Back to Journals » Therapeutics and Clinical Risk Management » Volume 11
Circulating thymus and activation-regulated chemokine/CC chemokine ligand 17 is a strong candidate diagnostic marker for interstitial lung disease in patients with malignant tumors: a result from a pilot study
Authors Yamane H , Ochi N , Yamagishi T, Honda Y, Takeyama M, Takigawa N
Received 17 February 2015
Accepted for publication 2 April 2015
Published 17 June 2015 Volume 2015:11 Pages 949—959
DOI https://doi.org/10.2147/TCRM.S82995
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Hiromichi Yamane, Nobuaki Ochi, Tomoko Yamagishi, Yoshihiro Honda, Masami Takeyama, Nagio Takigawa
Department of General Internal Medicine 4, Kawasaki Medical School, Kita-ku, Okayama, Japan
Introduction: Serum Krebs von den Lungen-6 (KL-6) level is an established diagnostic marker of interstitial lung disease (ILD). However, it is also elevated in patients with non-small cell lung cancer (NSCLC). The significance of circulating thymus and activation-regulated chemokine (TARC)/CC chemokine ligand 17 (CCL17) in malignant diseases remains unknown.
Methods: We measured circulating TARC/CCL17 and KL-6 using enzyme-linked immunosorbent assay and electrochemiluminescence immunoassay, respectively, in 26 patients with malignant disease and six patients with benign lung disease (BLD). The cutoff levels were 500 U/mL for KL-6 and 450 pg/mL for TARC/CCL17. The significance of the markers was evaluated in relationship to the presence of ILD (n=10). The statistical significance was set at P<0.05.
Results: The KL-6 positive ratio was significantly higher in the patients with NSCLC (n=17) than in those with BLD. There was a significant difference in the KL-6 positive ratio between the patients with NSCLC without ILD and those with BLD without ILD. However, there were no significant differences in the TARC/CCL17 positive ratio between the patients with NSCLC and BLD or between those with NSCLC without ILD and those with BLD without ILD. The TARC/CCL17 positive ratio was significantly higher in the patients with malignancy and ILD than in those without ILD. There was also a significant difference in the TARC/CCL17 positive ratio between the patients with NSCLC without ILD and those with ILD.
Conclusion: TARC/CCL17 may be useful for the diagnosis of ILD in patients with malignancies. Confirmation of the results is warranted through a large-scale study.
Keywords: thymus and activation-regulated chemokine/CC chemokine ligand 17, Krebs von den Lungen-6, interstitial lung disease, biomarker
Introduction
Most anticancer drugs have the potential to induce pulmonary toxicity in the lung parenchyma, airways, pleura, and pulmonary circulation. Interstitial lung disease (ILD) is not uncommon, ranging from reversible benign pulmonary infiltration to life-threatening acute respiratory distress syndrome.1 The mainstay of treatment for drug-induced ILD is to first identify and eliminate the causative agent as soon as possible. The clinical patterns differ depending on the patient’s illness and drug-related factors, including the type of drug.2 Because more comprehensive insights into the mechanisms involved in the development of drug-induced ILD, aside from our knowledge regarding the risk factors, are yet to be clarified,3 the establishment of reasonable early diagnostic methods is crucial. ILD, including idiopathic pulmonary fibrosis (IPF), is strongly associated with increased lung cancer risk.4 Although chest high-resolution computed tomography (HRCT) is a powerful diagnostic tool for ILD, the risk of developing radiation-induced cancer following computed tomography screening is a concern for people without malignancies.5 Thus, convenient diagnostic markers in serum are necessary.
