Back to Journals » Cancer Management and Research » Volume 13
Circulating Tumor Cells in Esophageal Squamous Cell Carcinoma – Mini Review
Authors Shi Y, Ge X, Ju M, Zhang Y, Di X, Liang L
Received 8 September 2021
Accepted for publication 24 October 2021
Published 5 November 2021 Volume 2021:13 Pages 8355—8365
DOI https://doi.org/10.2147/CMAR.S337489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bilikere Dwarakanath
Yujing Shi,1 Xiaolin Ge,2 Mengyang Ju,3 Yumeng Zhang,4 Xiaoke Di,2 Liang Liang1
1Jurong People’s Hospital, Zhenjiang, 212400, People’s Republic of China; 2Jiangsu Provincial People’s Hospital, Nanjing, 212000, People’s Republic of China; 3Department of Radiation Oncology, Osaka University Graduate School of Medicine, Suita, 5650871, Japan; 4Nanjing Medical University, Nanjing, 212000, People’s Republic of China
Correspondence: Liang Liang
Jurong People’s Hospital, Huayang Town, Jurong City, People’s Republic of China
Tel +86 511-8730 0705
Email [email protected]
Abstract: Esophageal cancer has high incidence and mortality rates and a low five-year survival rate of < 15% owing to its strong capabilities of invasion, relapse and metastasis. The classic view holds that metastasis and diffusion is an advanced event during cancer progression, but recent studies show that distant diffusion of primary cancer cells may actually be an early event. Detection of circulating tumor cells (CTCs) in the circulation may indicate tumor spread, so CTCs are considered to be the key factor of metastatic cascade. In recent years, despite research progress on CTCs, there is a lack of systematic and important evidence to confirm the diagnostic, monitoring and prognostic values of CTCs in esophageal squamous cell carcinoma (ESCC). In this review, we clarify the relationship between CTC values and ESCC and provide more reliable evidence to improve the management and treatment of ESCC.
Keywords: esophageal squamous cell carcinoma, circulating tumor cells, diagnostic, prognostic
Introduction
The global incidence of esophageal cancer (EC) is increasing. EC is the seventh most common cancer worldwide and its mortality rate ranks the sixth.1 The two major histologic subtypes of EC are squamous cell carcinoma and adenocarcinoma. The former is more prevalent in East Asia, East and South Africa, and South Europe, whereas the latter is much more common in North America and other parts of Europe.2 In Asian countries, esophageal squamous cell carcinoma (ESCC) accounts for >90% of all cases of EC.3
EC patients at early stage have no typical symptoms or disease-related symptoms, so most EC patients are found with advanced disease that is often incurable or directly invades into adjacent organs and distant metastases.4,5 Radical resection of EC is still the main treatment, but because of high recurrence and mortality rates,6 postoperative symptoms such as appetite loss, early satiety and dysphagia can impair the quality of life of patients.7 Neoadjuvant chemoradiotherapy (CRT) followed by surgery or concurrent CRT (CCRT) is a standard approach for treatment of localized ECs and can improve overall survival compared to esophagectomy alone.8 However, although EC treatment has advanced greatly in recent decades, the treatment outcomes are still poor, and the five-year survival rate is less than 15%.09−13 The poor prognosis is largely due to the rapid progress of local recurrences and metastasis. Thus, tumor markers that can clarify the treatment response and early tumor recurrence/metastasis are needed. At present, the commonly-used tumor markers in clinic are serum biomarkers (eg carcinoembryonic antigen (CEA), SCC antigen, and cytokeratin 19 fragment), which have low sensitivity and specificity for early diagnosis or recurrence,14 however. Therefore, highly sensitive and specific biomarkers are urgently needed in clinical practice. There is an unmet clinical need to identify biomarkers that can sensitively detect residual disease and/or early progression in EC patients.15–17 Hence, useful non-invasive biomarkers in blood samples are considered to be valuable and convenient for the early detection and subsequent management of cancers.18
Circulating tumor cells (CTC) come from tumor cells that escape from the primary tumor, then circulate in the vascular system, and extravasate into distant organs to form metastases (Figure 1).19 CTCs were first described by Thomas Ashworth in 1869.20 The significance of CTCs in peripheral blood is extensively studied in various malignancies.21–25 The CTCs in peripheral blood are potentially correlated with tumor metastasis/recurrence of breast,21,22 prostate,23 lung,24 and colon rectal cancers.25 As the detection is relatively convenient, CTCs as liquid biopsies are an emerging noninvasive tool for cancer diagnosis, surveillance and treatment. Multiple studies prove the important diagnostic, prognostic and therapeutic implications of CTC monitoring.26–31 Nevertheless, systematic and significant evidence that validates the diagnostic, monitoring and prognostic values of CTCs in ESCC is lacking. In this review, we clarify the relationship between CTCs and the values of ESCC patients, and provide more reliable evidence that may improve the management and treatment of ESCC.
