Back to Journals » Psoriasis: Targets and Therapy » Volume 10
Circulating Cell-Free DNA as Inflammatory Marker in Egyptian Psoriasis Patients
Authors Anani HAA , Tawfeik AM , Maklad SS , Kamel AM , El-Said EE, Farag AS
Received 10 December 2019
Accepted for publication 19 March 2020
Published 21 May 2020 Volume 2020:10 Pages 13—21
DOI https://doi.org/10.2147/PTT.S241750
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Uwe Wollina
Haneya AA Anani,1 Amany M Tawfeik,1 Soheir S Maklad,1 Abeer M Kamel,2 Enas E El-Said,3 Asmaa S Farag2
1Departments of Microbiology and Immunology, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 2Dermatology Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 3Clinical Pathology Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt
Correspondence: Haneya AA Anani Email [email protected]
Background: Cell lesion and apoptosis with release of cell-free DNA (CFD) in circulation are associated with chronic inflammation of psoriasis.
Objective: The objective of this study was to determine the CFD concentrations in sera of patients with psoriasis, to assess its relationship with disease severity as defined by Psoriasis Area Severity Index (PASI) and other inflammatory biomarkers (C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR)) levels, and to monitor the efficacy of treatment.
Patients and Methods: Thirty adult patients with different types of psoriasis (25 vulgaris; 10 mild, 15 moderate and 5 erythroderma; severe) were evaluated during the exacerbation phase of the disease, before starting (T0) and after 12 weeks (T12) of treatment with topical therapy for mild cases, narrowband-ultraviolet light B (NB-UVB) for moderate cases and methotrexate for severe cases. Twenty healthy controls were also involved in the study. The concentrations of CFD in sera were measured before and after treatment by quantitative real time PCR (qPCR) using primers of the human β-globin gene.
Results: At T0, all patients presented significant higher levels of ESR (P=0.05) and CFD (P=0.001) compared with controls. Highly significant elevations of all parameters were observed in severe disease (erythroderma) compared to mild/moderate disease (vulgaris). Methotrexate treatment induced highly significant reductions in all inflammatory markers including CFD (P= 0.042) while topical and UV irradiation therapies had no effects. CFD concentrations showed positive correlations with both PASI (r=0.422, P=0.020) and ESR (r=0.321, P=0.023) only before the start of treatment.
Conclusion: The level of circulating CFD could be used to monitor psoriasis severity. However, its level cannot be stated for the treatment, except in severe erythrodermic patients upon successful treatment with methotrexate. We recommend validation of a convenient and accurate DNA assay applied directly to biological samples which does not require prior DNA extraction and amplification.
Keywords: circulating cell-free DNA, Psoriasis Severity Index, C-reactive protein, psoriasis therapy
Introduction
Psoriasis is a chronic inflammatory disease with a prevalence of 1–3% in the general population.1 The pathogenesis of the disease involves enhanced proliferation and shortened duration of maturation of keratinocytes, perivascular infiltration of T cells, dendritic cells, macrophages, neutrophils, and imbalance in apoptosis pathways. During apoptotic cell death, nucleosomes which are formed by DNA and histone proteins are packed into membrane-bound bodies that are eliminated by macrophages to prevent excessive inflammation.2
In cases with a high rate of apoptosis, these homeostatic mechanisms are impaired. So, as a consequence, higher concentrations of nucleosomes in the circulation are observed, which possibly participating in the development of immunopathology. In blood, apoptotic nucleosomes are detected either as oligonucleosome-protected fragments or nucleosomal circulating cell-free DNA (CFD).3 Once released into the circulation, cell-free DNA stimulates the innate and adaptive immune responses.1