Krebs von den Lungen-6 (KL-6), a human MUC1 mucin, has been extensively investigated by Kohno et al.6 Surfactant protein-D (SP-D) and surfactant protein-A (SP-A) belong to the collectin subgroup of the C-type lectin super family.7,8 KL-6, SP-A, and SP-D are regarded as type II pneumocyte biomarkers and have been shown to correlate with clinical manifestations of IPF and inflammation and reflect the disease status of various ILDs.7–10
Previous studies had shown that circulating KL-6 level was elevated in patients with non-small cell lung cancer (NSCLC), although the cellular origin of circulating KL-6 and the mechanism of its increase in serum have not been clearly demonstrated.11 Thus, KL-6 may not be useful as an early diagnostic biomarker of ILD in patients with NSCLC.
Thymus and activation-regulated chemokine (TARC)/CC chemokine ligand 17 (CCL17), a functional ligand for CC chemokine receptor type 4, was reported to be elevated in the bronchoalveolar lavage fluids of patients with acute eosinophilic pneumonia (AEP).12 In addition, serum levels of TARC/CCL17 have been shown to be elevated and associated with the disease activity of AEP; allergic diseases, such as atopic dermatitis and bronchial asthma; and lymphoid malignancies, such as classical Hodgkin’s lymphoma and mycosis fungoides.13,14
TARC/CCL17 in sera was thought to reflect in situ immunological reactions induced by Th2 cytokines in various diseases. The pathological presentation of AEP sometimes closely resembles that of acute lung injury/acute respiratory distress syndrome, including its idiopathic form; acute interstitial pneumonia; and drug-induced ILD.2 Furthermore, it has been shown that TARC/CCL17 predominantly localized to the epithelial cells in a mouse model of bleomycin-induced fibrosis and in human IPF lung tissue.15 These previous reports suggest that TARC/CCL17 can be a biomarker for the detection or pretreatment of drug-induced ILD.
In this study, we evaluated the possibility of using TARC/CCL17, compared with KL-6, SP-D, and SP-A, for the diag-nosis of ILD.
Materials and methods
Study population
We recruited 32 patients (age: 51–88 years, 18 males and 14 females, mean ± standard deviation (SD): 70.5±9.4 years) between January 2013 and May 2013 who were hospitalized in the Department of General Internal Medicine 4, Kawasaki Medical School Hospital. The inclusion criteria for patient recruitment were as follows: 1) diagnosis or high clinical suspicion of malignant tumor and 2) undergoing systemic chemotherapy in cases with advanced malignant tumors. Inclusion criteria were not based on age, sex, or smoking habits.
The diagnosis of IPF was made based on history, physical examinations, pulmonary function studies, arterial blood gas analysis, and chest HRCT. Eight patients met the American Thoracic Society criteria for IPF.16 Peripheral venous blood samples that were collected from the patients on their initial admission were stored at −80°C until use and subsequently analyzed in a blinded fashion with regard to the patient’s clinical status. This study was approved by the Ethics Committee of the Kawasaki Medical University (No 1605) and conformed to the Declaration of Helsinki (1975). Written informed consent was provided by all the participants before initiating treatment. None of the patients who provided consent were excluded from this study.
Measurement of serum KL-6 concentration
The serum concentration of KL-6 was measured by a sandwich-type electrochemiluminescence immunoassay at BML, Inc., Tokyo, Japan. The cutoff level was set as the level that resulted in the optimal diagnostic accuracy, according to the previous results for healthy volunteers: 500 U/mL (mean + 2SD for healthy volunteers).17
Measurement of serum SP-D, SP-A, and TARC/CCL17 concentrations
Commercially available specific kits were used to measure the level of each marker, according to the protocol of each manufacturer. Sandwich-type enzyme-linked immunosorbent assay (ELISA) kits (Human SP-D Quantikine ELISA Kit; R&D Systems, Inc., Minneapolis, MN, USA; Human SP-A ELISA; Funakoshi Co., Ltd., Tokyo, Japan) were used to measure the concentrations of SP-D and SP-A. The cutoff levels were set as the levels that resulted in optimal diagnostic accuracy according to the previous results for healthy volunteers: 110 ng/mL for SP-D and 43.8 ng/mL for SP-A (mean + 2SD for healthy volunteers).7 ELISA (ITSI-Biosciences, LLC, Johnstown, PA, USA) was used to measure the concentration of TARC/CCL17. The cutoff level was set as the level that resulted in the optimal diagnostic accuracy according to the previous results for healthy volunteers: 450 pg/mL (mean + 2SD for healthy volunteers).17
Statistical analysis
Student’s t-test or the Mann–Whitney U-test, if applicable, was used to analyze the differences in the levels of various serum markers between the subject groups. The chi-squared test for goodness of fit or Fisher’s exact probability test was used to test positive quantitative differences between the groups. The significance of the positive ratio of KL-6 and TARC/CCL17 was tested using the chi-squared test. Multivariate analysis was performed using STATA software (Light Stone Corp., Tokyo, Japan). All P-values corresponded to two-sided tests, and the significance was set at P<0.05.