 |
Figure 1 Production and metastasis of circulating tumor cells. |
Circulating Tumor Cells Capture
CTCs are released from primary tumors or metastatic sites into the bloodstream.20 Most exfoliated tumor cells may die in the circulatory system due to physical and anatomical conditions, but some residual CTCs with particular malignant potential acquire stem cell characteristics and eventually develop metastatic tumors.32 Hence, CTCs exist as rare cells in the blood (one CTC in 106–109 blood cells).33 Isolation and subsequent quantitative and qualitative analysis across different stages of the disease prove the prognostic and predictive significance of CTCs in different malignancies.34 Methods to detect CTCs mainly include reverse transcription polymerase chain reaction (RT)-PCR, the CellSearch® detection system, isolation by size of epithelial tumor cells (ISET),35 and fluid-assisted separation technique (FAST).36 PCR-based methods are widely applied in CTC detection, but are mainly limited by the inability in visualization, enumeration or evaluation of viability of CTCs, which are destroyed during RNA isolation.37 The CellSearch® system developed and approved by the US Food and Drug Administration in 2004 still has no standard method or protocol for identification or isolation of CTCs because of the relatively low detection efficiency.3,38–41 Studies show that CTCs during metastasis often experience epithelial mesenchymal transformation, including the loss of epithelial markers and transformation into interstitial components, leading to the escape of cells from the detection system based on epithelial markers.42 Therefore, in clinical practice, the detection technology should be improved to avoid this disadvantage. ISET with higher sensitivity and specificity assesses differences in the diameters of tumors and normal blood cells, and separates CTCs and/or circulating tumor microemboli from normal blood cells with a membrane filter. FAST is also recognized in clinical practice.3,35,39 It proves FAST is capable of highly sensitive, selective, rapid and label-free isolation of CTCs from the whole blood directly without prior sample manipulation.36,43,44
Circulating Tumor Cells in ESCC Diagnosis
Most ESCC patients experiencing poor outcomes are mainly due to delayed diagnosis as a result of late presentation of symptoms or structural changes. Specific clinical symptoms and signs are usually unhelpful in making early diagnosis and results of most diagnostic studies are unreliable. Moreover, widespread screening is usually impossible.45 CTCs isolated from the peripheral blood of primary tumors are increasingly studied owing to their prognostic value in many tumors and are recognized as a key factor in tumor metastasis.46,47 However, there are few studies on CTCs in the early diagnosis of EC, because the CTCs are rarely or even not detected in healthy controls or benign disease.3,16,36,48–53
Li et al3 detected the CTC values in peripheral blood of 61 ESCC patients and 22 normal control subjects using CellSearch and ISET, and compared the sensitivity and specificity of the two methods. However, neither method detected CTCs in the healthy controls. Similarly, Qiao et al52 studied 103 peripheral blood samples from 59 ESCC patients and 25 healthy subjects, evaluated the CTC diagnostic value and optimal CTC cut-off level of overall survival (OS) and relapse-free survival (RFS) in ESCC patients. The CTC count was>3 in 24 patients (54.5%) and >5 in 14 patients (31.8%), but no CTCs were found in the blood samples of healthy subjects. Also Su et al50 studied the changes of CTCs before and after treatment and the relationship of CTCs with prognosis in 75 ESCC patients treated CCRT. It was concluded similarly that the CTC numbers of 57 EC patients were significantly higher than those of 20 healthy donors. Allard et al16 used a CellSearch® system to detect the value of peripheral blood CTC in 199 patients with benign diseases, 964 patients with metastatic cancers, and 145 healthy controls. Of the 344 healthy and non-malignant subjects, only one subject (0.3%) had > 2 CTCs per 7.5 mL of blood. In 2183 blood samples from 964 patients, the range of CTCs in patients with metastatic cancers was 0 to 23,618 CTC per 7.5 mL (mean 60 ± 693 CTC per 7.5 mL), and 36% (781 of 2183 samples) had more than 2 CTCs.