Circulating cell-free DNA appears following cell damage, and its levels are increased in pathological conditions characterized by marked inflammation such as cancer, severe sepsis, cerebral stroke, systemic lupus erythematosus, rheumatoid arthritis, traumatic and burn injuries, and acute rejection of transplants.3,4 Measuring CFD levels have been recognized as a diagnostic potential tool in the management of patients with various dynamic clinical situations reflecting cell necrosis and apoptosis and can be used to assess the activity and severity of the disease.5 In patients on haemodialysis, CFD levels were found to be positively correlated with interleukin IL-6 (IL-6) which is a biomarker of inflammation.4,6
Numerous studies5,7,8 had reported elevated levels of different biomarkers of inflammation such as C-reactive protein (CRP), interferon-γ (IFN-γ), tumor necrosis factor (TNF-α), IL-6, and leptin in Psoriatic patients. Activation of dendritic and T cells induces the production of typical biomarkers of inflammation by keratinocytes. These markers are found in the lesions and blood of Psoriatic patients, and are reflecting the severity of the disease that can be assessed by Psoriasis area and severity index (PASI). A limited studies are available concerning CFD levels in patients with psoriasis worldwide.9
In Egypt, studies dealing with CFD are not reached by searching the internet. However, there are several studies are available dealing with other factors that play roles in the pathogenesis, their correlation to disease severity or treatment of psoriasis.10–12 There are some studies also concerning the different protocols for the management of psoriasis at Kasr Al-Ainy’s Psoriasis Unit, Cairo,13 either topical and systemic therapies or biological therapies.14
We hypothesized that the inflammatory and hyperproliferative features of psoriasis may be associated with increased levels of CFD that may provide a new inflammatory marker to monitor psoriasis, its severity and treatment in Egyptian patients. Thus, the objectives of this work were to determine the CFD concentrations in sera of patients with psoriasis as indicator of apoptosis and inflammation, to assess its relationship with disease severity (PASI) and other inflammatory biomarkers (CRP) and (ESR) levels as well as to monitor the efficacy of treatment.
Patients and Methods
Thirty Egyptian psoriasis patients including 21 females and 9 males aged 22–64 years with different types of psoriasis (25 vulgaris, 5 erythroderma) were recruited from Dermatology Department at Al-Zahraa Hospital, Cairo, Egypt. All patients were clinically studied in the exacerbation phase of the disease. The severity of the disease was assessed from the basic characteristics of disease status (erythema, desquamation, and skin infiltration) and expressed as PASI score. According to PASI patients were classified into: mild cases (scores of <10), moderate (scores 10–20) and severe (scores >20).9 The duration of disease was also reported.
None of the patients received any treatment at least 2 weeks before inclusion. They were examined before starting (T0) and 12 weeks after treatment (T12). The type of treatment was determined according to disease severity and the clinical and therapeutic history of the patients. Mild cases (n = 10; vulgaris) were treated with topical corticosteroids and Calcipotriol, moderate cases (n = 15; vulgaris) were treated with narrowband ultra-violet light B irradiation (NB-UVB, 311 nm) in a dose of 150 mJ/m2 using a UVB Meter Waldmann 7001 K cabin (UVA⁄ UVB-TL01; Germany), National biological corporation 1532 Enterprise Parkway Twinsburg, Ohio 44087, 3 sessions per week for 2 months. The severe cases (n = 5; erythroderma) received methotrexate 15 mg/week for 2 months.15
Twenty healthy control volunteers matched with patients and with normal haematological and biochemical values were also included. They were 16 females and 4 males aged (18–50 years). The exclusion criteria of both patients and controls included other skin diseases, diabetes mellitus, inflammatory or infectious diseases, cardiovascular, liver or kidney diseases. Both patients and controls were investigated for CRP and ESR. The used protocol was approved by the Committee of Ethics at Al-Azhar University; the approval number is 201,910,227. An informed written consent was obtained from each patient and control subject. This study was conducted in accordance with the Declaration of Helsinki.
Sample Collection
Five milliliters of venous blood were obtained from each patient and control subject with a sterile syringe without anticoagulant, sera were collected for laboratory investigations and aliquot was stored at −20°C till the time of DNA extraction.