Results
Demographic characteristics of the study population
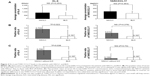
The characteristics of the patients in this study are outlined in Table 1. There were six patients with benign lung disease (BLD) and 26 patients with malignancies. The distribution of the primary diagnoses is shown in Figure 1A. Among our recruited patients, 59.6% (19/32) had lung cancer. In the six patients with BLD, three had infectious pneumonia, two had nontuberculous mycobacterium infection, and one patient had organizing pneumonia after infectious pneumonia. None of the patients with BLD had ILD. There were 68.8% (22/32) current smokers or ex-smokers, and ten patients suffered from chronic obstructive pulmonary disease (COPD). Among the ten patients with a history of ILD, eight patients were diagnosed as having IPF, one patient radiation pneumonitis, and one patient drug-induced ILD. The case of drug-induced ILD is described in the latter half of this section. All the ILD patients had concurrent malignancies (seven, NSCLCs; one, small cell lung cancer; one, ovarian cancer; and one, primary unknown cancer). We considered the patients with BLD as the control group in this study and compared the ability of each marker to detect ILD among the various disease categories. Previously reported values of SP-A, SP-D,7 KL-6, and TARC/CCL1717 in Japanese healthy volunteers were used for reference in this study (Table S1). The total number of diseases and the rate for each are shown in Table 1.
Serum levels of circulating KL-6, SP-D, SP-A, and TARC/CCL17
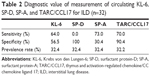
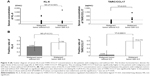
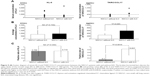
The sensitivity, specificity, and prevalence rates associated with the cutoff levels of each marker in this study are shown in Table 2. The receiver-operating characteristic curve of each marker clearly revealed the superiority of TARC/CCL17 as the surrogate marker for detecting ILD compared to KL-6, SP-D, and SP-A (Figure S1). For further analysis, KL-6, which is thought to be a standard surrogate marker to detect ILD, and TARC/CCL17 were used. SP-D and SP-A were excluded because of the low sensitivity of SP-D and low specificity of SP-A. Circulating KL-6 and TARC/CCL17 levels according to disease are shown in Figure 1B and 1C. There were no significant differences in the serum concentrations and positive ratios of KL-6 and TARC/CCL17 between the patients with malignant tumors and those with BLD (Figure 2). Next, we examined KL-6 and TARC/CCL17 levels in the patients with NSCLC and BLD (Figure 3). The circulating KL-6 levels tended to be higher in the patients with NSCLC than those observed in the patients with BLD, although the difference was not significant (P=0.254) (Figure 3A). In contrast, the circulating KL-6 positive ratio (0.705; 95% confidence interval: 0.44–0.896) was significantly higher in patients with NSCLC than in those with BLD (0.167; 95% confidence interval: 0.0042–0.64; P=0.022), but no significant difference was observed in the circulating TARC/CCL17 positive ratio (Figure 3B). This tendency was also observed in the patients with NSCLC without ILD and those with BLD without ILD (Figure 3C). These results indicated that KL-6 may not be suitable as a biomarker for ILD in patients with NSCLC. To assess the possibility of using TARC/CCL17 as an early diagnostic biomarker for ILD, we compared the serum KL-6 and TARC/CCL17 concentrations between the patients with malignancy with and without ILD (Figure 4). The TARC/CCL17 concentration was significantly higher in the patients with malignant tumors and ILD (575.6±315.2 pg/mL; n=9) than in the patients without ILD (252.8±110.7 pg/mL; n=17; P=0.015) (Figure 4A). In contrast, there was no significant difference in the concentration of circulating KL-6 between the patients with malignancy with