRC) is a common solid and malignant tumor with an incidence ranking the third place worldwide.1,2 Due to poor prognosis, CRC is considered as the second most common cause of cancer-caused death.3 Surgery can be applied on CRC localized to the bowel with a cure rate of 50%.4 However, although many treatment options, such as radiation therapy, chemotherapy, and immunotherapy, can be applied on CRC patients with metastatic tumors to temporarily slow down tumor growth and spread,5 only fewer than 16% of patients can survive five years.6,7
Treatment of CRC is complicated, and certain treatment options, such as surgical resection, may cause severe side effects, such as bowel dysfunction.8 Therefore, safer and more effective approaches are required to improve patients’ survival. Therapies targeting cancer-related pathways to affect the expression and accumulation of RNAs and proteins with critical regulatory functions in cancers are emerging novel options to treat cancers.9 For instance, Wnt/β-catenin signaling can be targeted to suppress CRC progression.10 Although circRNAs and miRNAs have limited or even no protein-coding capacity, they can regulate downstream gene expression and protein accumulation to affect cancers.11,12 Therefore, circRNAs and miRNAs may be targeted to treat CRC. CirRNA F-circEA-2a and miR-3613-3p are two recently identified novel cancer-related RNAs.13–15 Their participation in CRC is unknown. Our preliminary sequencing analysis revealed the altered expression of F-circEA-2a and miR-3613-3p in CRC. In addition, F-circEA-2a and miR-3613-3p were predicted to interact with each other. Therefore, it is reasonable to hypothesize that F-circEA-2a and miR-3613-3p may form crosstalk to participate in CRC. Therefore, we analyzed their participation in CRC.
Materials and Methods
Participants and Follow-Up
Both CRC patients (n=64, 24 females and 40 males, 55.5 ± 6.6 years) and healthy controls (HCs, n=64, 24 females and 40 males, 55.4 ± 6.7 years) were enrolled at the First Affiliated Hospital of Dalian Medical University from May 2014 to August 2016 after the ethics approval was obtained from this hospital’s Ethics Committee. Recurrent patients and patients with therapies initiated three months prior to admission were excluded. People who were admitted to the hospital for systemic physiological examinations and showed normal physiological functions were also enrolled as the healthy controls. The 64 CRC patients were followed up for 60 months or until death to record their survival conditions. All participants signed the Informed consent.
Plasma and Tissue Samples
Blood (3 mL) extracted from all participants under fasting condition was transferred to EDTA tubes and centrifuged at 1200g for 12min to separate supernatant (plasma). CRC and paired non-tumor tissues were obtained from all patients either through biopsy or by collecting and dissecting resected tumors.
Cells
Human CRC cell lines RKO and HCT8 from ATCC (USA) were cultured in RPMI-1640 with 10% fetal bovine serum (FBS) at 37°C in an incubator with 5% CO2 and 95% humidity and sub-cultured at a 1:10 ratio following the instructions from ATCC.
Transfection
F-circEA-2a vector, miR-3613-3p mimic, and their negative controls (NCs) were prepared by GenePharma (Shanghai, China) and transfected or co-transfected into cells using lipofectamine 3000 to increase cellular F-circEA-2a and miR-3613-3p RNA levels. Transfection was confirmed at 48h after transfecting vectors and/or miRNAs. In cases of co-transfection, vector and miRNA mimic were transfected at the same time.
RNA Preparation
RNA samples were prepared using TRI Reagent (Sigma-Aldrich) from cells and tissues. In brief, cells and tissues were collected, washed with ice-cold PBS, and lysed in more than ten volumes of TRI Reagent for 30 min at room temperature. After that, the lysates were transferred to 1.6 mL tubes and centrifugated at 12000g for 10 min to remove cell debris. The supernatants were transferred to new 1.6 mL tubes and mixed with chloroform to remove protein contamination. After that, samples were centrifugated at 12000g, and the supernatants were transferred to new 1.6 mL tubes. The supernatants were mixed with an equal volume of methanol and centrifugated at 12000g for 30 min to precipitate RNAs. RNA samples were washed and resuspended in RNase-free water.