Methods
The following investigations were carried out before treatment (T0) and 12 weeks after treatment (T12).
Measurement of C-Reactive Protein Levels
CRP levels were measured by immunonephelometry; IMMAGE 800 (Beckman Coulter, USA) with the detection limit of 1.0 mg/l of sera.
Measurement of Erythrocyte Sedimentation Rate (mm/hr)
Westergren method was used.
Psoriasis Area and Severity Index Score
The severity of the disease was assessed from the basic characteristics of the disease status (erythema, desquamation, and skin infiltration) and expressed as PASI score.9
Measurement of Circulating Cell-Free DNA
DNA Extraction from Sera
DNA extraction from sera of patients and controls was done by (QIA amp R Blood Mini) Kit (Qiagen, Hilden, Germany) Cat No. 51304, according to the manufacturer’s protocol. The extracts were stored at −70°C till used for the detection of cell-free DNA by quantitative real-time PCR (qPCR).
Measurement of the Extracted Double-Stranded DNA (dsDNA) Concentrations Using DeNovix dsDNA Ultra High Sensitivity Assay
The Kit contains: Accublue dye (400x), Accublue Enhancer (100x), and 2 dsDNA standards (calf thymus); 300 pg/μL dsDNA Standard and 0 pg/μL dsDNA Standard to construct a standard curve to ensure that all sample concentrations fall within the limits of the reagent kit for accurate results. The detection range of this kit is from 5 pg to 3 ng total mass per assay tube. This is equivalent to sample concentrations of 0.5 pg/μL to 300 pg/μL. The concentration was measured by fluorometer Cat#: KIT- DSDNA-ULTRA-1.
Unfortunately, the concentrations of our samples were out of range. As recommended by the manufacturer's instruction that if the Ultra High Sensitivity Assay does not cover the concentration range of the unknown samples, consider using an alternate DeNovix dsDNA Broad Range Assay with detection range of 100 pg/μL to 2000 ng/μL. Ten-fold serial dilutions of standard ranging from 2 to 2000 ng/ul were done. The measurement was carried out in duplicate and the mean was recorded. The sample with highest concentration was used as a positive control in the measurement of CFD concentrations in sera by (qPCR).
Quantitative Real-Time PCR and Analysis of CFD
qPCR assay was performed using DT Lite 4 DNA technology based on amplification of β-globin gene and primers which were selected from published sequences. The amplification mixture contained: 1.5 μL of each primer (Invitrogen, Germany), 10 μL of SYBER Green Master Mix (Promega), 7 μL of the extracted DNA, 0.2 μL Rox dye, and water to a final volume of 27 μL. The preparation of the primers was done according to manufacturer’s instructions. The sequence of primers used for amplification of β-globin gene was (Forward primer: 5′-ACACAACTGTGTTCACTAGC-3′ and (Reverse primer: 5′- CAACTTCATCCACGTTCACC-3′16 Amplification conditions included: an initial denaturation step at 95°C for 15 min followed by 45 cycles of denaturation at 95°C for 15 s, annealing at 56°C for 20 s and extension at 72°C for 15 s.17
Statistical Analysis
Microsoft Excel Sheet was used and the results were analyzed by SPSS (version 20) computer program and the results are presented in tables and figures. Comparison between groups was done using the Chi-square test. Non-parametric Mann–Whitney U-test was used to compare between two groups. ∆ Comparison between before and after treatment, using Wilcoxon Rank test.
Results
Patients
The results demonstrated that (70%) of females and (30%) of males have psoriasis. Twenty-five were vulgaris and 5 were erythroderma. According to the PASI score which ranged (4–40), median value 8.5 at T0 before the start of therapy, patients were classified into mild disease (10 vulgaris cases; 33.3%), moderate disease (15 vulgaris cases; 50%) and only 5 erythroderma; (16.7%) have severe disease. The mild cases were treated with topical agents, the moderate cases were treated with UV irradiation (NBUV) while the severe cases received Methotrexate treatment (Table 1, Figure 1A and B).