Due to the limitations of CTC detection technology, the detection rate of CTCs is often low. In clinic, special markers detected by PCR represent CTC, and related studies are conducted, bringing similar conclusions. Kaganoi et al49 detected the peripheral blood CTCs (CEA and SCCA mRNA) of 70 EC patients, and showed that 23 patients (33%) were positive for SCCA mRNA on admission, but SCCA mRNA expression was undetected in blood samples from either healthy volunteers or patients with benign disease. Andolfo et al51 studied the changes of serum CTC (copy-number variations of erbB2) in 41 ESCC patients and 34 healthy volunteers, and found that 24 ESCC patients had copy-number variations of erbB2 (CN) ≤2 (58.5%), while 17 ESCC patients had CN >2 (41.4%) with a median CN of 2 ± 5.02, but the 34 healthy control subjects showed a median erbB2 CN of 1± 0.16. Similarly, Liu et al48 discussed the serum levels of CEA mRNA gene expression in 53 EC patients before surgery, immediately after surgery, and the 3rd day postoperatively quantified by PCR, and detected the changes of CEA mRNA before and after surgery, and the prognosis of patients with preoperative and postoperative positive CEA mRNA in comparison with 22 cases of benign esophageal tumors and 30 healthy controls. It was found the cells expressing CEA mRNA were below the detection limit in 22 benign patients or 30 healthy volunteers at all three time points. Different CTC detection techniques draw the same conclusion. Choi et al36 detected CTCs in 73 ESCC cases and 31 healthy controls by FAST, and found CTCs in 3 healthy controls (9.6%) and 63 ESCC patients (86.3%), and the 63 ESCC patients had CTC ≥ 2 CTC per 7.5 mL of blood. Based on the above studies, we have reason to believe that the serum CTC contents in benign tumors and normal people are extremely low or undetectable. Therefore, when CTCs are detected, the possibility of tumor is often considered. CTC detecting can be considered before endoscopic examination for patients with suspected esophageal cancer. Combination of the two may improve diagnosis rates and even clarify tumor load (Table 1).
 |
Table 1 Circulating Tumor Cells in Esophageal Squamous Cell Carcinoma Diagnosis |
Circulating Tumor Cells in ESCC Surveillance
In the treatment of EC, evaluation of treatment effect and monitoring of tumor status are important. Traditionally, clinical evaluations and imaging (eg endoscopy, endoscopic ultrasonography, computed tomography, magnetic resonance imaging, even PET-CT) are insufficient for independent evaluation of treatment effect.54,55 With CTC monitoring before and after treatment, multiple prospective studies show that the changes of CTCs before and after treatment are related to tumor stage, lymph node metastasis status and hematogenous metastasis, and moderately reflect tumor status.26,50,53,56–58 These studies prove that CTCs as independent predictors of metastasis and recurrence may allow better stratification of patients than classic parameters (eg TNM classification) and imaging methods.
Yin et al26 detected the positive rates of peripheral blood CTCs (CEA, CK19 and Survivin) of 72 ESCC patients before and after radiotherapy using PCR, and found poor radiotherapy efficacy was significantly correlated with CTC(+) pro-radiotherapy, but not with CTC(+) pre-radiotherapy. In addition, the role of survivin in monitoring ESCC was also studied. Cao et al58 explored the serum expressions of circulating cancer cells (CCCs) (survivin mRNA) in 108 ESCC patients before and after treatment by using PCR. It was found the survivin expression of CCCs was a significant risk factor only for metastasis, and thus may be an indicator of metastasis in ESCC patients. Moreover, the cumulative recurrence rate of survivin-positive patients was significantly higher than that of negative patients. Besides, Tanaka et al53 investigated the changes of CTCs (CEA mRNA and SCC mRNA) in EC patients before and after operation by PCR, and found that CTC positive patients after chest surgery had significantly more metastatic lymph nodes and higher degree of lymph infiltration.
Similar research indicates that CTCs with markers of tumor initiating cells or cancer stem cells are responsible for recurrence and metastasis. Nakashima et al57 also detected the changes of serum CTCs (CEA mRNA) in 50 ESCC patients after anesthesia, preoperatively and postoperatively by PCR, and obtained similar results. They illustrated the incidence of lymph node metastasis in the CTC(+) expression group was significantly higher than that in the non-expression group, and the positive rate significantly increased with the increment of tumor stage. Therefore, researchers believe that the value of CTC detection is greater at the later tumor stage. Yet, the recurrence rate of CTC(+) patients was significantly higher than that of CTC(-) patients. A case study was conducted by Qiao et al56 to understand the changes of CTCs in pre- and post-chemotherapy, pre- and pro-radiotherapy, recurrence, and disease stability for ESCC. It was found the CTC changes were consistent with imaging results. Furthermore, the postoperative CTC increased, and CTC remained at a high level after radiotherapy and chemotherapy, the number of CTCs decreased after comprehensive treatment, the CTC monitored for many times was 0, and the tumor condition was stable. Finally, it was confirmed positive postoperative CTC was associated with poor prognosis of patients.