RT-qPCR
After analysis of concentration, purity, and integrity using 2100 Bioanalyzer, RNA samples were converted to cDNA samples and subjected to RT-qPCR to analyze F-circEA-2a and miR-3613-3p expression levels with 18s RNA as the internal control. Data analysis was performed using the 2−ΔΔCt method. To determine mature miR-3613-3p levels, RNA samples were subjected to polyadenylation prior to the preparation of cDNA samples. Primer sequences were 5’-CAACTACTGCTTTGCTGGC-3’ (forward) and 5ʹTGCTCGAATTCAGAGCACA-3’ (reverse) for F-circEA-2a; 5’-CTCCTCTAACCATGTTTACAACT-3’ (forward) and 5’-CGGTTTTGACACTCTGAACTAC-3’ (reverse) for EZH2; 5’-TAACCCGTTGAACCCCATT-3’ (forward) and 5’-CATCCAATCGGTAGTAGCG-3’ (reverse) for 18S rRNA; and 5’-ACAAAAAAAAAAGCCCAACCCTT-3’ (forward) and oligo d (T) for mature miR-3613-3p.
RNA-RNA Pulldown Assay
Biotinylated wild type F-circEA-2a (F-circEA-2a-wt) or mutant F-circEA-2a (F-circEA-2a-mut) and NC were prepared through the following steps: 1) preparation of in vitro transcripts using T7 RNA Polymerase (NEB); 2) purification of the in vitro transcripts using 70% ethanol; 3) biotin labeling of the in vitro transcripts using Biotin RNA Labeling Mix (Sigma-Aldrich); and 4) purification of the labeled RNAs using 70% ethanol. The labeled RNAs, including Bio-F-circEA-2a-wt, Bio-F-circEA-2a-mut, and Bio-NC, were transfected into cells. At 48 h of post-transfection, cells were lysed. RNA complexes were then pulled down by streptavidin-coated magnetic beads, purified, and subjected to RT-PCR to determine miR-3613-3p levels.
BrdU Assay
Transfected cells were collected at 48h after transfection and cultured again in a 24-well plate with 100,000 cells per well. BrdU reagent was added to 10 mg per mL after cells were cultured for an additional 48h. The cultured cells were incubated with anti-BrdU antibody for 2 h. At last, cells were incubated with tetramethylbenzidine for 1h, and OD values at 450 nm were measured to analyze cell proliferation.
Western Blot
Protein isolation and quantification were performed using RIPA solution and BCA assays (Invitrogen), respectively. Extracted protein samples were denatured and subjected to 5% urea-PAGE analysis. After gel transfer to membranes, proteins were incubated with EZH2 (ab186006, Abcam) and GAPDH (ab8245, Abcam) primary antibodies and IgG-HRP secondary antibody (1:1000, MBS435036, MyBioSource), and signals were developed using ECL (Thermo Fisher Scientific).
Statistical Analysis
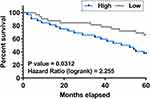
All statistical analyses were carried out using GraphPad Prism 8. Two and multiple groups were compared by Student’s t-test and ANOVA Turkey’s test, respectively. To study the role of plasma F-circEA-2a in predicting the survival of CRC patients, patients were divided into two plasma F-circEA-2a level groups (low and high, n=32), and their survival curves were plotted using follow-up data. Differences with p values smaller than 0.05 were considered statistically significant.
Results
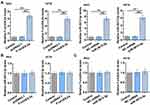
F-circEA-2a and miR-3613-3p Expression Analysis in CRC
Plasma and paired CRC and non-tumor tissues from CRC patients (n=64) and plasma samples from healthy controls (HCs, n=64) were collected, and F-circEA-2a and miR-3613-3p levels in these samples were analyzed using RT-qPCR. It was observed that F-circEA-2a was over-expressed in both plasma (Figure 1A, p<0.01) and CRC tissues (Figure 1B, p<0.01) from CRC patients. In contrast, miR-3613-3p was under-expressed in both plasma (Figure 1C, p<0.01) and CRC tissues (Figure 1D, p<0.01) from CRC patients. Correlations between F-circEA-2a and miR-3613-3p across CRC plasma and tissue samples were analyzed by Pearson’s correlation coefficient. F-circEA-2a and miR-3613-3p levels were not closely correlated with each other across CRC plasma (Figure 1E) and tissue (Figure 1F) samples.
The Role of Plasma F-circEA-2a in Predicting the Survival of CRC Patients
To study the role of plasma F-circEA-2a in predicting the survival of CRC patients, patients were divided into low and high plasma F-circEA-2a level groups (n=32), and their survival curves were plotted using follow-up data. We found that most patients who died during the follow-up had high F-circEA-2a levels (Figure 2).