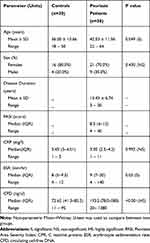 |
Table 1 Demographic and Clinical Data of Patients and Controls Before the Start of Treatment |
Inflammatory Markers Before the Start of Treatment (T0)
In patient’s group (n=30) the median (IQR) levels of inflammatory markers (CRP, ESR) as well as the indicator of apoptosis (CFD) at T0 were 3.05 mg/l (2.5–4.2), 9 mm/hr7–20 and 193.5 ng/ul (78.0–580) compared to 3.45 mg/l (3–4.01), 8 mm/hr (5–9.5) and 72.65 ng/ul (41.3–85.5) respectively, in controls, with significant difference (P = 0.05) in ESR, highly significant difference (P = 0.001) in CFD and insignificant difference in CRP levels (Table 1)
Relationship Between Inflammatory Markers, Disease Severity and Type of Psoriasis Before the Start of Treatment (T0)
In both forms of disease: vulgaris (mild/moderate disease) and erythroderma (severe disease) the median PASI score were (8 vs 37.5, P = 0.0001), ESR (9 vs 122.5 mm/hr, P = 0.001), CRP (3 vs 9 mg/l, P = 0.002), and CFD (170 vs 405 ng/ul, P = 0.002). Highly significant elevations of all parameters were observed in psoriasis patients (vulgaris and erythroderma) compared to controls and between erythroderma (severe disease) and vulgaris (mild/moderate disease), Table 2 and Figure 2 shows the result of CFD.
 |
Table 2 Relationship Between Inflammatory Markers, Disease Severity and Type of Psoriasis Before the Start of Treatment |
Influence of Different Types of Therapy on Inflammatory Markers
Topical and NBUV treatment in mild/moderate vulgaris patients induced highly significant reductions only in PASI score (P = 0.004) and (P = 0.001) respectively. Methotrexate treatment induced highly significant reductions in all inflammatory markers including CFD ng/ul (P values were: 0.027, 0.028, 0.046, and 0.042) respectively, Table 3.
 |
Table 3 Influence of Different Types of Therapy on Inflammatory Markers |
Correlations Between CFD Levels, Disease Severity and Other Inflammatory Markers
Positive correlations were found between CFD levels ng/ul and PASI score (r = 0.422, P = 0.020) and with ESR mm/hr (r = 0.321, P = 0.023) only before the start of treatment, Figure 3.
Discussion
In the present study, psoriasis in Egyptian patients showed an increased frequency in females (70%) than in males which is matched with previous results of De Oliveira et al,18 who found a positive association of psoriasis with Caucasian females.
The current study demonstrated that Psoriatic patients presented with different degrees of severity dependent on the PASI score which is ranged 4–40 with median 8.5. The inflammatory markers; ESR and CFD showed higher levels in Psoriatic patients compared to controls. Although CRP is known to mediate the acute phase response of inflammation yet its levels showed insignificant elevation in our study which may be explained by increase number of patients with mild and moderate disease and the age of our patients. Nevertheless, other studies19,20 could not confirm the association between some inflammatory markers such as TNF-α and PASI score. Many factors could be the cause of these controversial results such as degree of systemic inflammation which can affect the level of CRP in the circulation. Age of patients is another variable that affects the results of CRP. In the current study, the majority of our patient’s age ranged 22–64 years old. De Oliveira et al,18 documented that in subjects with age 50 years or older, CRP level was about two-fold higher than in the younger subjects. Taking into consideration, the non-specificity of CRP which might reflect the tendency to increase CRP with advancing age as a part of low-grade inflammation (inflammaging). In addition, Martin et al,7 detected a significant relationship between age and CRP levels in psoriasis patients.