The effects of positive CTC after treatment were identified, so what is the impact of routine pre-treatment CTC test on the formulation of follow-up treatment plan? Matsushita et al59 suggested that early detection of CTCs may provide important information for treatment, including surgery, chemotherapy, and CRT. Neoadjuvant therapy may be effective in the case of pro-surgery CTC(+). Moreover, the pro-surgery CTC(+) rate in patients receiving chemotherapy alone was significantly higher in comparison with CCRT. Zhao et al60 monitored preoperative and postoperative CTCs of 115 ESCC patients, and found that CTC(-) patients upon admission were not significantly different in 2-year PFS or OS between the preoperative chemotherapy group and the non-chemotherapy group (PFS: 53.33% vs 58.06%; OS: 38.88% vs 66.63%). But for patients with CTC(+) upon admission, the 2-year PFS of patients receiving preoperative chemotherapy was significantly better than that of patients not receiving preoperative chemotherapy (71.90% vs 38.73%). Besides, Klein et al61 showed that the detection of serum CTCs in patients with early-stage cancer often indicates the occurrence of hematogenous spread prior to lymph node metastasis. Therefore, monitoring the changes of CTCs in peripheral blood of ESCC patients can dynamically understand the tumor changes and development in patients in real time, so as to develop the appropriate treatment plan and achieve a real sense of personalized treatment (Table 2).
 |
Table 2 Surveillance Value of Circulating Tumor Cells in the Treatment of Esophageal Squamous Cell Carcinoma |
Circulating Tumor Cells in ESCC Prognostic Value
The impact of CTC presence in the peripheral blood of ESCC patients on prognostic value is already evaluated.26,33,35,36,53 In fact, studies also show that the prognosis of CTC(+) patients is worse than that of CTC(-) patients. Yin et al26 confirmed the 2-year PFS of CTC(+) ESCC patients before or after radiotherapy was significantly lower than that of CTC(-) patients (mean 18.3 months (95% confidence interval [CI]: 16.7–19.9) vs 21.5 months (19.5–23.6), mean 16.3 months (14.4–18.2) vs 22.8 months (21.8–23.8). Han et al35 collected peripheral blood from 60 primary EC patients before treatment, and captured CTC ISET, with a CTC(+) ratio of 33.3%. Results showed that CTC(+) significantly shortened PFS than CTC(-) did. PFS was negatively correlated with the number of CTCs. Multivariate analyses showed that a CTC count >2 was a strong independent prognostic indicator of tumor recurrence (hazard ratio [HR] 5.63; 95% CI 1.77–17.89). Subgroup analysis of 50 patients undergoing R0 resection and postoperative adjuvant radiotherapy or chemotherapy demonstrated CTC was a strong independent prognostic indicator of tumor recurrence (HR10.70; 95% CI, 1.40–8 1.91). Similarly, Reeh et al33 evaluated 100 patients with EC peripheral blood CTC values by a CellSearch® system, and found the overall CTC detection rate was 18.0%, and the CTC counts ranged from 1 to 56 cells/7.5mL blood. Furthermore, CTC detection may be a stronger indicator of OS and RFS than pathological LN stage. The risk of tumor recurrence was 5.1 times significantly higher if CTCs were detected (HR, 5.063; 95% CI, 2.233–11.480). Patients with CTCs did significantly suffer from worse OS and RFS compared with patients without CTCs, and CTC(+) patients had significantly worse OS and RFS than patients with pN+, M0, CTC(-). As for LN-negative patients, CTC detection showed significant prognostic impact on OS and RFS. Tanaka et al53 also confirmed that disease-free survival of CTC(-) patients after chest surgery was significantly better than CTC(+) patients. Multivariate analysis found that postoperative CTC status was a significant independent prognostic factor for EC (HR=1.647; 95% CI, 1.032–2.629). But there was no significant difference in OS between patients with preoperative CTC (+) and CTC(-). However, studies also showed that CTC(+) before treatment also affected OS and PFS. Qiao et al52 confirmed the OS and PFS of patients with CTC counts >3 or >5/7.5 mL of peripheral blood before surgery were significantly shorter than those of patients with CTC counts<3 or<5/7.5 mL. Su et al50 showed that the number of CTCs pre-CCRT can be used as a significant prognostic factor for disease-specific PFS and OS in advanced ESCC patients. The number of CTC pre-CCRT showed an independent prognostic effect on disease-specific PFS and OS (HR (95% CI, 3.113 (1.427–6.791) and 1.002 (1.000–1.004), respectively).
Similar results indicating the prognostic significance of CTCs in EC patients were already published in meta-analyses. Qiao et al44 proved CTCs were significantly associated with poor OS (HR (95% CI) =1.71 (1.30, 2.12)) and PFS (1.67 (1.19, 2.15)) in 1260 EC patients. Subgroup analysis indicated that presence of CTCs was closely associated with worse OS (Asian: HR (95% CI) =1.66 (1.24–2.08), SCC: 1.66 (1.24–2.08)) and PFS (Asian: 1.63 (1.15–2.12), SCC: 1.63 (1.15–2.12)).