and without ILD (malignant tumor with ILD [n=10]: 817.7±976.8 U/mL vs malignant tumor without ILD [n=17]: 619.4±591.6 U/mL; P=0.507). The positive ratio of circulating TARC/CCL17 was significantly higher in the patients with malignancy and ILD than in those without ILD (P=0.00011), but no significant difference in the circulating KL-6 positive ratio was observed between the patients with malignancy with and without ILD (P=0.31) (Figure 4B). Among those with NSCLC or all recruited patients, similar results were obtained (Figures 5 and 6). These results suggest that TARC/CCL17, but not KL-6, can be a useful biomarker for the detection of ILD. Subsequently, we performed multivariate analysis using logistic regression models to confirm the superiority of TARC/CCL17 as a surrogate marker to detect ILD. The result revealed that TARC/CCL17 was an independent factor for the detection of ILD (Table S2).
Discussion
KL-6 is believed to be a reliable serum biomarker for the diagnosis and management of ILD.18 In this study, we showed that TARC/CCL17 may be also useful as a diagnostic marker of ILD. In particular, TARC/CCL17 may be a better marker than KL-6 in patients with NSCLC.
Tanaka et al showed that circulating KL-6 was elevated both at diagnosis and before chemotherapy for NSCLC and that KL-6 was highly expressed on the cell surface of tumors, although there was no assessment of its diagnostic value for concurrent ILD.11 In addition, elevation of circulating KL-6 was associated with subsequent lung cancer risk19 and was reported to be a prognostic marker for patients with NSCLC, harboring epidermal growth factor receptor active mutation with no relationship to ILD.20 These results indicated an unclear cellular origin of KL-6 in NSCLC. Both tumor tissue and type II pneumocytes regenerated in the normal lung could be the main source of circulating KL-6. Thus, KL-6 seems to be unreliable as a comprehensive biomarker for ILD, particularly in patients with NSCLC.
The characteristics of the study population might be one of the reasons for the low sensitivity of SP-D (0.0%) and low specificity of SP-A (30.4%). All the patients with ILD in this study had ILD-negative chest X-ray images but ILD-positive findings on HRCT. In addition, the proportion of smokers (68.8%) was more than two-thirds. Circulating SP-D could be closely associated with alveolitis but not fibrosis, and the SP-A level is affected by smoking.6,21 Thus, the characteristics of our subjects may have influenced the sensitivity of SP-D and specificity of SP-A.
Numerous factors that regulate immune and inflammatory responses have been implicated in the pathogenesis of IPF.22–24 Regardless of the initial inciting agent, the hallmark of IPF is chronic inflammation and deposition of extracellular matrix.25 The phenotype of this chronic inflammation appears to be highly associated with a Th2 phenotype of cytokine expression.26 Considering the TARC/CCL17 findings from previous15,26 studies and our small studies, this marker may have a key role in the development of IPF and drug-induced ILD. Kawashima et al had reported that serum TARC/CCL17 levels were apparently increased in patients with dermatomyositis and ILD compared to patients with dermatomyositis and without ILD;27 this report strongly supports our results. In addition, factors affecting the circulating TARC/CCL17 concentration should be considered. Serum levels of TARC/CCL17 are elevated in patients with atopic dermatitis, AEP, bronchial asthma, classical Hodgkin’s lymphoma, and mycosis fungoides.13,14,28 In this study, we encountered an NSCLC patient who had a high level of circulating TARC/CCL17 (473.3 pg/mL) without ILD. She had a history of atopic dermatitis and had used a steroid skin lotion for 3 years. It is important to clarify the disease background in order to properly evaluate circulating TARC/CCL17.