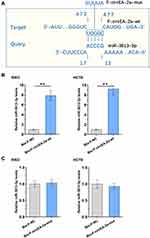
The Direct Binding of miR-3613-3p to F-circEA-2a
IntaRNA 2.0 program was applied to predict the direct binding of miR-3613-3p to F-circEA-2a. We showed that miR-3613-3p was able to bind to F-circEA-2a (Figure 3A). Mutant F-circEA-2a (F-circEA-2a-mut) was designed and indicated by blue letters (Figure 3A). The direct interaction of miR-3613-3p to wild type F-circEA-2a (F-circEA-2a-wt) or mutant F-circEA-2a (F-circEA-2a-mut) was analyzed using RNA-RNA pulldown assay in both RKO and HCT8 cell lines. F-circEA-2a-wt (Figure 3B, p<0.01), but not F-circEA-2a-mut (Figure 3C, p<0.01), directly interacted with miR-3613-3p.
Participation of miR-3613-3p and F-circEA-2a in Regulating Each Other’s Expression
The roles of miR-3613-3p and F-circEA-2a in regulating each other’s expression were analyzed with overexpression assay. The transfection of miR-3613-3p and F-circEA-2a vector in both RKO and HCT8 cell lines was confirmed by RT-qPCR (Figure 4A, p<0.01). F-circEA-2a overexpression did not regulate miR-3613-3p expression (Figure 4B). MiR-3613-3p mimic transfection also showed no role in regulating F-circEA-2a expression (Figure 4C).
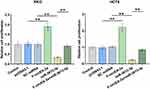
Roles of miR-3613-3p and F-circEA-2a in Regulating CRC Cell Proliferation
The participation of miR-3613-3p and F-circEA-2a in regulating CRC cell proliferation was analyzed with BrdU assay. F-circEA-2a increased cell proliferation, while miR-3613-3p decreased cell proliferation. Moreover, F-circEA-2a reduced the inhibitory effects of miR-3613-3p on cell proliferation (Figure 5, p<0.01).
Role of F-circEA-2a in Regulating EZH2 Expression
It is known that miR-3613-3p can target EZH2,14 a critical player in CRC cell proliferation.16 Therefore, the role of F-circEA-2a in regulating EZH2 expression was analyzed using RT-qPCR and Western blot. It was observed that F-circEA-2a upregulated EZH2 at both mRNA (Figure 6A, p<0.01) and protein (Figure 6B, p<0.01) levels.
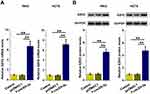 |
Figure 6 Role of F-circEA-2a in regulating EZH2 expression The role of F-circEA-2a in regulating EZH2 expression was analyzed by RT-qPCR (A) and Western blot (B) analyses. **p<0.01. |
Discussion
This study mainly explored the participation of F-circEA-2a and miR-3613-3p in CRC. We showed F-circEA-2a and miR-3613-3p levels were altered in CRC, and F-circEA-2a might absorb miR-3613-3p to suppress its role in CRC cell proliferation.
F-circEA-2a is a recently identified circRNA derived from EML4-ALK fusion gene.13 It was reported that F-circEA-2a is over-expressed in lung cancer and promotes cancer cell movement to increase cancer progression.13 The involvement of F-circEA-2a in other cancers is unclear. This study presented an increased F-circEA-2a expression in both plasma and tumor tissues from CRC patients. In addition, increased F-circEA-2a expression in two CRC cell lines resulted in increased cell proliferation. Moreover, the follow-up study analysis showed that most CRC patients who died during the follow-up had high plasma F-circEA-2a levels. Therefore, F-circEA-2a is overexpressed in CRC and may promote cell proliferation to increase cancer progression, which is related to high mortality.