As for the effect of CTC on ESCC, a similar situation exists in EAC. Sclafani et al62 included obtained the peripheral blood CTC from 22 cases of locally advanced or metastatic gastroesophageal junction adenocarcinoma before and after chemotherapy and at the time of progression through CellSearch system, and clarified the changes and significance of serum CTC before and after treatment.The number of CTCs detected during chemotherapy decreased in all patients with baseline CTCs>2, which reflects the response to chemotherapy to some extent. Overall median progression-free survival was 5.5 months, and was 6.1 months in the patients with < 2 CTCs and 5.2 months in the patients with >2 CTCs (HR 1.06; 95% CI, 0.37–3.03). Median OS was 8.3 months and was 10.5 months in the patients with < 2 CTCs and 6.1 months in the patients with >2 CTCs (HR, 0.52; 95% CI, 0.18–1.50; p=0.23). Similar results were found in studies of other tumors. Tsai et al63 detected CTCs in the two groups of breast cancer xenograft mice at different growth time points, and found the proportion of CTC detection increased with tumor growth. Furthermore, the CTC number, tumor size, and vascular density all increased significantly with the time of tumor progression, while the correlation of CTCs to vascular density was more significant than to tumor size. Thus, we think noninvasive monitoring of CTC changes is of great significance for clinical practice (Table 3).
 |
Table 3 Prognostic Value of Circulating Tumor Cells in Esophageal Squamous Cell Carcinoma |
The above studies show that with continuous growth of tumors, the CTCs in peripheral blood increase continuously, and the the value of CTCs is greater and the prognosis is worse at higher tumor stage. Therefore, routine detection of CTCs during treatment and at the end of treatment can be used to clarify the tumor situation. Studies also show that changes in CTCs before and after treatment partly reflect tumor response to treatment, so relapse or metastasis can be identified by monitoring the dynamic changes of CTCs.
Circulating Tumor Cells Cutoff Values in ESCC
Due to the limitations of the existing CTCs detection methods and the extremely low content of CTCs in circulating blood, the detection rate of CTC is low. The cutoff value for the clinical impact of CTCs may vary among different detection methods. In fact, different methods produce different recovery and purity rates.50 Noticeably, the numbers of CTCs obtained by different methods should not be compared for clinical significance. In addition, the cutoff values differ among CTCs detection methods. Hence, we need to know how much value of CTC in serum is meaningful.
Su et al50 used CD45+ cell removal and positive selective flow cytometry for EpCAM and cytokeratin to detect CTCs and found CTCs ≥21/mL played independent prognostic roles. Andolfo et al51 conformed that CTC (erbB2 CN) >2 was significantly negatively correlated with survival rate in EC patients by real-time PCR. Han et al35 showed that CTCs>2 was an independent prognostic marker for PFS (HR 3.88; 95% CI 1.42–10.56) based on ISET. Multivariate analysis by Qiao et al52 showed that peripheral blood CTCs >5/7.5 mL was a strong prognostic indicator of OS (HR 12.478; 95% CI, 8.2–34.3) and PFS (HR 6.524; 95% CI, 1.2–34.3). The CTCs were detected by CellSearch system. With FAST and a threshold of CTCs≥2/7.5 mL of blood, Choi et al36 found the sensitivity and specificity to differentiate ESCC patients from healthy controls were 86.3% and 90.3%, respectively. Lee et al64 used FAST to detect CTCs in ESCC patients and the expression of CTCs with positive TWIST, and also set the cut-off CTCs≥2/7.5 mL of blood. Choi et al36 evaluated the CTCs in peripheral blood of 63 ESCC patients and 28 healthy controls before treatment by FAST, and clarified the role of CTCs in early diagnosis of EC according to the distribution of CTC value in the two groups. It was also confirmed the CTCs cut-off value of≥2/7.5mL blood will help to distinguish ESCC patients from healthy controls (Table 4).
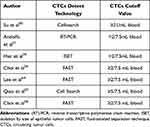 |
Table 4 Cut off Value of CTCs with Different Detection Techniques |
Clinical Use Limitations of Circulating Tumor Cells
Circulating tumor cells (CTCs) can dynamically monitor the tumor state. However, the clinical application of CTCs shall be focused on five aspects: high heterogeneity and defects with epithelial mesenchymal transformation (EMT), low detection rate, low specificity, large individual difference, and high cost, which limit the wide use of CTCs in clinic. In addition, cell-free DNA (cfDNA) can originate directly from the viable tumor cells or from CTCs by apoptosis, necrosis, autophagy, microenvironmental stress, mitotic catastrophe, trauma, and treatment procedure.65 CfDNA retains some properties of nuclear chromatin during DNA release.66 The cfDNA of tumor patients contains various somatic mutations the same as tumor gene mutations, such as oncogene and tumor suppressor gene mutations, microsatellite changes, promoter methylation and loss of heterozygosity. Therefore, cfDNA has high specificity and becomes a hit in the research of many medical disciplines.67 Nevertheless, its detection methods need to rely on cutting-edge technologies, such as second-generation sequencing, and qRT- PCR. At the same time, its detection has high requirements for testers, which limits its wide application in clinic.