This study had a number of limitations. Because of the small sample size and a potential patient selection bias, confirmation of the present results by a large-scale study is needed to ensure the generalizability of our data. In addition, the association between the circulating TARC/CCL17 level and ILD disease activity should be analyzed. Furthermore, drug-induced ILD was found in only one case. Whether sequential measurement of circulating TARC/CCL17 level is useful for the early detection of drug-induced ILD should be clarified in a prospective study.
Conclusion
In conclusion, TARC/CCL17 can be useful as a diagnostic marker of ILD in patients with malignant tumors, especially in patients with NSCLC. Confirmation of the results in a prospective large-scale study is warranted.
Acknowledgment
This work was supported in whole by a research project grant, 24-B-67, from the Kawasaki Medical School.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
We declare that no conflicts of interest exist, including any financial and personal relationships with other people or organizations that could inappropriately influence our work.
References
Saito Y, Gemma A. Current status of DILD in molecular targeted therapies. Int J Clin Oncol. 2012;17(6):534–541. | ||
Camus P, Kudoh S, Ebina M. Interstitial lung disease associated with drug therapy. Br J Cancer. 2004;91(2):S18–S23. | ||
Hotta K, Kiura K, Takigawa N, et al. Comparison of the incidence and pattern of interstitial lung disease during erlotinib and gefitinib treatment in Japanese patients with non-small cell lung cancer: the Okayama Lung Cancer Study Group experience. J Thorac Oncol. 2010;5(2):179–184. | ||
Samet JM. Does idiopathic pulmonary fibrosis increase lung cancer risk? Am J Respir Crit Care Med. 2010;161(1):1–2. | ||
Shah DJ, Sachs RK, Wilson DJ. Radiation-induced cancer: a modern view. Br J Radiol. 2012;85(1020):1166–1173. | ||
Kohno N, Kyoizumi S, Awaya Y, Fukuhara H, Yamakido M, Akiyama M. New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL-6. Chest. 1989;96(1):68–73. | ||
Takahashi H, Kuroki Y, Tanaka H, et al. Serum levels of surfactant proteins A and D are useful biomarkers for interstitial lung disease in patients with progressive systemic sclerosis. Am J Respir Crit Care Med. 2000;162(1):258–263. | ||
Greene KE, King TE Jr, Kuroki Y, et al. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J. 2002;19(3):439–446. | ||
Sakamoto K, Taniguchi H, Kondoh Y, et al. Serum KL-6 in fibrotic NSIP: correlations with physiologic and radiologic parameters. Respir Med. 2010;104(1):127–133. | ||
Ohnishi H, Yokoyama A, Kondo K, et al. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am J Respir Crit Care Med. 2002;165(3):378–381. | ||
Tanaka S, Hattori N, Ishikawa N, et al. Krebs von den Lungen-6 (KL-6) is a prognostic biomarker in patients with surgically resected non-small cell lung cancer. Int J Cancer. 2012;130(2):377–387. | ||
Miyazaki E, Nureki S, Fukami T, et al. Elevated levels of thymus and activation-regulated chemokine in bronchoalveolar lavage fluid from patients with eosinophilic pneumonia. Am J Respir Crit Care Med. 2002;165(8):1125–1131. | ||
Miyazaki E, Nureki S, Ono E, et al. Circulating thymus and activation-regulated chemokine/CCL17 is a useful biomarker for discriminating acute eosinophilic pneumonia from other causes of acute lung injury. Chest. 2007;131(6):1726–1734. | ||