Although the functions of F-circEA-2a are related to various pathways, its direct downstream target is unclear.13 We predicted and confirmed the direct interaction between F-circEA-2a and miR-3613-3p by RNA-RNA pulldown assay. MiR-3613-3p is under-expressed in breast cancer, and its overexpression suppresses cancer progression and increases the sensitivity of cancer cells to CDK4/6 inhibitor Palbociclib.14 We observed a decreased miR-3613-3p accumulation in both plasma and tissue samples from CRC patients. In addition, miR-3613-3p suppresses CRC cell proliferation. Therefore, miR-3613-3p is also a tumor suppressor in CRC. Although the downstream signal pathways of miR-3613-3p in cancer biology have been widely studied,14,15 its upstream regulators in cancer development and progression are poorly understood. We showed that miR-3613-3p directly binds to F-circEA-2a, F-circEA-2a and miR-3613-3p do not affect each other’s expression, and F-circEA-2a suppresses the role of miR-3613-3p in cell proliferation. It is known that miR-3613-3p targets EZH2,14 a critical player in CRC cell proliferation.16 Moreover, F-circEA-2a upregulates EZH2 at both mRNA and protein levels. Therefore, F-circEA-2a may sponge tumor-suppressive miR-3613-3p to play an oncogenic role by upregulating EZH2.
The study only enrolled 64 CRC patients. Therefore, F-circEA-2a and miR-3613-3p expression in CRC and the role of F-circEA-2a in predicting the survival of CRC patients should be further analyzed in future studies with more patients enrolled. Moreover, the in vivo interaction between F-circEA-2a and miR-3613-3p has not been explored and needs to be further explored in animal models.
In conclusion, F-circEA-2a is overexpressed in CRC and predicts poor survival of CRC patients. MiR-3613-3p is under-expressed in CRC. F-circEA-2a may sponge tumor-suppressive miR-3613-3p to promote CRC cell proliferation by upregulating EZH2.
Abbreviations
CRC, colorectal cancer; HCs, healthy controls.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The present study was approved by the Ethics Committee of the First Affiliated Hospital of Dalian Medical University and carried out in accordance with the World Medical Association Declaration of Helsinki. All patients and healthy volunteers provided written informed consent prior to their inclusion in the study.
Consent for Publication
All authors have read and approved the final manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, data acquisition, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the manuscript; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–164. doi:10.3322/caac.21601
2. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89–103. doi:10.5114/pg.2018.81072
3. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713–732. doi:10.1038/s41575-019-0189-8
4. Brouwer NPM, Bos A, Lemmens V, et al. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer. 2018;143(11):2758–2766. doi:10.1002/ijc.31785
5. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669–685. doi:10.1001/jama.2021.0106
6. Kather JN, Krisam J, Charoentong P, et al. Predicting survival from colorectal cancer histology slides using deep learning: a retrospective multicenter study. PLoS Med. 2019;16(1):e1002730. doi:10.1371/journal.pmed.1002730
7. van Zutphen M, Kampman E, Giovannucci EL, van Duijnhoven FJB. Lifestyle after colorectal cancer diagnosis in relation to survival and recurrence: a review of the literature. Curr Colorectal Cancer Rep. 2017;13(5):370–401. doi:10.1007/s11888-017-0386-1
8. Hashiguchi Y, Muro K, Saito Y, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1–42. doi:10.1007/s10147-019-01485-z
9. Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5(1):22. doi:10.1038/s41392-020-0116-z
10. Cheng X, Xu X, Chen D, Zhao F, Wang W. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed Pharmacother. 2019;110:473–481. doi:10.1016/j.biopha.2018.11.082
11. Hicks CC, Cohen PJ, Graham NAJ, et al. Harnessing global fisheries to tackle micronutrient deficiencies. Nature. 2019;574(7776):95–98. doi:10.1038/s41586-019-1592-6
12. Sun Z, Shi K, Yang S, et al. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer. 2018;17(1):147. doi:10.1186/s12943-018-0897-7
13. Tan S, Sun D, Pu W, et al. Circular RNA F-circEA-2a derived from EML4-ALK fusion gene promotes cell migration and invasion in non-small cell lung cancer. Mol Cancer. 2018;17(1):138. doi:10.1186/s12943-018-0887-9
14. Yu Y, Liao H, Xie R, et al. Overexpression of miRNA-3613-3p enhances the sensitivity of triple negative breast cancer to CDK4/6 inhibitor palbociclib. Front Oncol. 2020;10:590813. doi:10.3389/fonc.2020.590813
15. Chen C, Pan Y, Bai L, et al. MicroRNA-3613-3p functions as a tumor suppressor and represents a novel therapeutic target in breast cancer. Breast Cancer Res. 2021;23(1):12. doi:10.1186/s13058-021-01389-9
16. Fussbroich B, Wagener N, Macher-Goeppinger S, et al. EZH2 depletion blocks the proliferation of colon cancer cells. PLoS One. 2011;6(7):e21651. doi:10.1371/journal.pone.0021651
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.