Conclusions
This paper reviews the roles of CTCs in diagnosis, monitoring and prognosis of ESCC, and provides a powerful basis for early diagnosis, recurrence monitoring and prognosis evaluation of ESCC as well as the possibility for individualized treatment of ESCC. The content of CTCs in ESCC is higher than that in normal controls, and is closely related to the depth of tumor invasion, lymph node metastasis and disease stage. A large number of experiments show that CTCs are associated with poor PFS and OS especially when CTC>2. Early monitoring of CTCs may reduce the risks of recurrence and metastasis, and thus improve the prognosis of ESCC patients. Therefore, real-time non-invasive CTC monitoring is of great significance for the diagnosis and treatment of ESCC. The changes of CTC values before and after treatment can guide clinical practice and further help to realize individualized treatment. However, due to the limitations of CTC detection technology and the interference of EMT, which limit the wide use of CTCs in clinic.But for the unable to second-generation sequencing or qRT-PCR institutions, CTC detection is relatively simple.Although the roles of CTC are studied in various tumors and are guiding, further large-scale research on CTCs in ESCC is still needed to realize standardized management.
Acknowledgments
This work was supported by a grant-in-aid (2020SA00112) from the Jurong City livelihood science and technology project and supported by National Natural Science Foundation of China(82003228). Yujing Shi write this manuscript, the authors thank Xiaolin Ge for checking and reading this manuscript, Yumeng Zhang, Xiaoke Di and Mengyang Ju for search papers and chose the papers, Liang Liang for modify this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
2. Sclafani F, Smyth E, Cunningham D, et al. Assessing the incidence and clinical significance of circulating tumor cells in esophagogastric cancers. Clin Colorectal Cancer. 2014;13(2):94–99. doi:10.1016/j.clcc.2013.11.003
3. Li H, Song P, Zou B, et al. Circulating tumor cell analyses in patients with esophageal squamous cell carcinoma using epithelial marker-dependent and -independent approaches. Medicine (Baltimore). 2015;94(38):e1565. doi:10.1097/MD.0000000000001565
4. Kato H, Kuwano H, Nakajima M, et al. Usefulness of positron emission tomography for assessing the response of neoadjuvant chemoradiotherapy in patients with esophageal cancer. Am J Surg. 2002;184(3):279–283. doi:10.1016/S0002-9610(02)00932-7
5. Ikoma D, Ichikawa D, Ueda Y, et al. Circulating tumor cells and aberrant methylation as tumor markers in patients with esophageal cancer. Anticancer Res. 2007;27(1B):535–539.
6. Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg. 2014;260(2):259–266. doi:10.1097/SLA.0000000000000644
7. Elliott JA, Docherty NG, Eckhardt H-G, et al. Weight loss, satiety, and the postprandial gut hormone response after esophagectomy: a prospective study. Ann Surg. 2017;266(1):82–90. doi:10.1097/SLA.0000000000001918
8. Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281(17):1623–1627. doi:10.1001/jama.281.17.1623
9. Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412. doi:10.1016/S0140-6736(12)60643-6
10. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi:10.3322/caac.20006
11. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013. Bethesda, MD, USA: National Cancer Institute; 2016.
12. Lightdale CJ. Esophageal cancer. Am J Gastroenterol. 1999;94:20–29. doi:10.1111/j.1572-0241.1999.00767.x
13. Faivre J, Forman D, Esteve J, Gatta G. Survival of patients with oesophageal and gastric cancers in Europe. EUROCARE Working Group. Eur J Cancer. 1998;34:2167–2175. doi:10.1016/S0959-8049(98)00329-3
14. Kosugi S, Nishimaki T, Kanda T, Nakagawa S, Ohashi M, Hatakeyama K. Clinical significance of serum carcinoembryonic antigen, carbohydrate antigen 19-9, and squamous cell carcinoma antigen levels in esophageal cancer patients. World J Surg. 2004;28(7):680–685. doi:10.1007/s00268-004-6865-y
15. Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007;253(2):180–204. doi:10.1016/j.canlet.2006.12.014
16. Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi:10.1158/1078-0432.CCR-04-0378
17. Maheswaran S, Haber DA. Circulating tumor cells: a window into cancer biology and metastasis. Curr Opin Genet Dev. 2010;20:96–99. doi:10.1016/j.gde.2009.12.002
18. Van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi:10.1056/NEJMoa1112088
19. Matsushita D, Arigami T, Okubo K, et al. The diagnostic and prognostic value of a liquid biopsy for esophageal cancer: a systematic review and meta-analysis. Cancers (Basel). 2020;12(10):3070. doi:10.3390/cancers12103070
20. Galvis MM, Romero CS, Bueno TO, Teng Y. Toward a new era for the management of circulating tumor cells. Adv Exp Med Biol. 2021;1286:125–134.