Jones K, Vari F, Keane C, et al. Serum CD163 and TARC as disease response biomarkers in classical Hodgkin lymphoma. Clin Cancer Res. 2013;19(3):731–742. | ||
Belperio JA, Dy M, Murray L, et al. The role of the Th2 CC chemokine ligand CCL17 in pulmonary fibrosis. J Immunol. 2004;173(7):4692–4698. | ||
American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med. 2000;161(2pt1):646–664. | ||
Kakinuma T, Wakugawa M, Nakamura K, Hino H, Matsushima K, Tamaki K. High level of thymus and activation-regulated chemokine in blister fluid and sera of patients with bullous pemphigoid. Br J Dermatol. 2003;148(2):203–210. | ||
Ishikawa N, Hattori N, Yokoyama A, Kohno N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig. 2012;50(1):3–13. | ||
Shiels MS, Chaturvedi AK, Katki HA, Gochuico BR, Caporaso NE, Engels EA. Circulating markers of interstitial lung disease and subsequent risk of lung cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2262–2272. | ||
Fujiwara Y, Kiura K, Toyooka S, et al. Elevated serum level of sialylated glycoprotein KL-6 predicts a poor prognosis in patients with non-small cell lung cancer treated with gefitinib. Lung Cancer. 2008;59(1):81–87. | ||
Kobayashi H, Kanoh S, Motoyoshi K. Serum surfactant protein-A, but not surfactant protein-D or KL-6, can predict preclinical lung damage induced by smoking. Biomarkers. 2008;13(4):385–392. | ||
Feghali-Bostwick CA, Tsai CG, Valentine VG, et al. Cellular and humoral autoreactivity in idiopathic pulmonary fibrosis. J Immunol. 2007;179(4):2592–2599. | ||
Zuo F, Kaminski N, Eugui E, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci U S A. 2002;99(9):6292–6297. | ||
Kurosu K, Takiguchi Y, Okada O, et al. Identification of annexin 1 as a novel autoantigen in acute exacerbation of idiopathic pulmonary fibrosis. J Immunol. 2008;181(1):756–767. | ||
Borchers AT, Chang C, Keen CL, Gershwin ME. Idiopathic pulmonary fibrosis an epidemiological and pathological review. Clin Rev Allergy Immunol. 2011;40(2):117–134. | ||
Wallace WA, Ramage EA, Lamb D, Howie SE. A type 2 (Th2-like) pattern of immune response predominates in the pulmonary interstitium of patients with cryptogenic fibrosing alveolitis (CFA). Clin Exp Immunol. 1995;101(3):436–441. | ||
Kawashima T, Tada Y, Asano Y, et al. Serum TARC/CCL17 levels are increased in dermatomyositis associated with interstitial lung disease. J Dermatol Sci. 2010;60(1):52–54. | ||
Saeki H, Tamaki K. Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J Dermatol Sci. 2006;43(2):75–84. |
Supplementary materials
  | Table S1 The relationship between average concentration of KL-6, SP-A, SP-D and TARC/CCL17 in healthy volunteers and benign lung disease in this study |
References
Kohno N, Kyoizumi S, Awaya Y, Fukuhara H, Yamakido M, Akiyama M. New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL-6. Chest. 1989;96(1):68–73. | ||
Takahashi H, Kuroki Y, Tanaka H, et al. Serum levels of surfactant proteins A and D are useful biomarkers for interstitial lung disease in patients with progressive systemic sclerosis. Am J Respir Crit Care Med. 2000;162(1):258–263. | ||
Kakinuma T, Wakugawa M, Nakamura K, Hino H, Matsushima K, Tamaki K. High level of thymus and activation-regulated chemokine in blister fluid and sera of patients with bullous pemphigoid. Br J Dermatol. 2003;148(2):203–210. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.