Although increased levels of circulating nucleosomes have been detected in patients with diseases characterized by systemic inflammation, yet only a few studies dealt with levels of nucleosomes and/or cell-free DNA in blood of Psoriatic patients. They suggested that circulating nucleosomes can be used as a new biomarker to monitor and follow-up of Psoriatic patients and to examine the effectiveness of drugs used in treatment.9
Circulating cell-free DNA (CFD) can be measured by several molecular biological techniques. In this study, real-time PCR based on the amplification of human β-globin gene fragments was used to determine the amount of nucleosome-derived (CFD) and to distinguish nucleosomal DNA from circulating mitochondrial or bacterial DNA.
Apoptotic pathways have been extensively studied in Psoriatic skin lesions, but little is known about the presence of several products of cell death circulating in blood. Apoptosis and necrosis promote the release of nucleosomes into the extracellular space with other nuclear damage-associated molecular patterns (nDAMPS), where fragments of DNA and histones exhibit proinflammatory activities. Nucleosomes can also be exported within neutrophil extracellular traps (NETs) during NETosis which is a unique form of cell death in neutrophils at sites of infection and inflammation.2
Coimbra et al,20 first proved higher levels of circulating cell-free DNA (CFD) in serum of patients with exacerbated psoriasis vulgaris and its significant correlation with serum levels of IL-6. Although,21 reported that Psoriatic patients displayed twofold higher plasma concentrations of CFD compared to controls, yet they did not find any correlation between CFD and serum IL-6 levels. The discrepancy could be explained by different specificities of quantitative real-time PCR and fluorescent analysis used in their studies.
Nishimoto et al,22 reported that relationships between CFD and cytokines or mediators participating in the pathogenesis of psoriasis except IL-6 have not been defined yet. Their data did not show any association of CFD and serum levels of TNF-α or CRP because of their several roles in biological processes such as inflammation, cell growth, differentiation, apoptosis, and many other processes that are exaggerated in psoriasis vulgaris.
Although the current study demonstrated significant elevation of CFD in sera of Psoriatic patients and positive correlations between CFD levels and both severity index; PASI (r = 0.422, P = 0.020) and the inflammatory marker; ESR (r = 0.321, P = 0.023) only before the start of treatment yet, there was no significant correlation between CFD and CRP. Our results are similar to Nishimoto et al.22 who found no correlations between CFD and several parameters such as CRP, TNF-α, IL-6, PASI score, age or Body Mass Index (BMI). Previously23 reported that IL-6 correlates with PASI score only in more severe forms of psoriasis. The discrepancy between the results, might be explained by differences in the criteria of the studied groups since the majority of their patients have severe psoriasis vulgaris and PASI score ranged 30–70. In contrast, most of our patients have mild/moderate vulgaris disease with PASI ranged 4–20 while in patients with severe erythrodermic psoriasis ranged from 20 to 40, which limited the possibility to find a significant association. These positive correlations with psoriasis severity, as defined by PASI, suggesting that CFD could be used as a useful marker to monitor psoriasis severity.
Dilek et al,9 suggested that circulating nucleosomes can be used as a new biomarker to follow up Psoriatic patients and to monitor the effectiveness of therapy.
The current study revealed that topical and NBUV treatment in mild and moderate cases induced highly significant reductions only in PASI levels (P = 0.004) and (P = 0.001) respectively. Patients with severe disease who were treated with methotrexate presenting a higher PASI score than patients treated with topical and NBUVB therapy. Methotrexate treatment-induced highly significant reductions in all inflammatory markers (P values were: 0.027 for PSAI, 0.028 for CFD, 0.046 for ESR, and 0.042 for CRP). Coimbra et al,20 explained that the significant reduction in PASI seems to reflect the inhibition of cellular proliferation and release of pro-inflammatory cytokines. However, a low-grade of inflammation persists which is a common finding in the course of the therapies then TNF-α further strengthens the role of inflammation in psoriasis.