21. Pierga JY, Bidard FC, Mathiot C, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a Phase II randomized trial. Clin Cancer Res. 2008;14:7004–7010. doi:10.1158/1078-0432.CCR-08-0030
22. Camara O, Rengsberger M, Egbe A, et al. The relevance of circulating epithelial tumor cells (CETC) for therapy monitoring during neoadjuvant (primary systemic) chemotherapy in breast cancer. Ann Oncol. 2007;18:1484–1492. doi:10.1093/annonc/mdm206
23. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302–6309. doi:10.1158/1078-0432.CCR-08-0872
24. Yousefi M, Ghaffari P, Nosrati R, et al. Prognostic and therapeutic significance of circulating tumor cells in patients with lung cancer. Cell Oncol (Dordr). 2020;43(1):31–49. doi:10.1007/s13402-019-00470-y
25. Cohen SJ, Punt CJ, Iannotti N, et al. Meropol NJ: prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol. 2009;20(7):1223–1229. doi:10.1093/annonc/mdn786
26. Yin XD, Yuan X, Xue JJ, Wang R, Zhang ZR, Tong JD. Clinical significance of carcinoembryonic antigen-, cytokeratin 19-, or survivin-positive circulating tumor cells in the peripheral blood of esophageal squamous cell carcinoma patients treated with radiotherapy. Dis Esophagus. 2012;25(8):750–756. doi:10.1111/j.1442-2050.2012.01326.x
27. Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi:10.1056/NEJMoa040766
28. Lucci A, Hall CS, Lodhi AK, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13(7):688–695. doi:10.1016/S1470-2045(12)70209-7
29. Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. 2011;29:1556–1563. doi:10.1200/JCO.2010.28.7045
30. Iinuma H, Watanabe T, Mimori K, et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. J Clin Oncol. 2011;29:1547–1555. doi:10.1200/JCO.2010.30.5151
31. Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–239. doi:10.1016/S1470-2045(08)70340-1
32. Reeh M, Effenberger KE, Koenig AM, et al. Circulating tumor cells as a biomarker for preoperative prognostic staging in patients with esophageal cancer. Ann Surg. 2015;261(6):1124–1130.
33. Joosse SA, Gorges TM, Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol Med. 2015;7(1):1–11. doi:10.15252/emmm.201303698
34. Liberko M, Kolostova K, Bobek V. Essentials of circulating tumor cells for clinical research and practice. Crit Rev Oncol Hematol. 2013;88(2):338–356. doi:10.1016/j.critrevonc.2013.05.002
35. Han L, Li YJ, Zhang WD, Song PP, Li H, Li S. Clinical significance of tumor cells in the peripheral blood of patients with esophageal squamous cell carcinoma. Medicine (Baltimore). 2019;98(6):e13921. doi:10.1097/MD.0000000000013921
36. Choi MK, Kim GH, H I, et al. Circulating tumor cells detected using fluid-assisted separation technique in esophageal squamous cell carcinoma. J Gastroenterol Hepatol. 2019;34(3):552–560.
37. Sleijfer S, Gratama JW, Sieuwerts AM, Kraan J, Martens JW, Foekens JA. Circulating tumour cell detection on its way to routine diagnostic implementation? Eur J Cancer. 2007;43:2645–2650. doi:10.1016/j.ejca.2007.09.016
38. Reeh M, Effenberger KE, Koenig AM, et al. Circulating tumor cells as a biomarker for preoperative prognostic staging in patients with esophageal cancer. Ann Surg. 2015;261(6):1124–1130.
39. Khoja L, Backen A, Sloane R, et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106:508–516. doi:10.1038/bjc.2011.545
40. Farace F, Massard C, Vimond N, et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer. 2011;105:847–853. doi:10.1038/bjc.2011.294
41. Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7:306–315. doi:10.1097/JTO.0b013e31823c5c16
42. Kuvendjiska J, Bronsert P, Martini V, et al. Non-metastatic esophageal adenocarcinoma: circulating tumor cells in the course of multimodal tumor treatment. Cancers (Basel). 2019;11(3):397. doi:10.3390/cancers11030397
43. Kim TH, Lim M, Park J, et al. FAST: size-selective, clog-free isolation of rare cancer cells from whole blood at a liquid-liquid interface. Anal Chem. 2017;89(2):1155–1162. doi:10.1021/acs.analchem.6b03534
44. Qiao GL, Qi WX, Jiang WH, Chen Y, Ma LJ. Prognostic significance of circulating tumor cells in esophageal carcinoma: a meta-analysis. Onco Targets Ther. 2016;31(9):1889–1897. doi:10.2147/OTT.S100005
45. Eisenberger CF, Knoefel WT, Peiper M, et al. Squamous cell carcinoma of the esophagus can be detected by microsatellite analysis in tumor and serum. Clin Cancer Res. 2003;9(11):4178–4183.
46. Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi:10.1038/nrc1098
47. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi:10.1126/science.1203543
48. Liu Z, Jiang M, Zhao J, Ju H. Circulating tumor cells in perioperative EC patients: quantitative assay system and potential clinical utility. Clin Cancer Res. 2007;13(10):2992–2997. doi:10.1158/1078-0432.CCR-06-2072
49. Kaganoi J, Shimada Y, Kano M, Okumura T, Watanabe G, Imamura M. Detection of circulating oesophageal squamous cancer cells in peripheral blood and its impact on prognosis. Br J Surg. 2004;91(8):1055–1060. doi:10.1002/bjs.4593
50. Su PJ, Wu MH, Wang HM, et al. Circulating tumour cells as an independent prognostic factor in patients with advanced oesophageal squamous cell carcinoma undergoing chemoradiotherapy. Sci Rep. 2016;6:31423. doi:10.1038/srep31423
51. Andolfo I, Petrosino G, Vecchione L, et al. Detection of erbB2 copy number variations in plasma of patients with esophageal carcinoma. BMC Cancer. 2011;11:126. doi:10.1186/1471-2407-11-126
52. Qiao Y, Li J, Shi C, et al. Prognostic value of circulating tumor cells in the peripheral blood of patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2017;3(10):1363–1373. doi:10.2147/OTT.S129004
53. Tanaka K, Yano M, Motoori M, et al. CEA-antigen and SCC-antigen mRNA expression in peripheral blood predict hematogenous recurrence after resection in patients with esophageal cancer. Ann Surg Oncol. 2010;17(10):2779–2786. doi:10.1245/s10434-010-1075-3
54. Schneider PM, Metzger R, Schaefer H, et al. Response evaluation by endoscopy, rebiopsy, and endoscopic ultrasound does not accurately predict histopathologic regression after neoadjuvant chemoradiation for esophageal cancer. Ann Surg. 2008;248:902–908. doi:10.1097/SLA.0b013e31818f3afb
55. Westerterp M, van Westreenen HL, Reitsma JB, et al. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy–systematic review. Radiology. 2005;236:841–851. doi:10.1148/radiol.2363041042
56. Qiao YY, Lin KX, Zhang Z, et al. Monitoring disease progression and treatment efficacy with circulating tumor cells in esophageal squamous cell carcinoma: a case report. World J Gastroenterol. 2015;21(25):7921–7928. doi:10.3748/wjg.v21.i25.7921
57. Nakashima S, Natsugoe S, Matsumoto M, et al. Clinical significance of circulating tumor cells in blood by molecular detection and tumor markers in esophageal cancer. Surgery. 2003;133(2):162–169. doi:10.1067/msy.2003.9
58. Cao M, Yie SM, Wu SM, et al. Detection of survivin-expressing circulating cancer cells in the peripheral blood of patients with esophageal squamous cell carcinoma and its clinical significance. Clin Exp Metastasis. 2009;26(7):751–758. doi:10.1007/s10585-009-9274-7
59. Matsushita D, Uenosono Y, Arigami T, et al. Clinical significance of circulating tumor cells in peripheral blood of patients with esophageal squamous cell carcinoma. Ann Surg Oncol. 2015;22:3674–3680. doi:10.1245/s10434-015-4392-8
60. Zhao Y, Han L, Zhang W, et al. Preoperative chemotherapy compared with postoperative adjuvant chemotherapy for squamous cell carcinoma of the thoracic oesophagus with the detection of circulating tumour cells randomized controlled trial. Int J Surg. 2020;73:1–8. doi:10.1016/j.ijsu.2019.11.005
61. Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–312. doi:10.1038/nrc2627
62. Sclafani F, Smyth E, Cunningham D, Chau I, Turner A, Watkins D. A pilot study assessing the incidence and clinical significance of circulating tumor cells in esophagogastric cancers. Clin Colorectal Cancer. 2014;13(2):94–99.
63. Tsai WS, Hung TF, Chen JY, Huang SH, Chang YC. Early detection and dynamic changes of circulating tumor cells in transgenic NeuN Transgenic (NTTg) mice with spontaneous breast tumor development. Cancers (Basel). 2021;13(13):3294. doi:10.3390/cancers13133294
64. Lee HJ, Kim GH, Park SJ, et al. Clinical significance of TWIST-positive circulating tumor cells in patients with esophageal squamous cell carcinoma. Gut Liver. 2021;15(4):553–561. doi:10.5009/gnl20194
65. Schwarzenbach H, Hoon DSB, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–437. doi:10.1038/nrc3066
66. Leng S, Zheng J, Jin Y, et al. Plasma cell-free DNA level and its integrity as biomarkers to distinguish non-small cell lung cancer from tuberculosis. Clin Chim Acta. 2018;477:160–165. doi:10.1016/j.cca.2017.11.003
67. Pinsky PF, Prorok PC, Kramer BS. Prostate cancer screening - a perspective on the current state of the evidence. N Engl J Med. 2017;376(13):1285–1289. doi:10.1056/NEJMsb1616281
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