Our results are similar to Coimbra et al,20 who found that topical therapy did not induce any significant change, except for TNF-α that was significantly reduced. They also revealed that after NBUVB treatment significant reductions in TNF-α and CRP. However, the differences observed at T0 seem to be related to the more severe disease. The therapy was successful if reduction occurred in PASI. However, the persistence of higher values of CRP reflect a persistently increased inflammation.
The finding that methotrexate treatment induced highly significant reductions in all inflammatory markers is in accordance with the results of20 who found that methotrexate treatment induced reduction of inflammation which is more prominent in TNFα, IL-6, CRP and adiponectin and presented a significant improvement.
Coimbra et al,20 found that exacerbation of the inflammatory process was associated with increasing CFD levels, compared with controls. After successful treatment, significant reduction of CFD levels was observed demonstrated by significant reduction in PASI score. The CFD increase seems to be linked to the inflammatory response, since CFD values were positively correlated with the inflammatory marker IL-6 which increases in active psoriasis. They suggested that CFD could be a consequence of inflammation in psoriasis, or itself contributes to the inflammatory process. The origin of CFD in psoriasis, has not been identified and might result from different inflammatory cells involved in Psoriatic lesions or leaking of DNA fragments from the nuclei of leucocytes.24
Limitations of the Study
The current study has some limitations related to the limited number of erythrodermic types to clarify the exact role of circulating cell-free DNA in psoriasis.
Conclusion
The level of circulating cell-free DNA (CFD) could be used to monitor psoriasis severity in Egyptian patients. However, its levels cannot be stated for the treatment, except in severe erythrodermic patients upon successful treatment with methotrexate.
Take Home Messages and Recommendation
We propose that the evaluation of circulating CFD may provide an additional inflammatory marker to monitor the severity of psoriasis in Egyptian patients, and to monitor the efficacy of treatment only in severe cases. We recommend validation of a convenient, simple, and accurate DNA assay applied directly to biological samples which does not require prior DNA extraction and amplification.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Frank MO. Circulating cell-free DNA differentiates severity of inflammation. Biol Res Nurs. 2016;18:477–488. doi:10.1177/1099800416642571
2. Penttilä AK, Rouhiainen A, Kylänpää L, et al. Circulating nucleosomes as predictive markers of severe acute pancreatitis. J Intensive Care. 2016;4:14. doi:10.1186/s40560-016-0135-6
3. Chen Q, Ye L, Jin Y, et al. Circulating nucleosomes as a predictor of sepsis and organ dysfunction in critically ill patients. Int J Infect Dis. 2012;16:e558–e564. doi:10.1016/j.ijid.2012.03.007
4. Tovbin D, Novack V, Wiessman MP, et al. Circulating cell-free DNA in hemodialysis patients predicts mortality. Nephrol Dial Transplant. 2012;27:3929–3935. doi:10.1093/ndt/gfs255
5. He L, Qin S, Dang L, et al. Psoriasis decreases the antioxidation and anti-inflammation properties of high-density lipoprotein. Biochim Biophys Acta. 2014;1841:1709–1715. doi:10.1016/j.bbalip.2014.09.008
6. Kohlova M, Ribeiro S, Do Sameiro-Faria M, et al. Circulating cell-free DNA levels in hemodialysis patients and its association with inflammation, iron metabolism, and rhEPO doses. Hemodial Int. 2013;17:664–667. doi:10.1111/hdi.12055
7. Beranek M, Fiala Z, Kremlacek J, et al. Changes in circulating cell‑free DNA and nucleosomes in patients with exacerbated psoriasis. Arch Dermatol Res. 2017;309:815–821. doi:10.1007/s00403-017-1785-5
8. Sereflican B, Goksugur N, Bugdayci G, Polat M, Haydar Parlak A. Serum visfatin, adiponectin, and tumor necrosis factor alpha (TNF-α) levels in patients with psoriasis and their correlation with disease severity. Acta Dermatovenerol Croat. 2016;24:13–19.
9. Dilek AR, Dilek N, Saral Y, Yüksel D. The relationship between severity of the disease and circulating nucleosomes in psoriasis patients. Arch Dermatol Res. 2013;305:483–487. doi:10.1007/s00403-013-1347-4
10. Zaher H, Shaker OG, EL‐Komy MH, El‐Tawdi A, Fawzi M, Kadry D. Serum and tissue expression of transforming growth factor in psoriasis. J Eur Acad Dermatol Venereol. 2009;23(4):406–409. doi:10.1111/j.1468-3083.2008.03064.x
11. Meki AR, Al-Shobaili H. Serum vascular endothelial growth factor, transforming growth factor β1, and nitric oxide levels in patients with psoriasis vulgaris: their correlation to disease severity. J Clin Lab Anal. 2014;28(6):496–501. doi:10.1002/jcla.21717
12. El-hadidi HH, Hassan AS, El-hanafy G, Amr KS, Abdelmesih SF, Abdelhamid MF. Transforming growth factor-β1 gene polymorphism in psoriasis vulgaris. Clin Cosmet Investig Dermatol. 2018;11:415–419. doi:10.2147/CCID.S171403
13. Rasheed H, El-Moaty HA, El-Komy MH, et al. Kasr Al-Ainy’s psoriasis unit protocol for the management of psoriasis, part I: topical and systemic therapies. J Egypt Women’s Dermatologic Soc. 2019;16:1–13. doi:10.4103/JEWD.JEWD_8_19
14. Rasheed H, El-Moaty HA, El-Komy MH, et al. Kasr AL-Ainy’s psoriasis unit protocol for the treatment of psoriasis, part II: biological therapies. J Egypt Women’s Dermatologic Soc. 2019;16:73–80. doi:10.4103/JEWD.JEWD_13_19
15. Coimbra S, Catarino C, Costa E, et al. Circulating cell free DNA levels in Portuguese patients with psoriasis vulgaris according to severity and therapy. Br J Dermatol. 2014;170:939–942. doi:10.1111/bjd.12738
16. Jung M, Klotzek S, Lewandowski M, et al. Changes in concentration of DNA in serum and plasma during storage of blood samples. Clin Chem. 2003;49(6):1028–1029. doi:10.1373/49.6.1028
17. Goldshtein H, Hausmann MJ, Douvdevani A. A rapid direct fluorescent assay for cell-free DNA quantification in biological fluids. Ann Clin Biochem. 2009;46(Pt 6):488–494. doi:10.1258/acb.2009.009002
18. de Oliveira MFSP, de Oliveira Rocha B, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9–20. doi:10.1590/abd1806-4841.20153038
19. Jylhävä J, Lehtimäki T, Jula A, et al. Circulating cell-free DNA is associated with cardiometabolic risk factors: the health 2000 survey. Atherosclerosis. 2014;233:268–271. doi:10.1016/j.atherosclerosis.2013.12.022
20. Coimbra S, Oliveira H, Reis F, et al. Circulating adipokine levels in Portuguese patients with psoriasis vulgaris according to body mass index, severity and therapy. J Eur Acad Dermatol Venereol. 2010;24:1386–1394. doi:10.1111/j.1468-3083.2010.03647.x
21. Muramatsu S, Kubo R, Nishida E, Morita A. Serum interleukin-6 levels in response to biologic treatment in patients with psoriasis. Mod Rheumatol. 2017;27:137–141. doi:10.3109/14397595.2016.1174328
22. Nishimoto S, Fukuda D, Higashikuni Y, et al. Obesity induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci Adv. 2016;2:e1501332. doi:10.1126/sciadv.1501332
23. Bevelacqua V, Libra M, Mazzarino MC, et al. Long pentraxin 3: a marker of inflammation in untreated psoriatic patients. Int J Mol Med. 2006;18:415–423.
24. Atamaniuk J, Kopecky C, Skoupy S, et al. Apoptotic cell-free DNA promotes inflammation in haemodialysis patients. Nephrol Dial Transplant. 2012;27:902–905. doi:10.1093/ndt/gfr695
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



