Back to Journals » Cancer Management and Research » Volume 12
CircRNA circUGGT2 Contributes to Hepatocellular Carcinoma Development via Regulation of the miR-526b-5p/RAB1A Axis
Authors Kong Q, Fan Q, Ma X, Li J, Ma R
Received 28 May 2020
Accepted for publication 25 September 2020
Published 15 October 2020 Volume 2020:12 Pages 10229—10241
DOI https://doi.org/10.2147/CMAR.S263985
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Qingling Kong,1 Qing Fan,2 Xianbin Ma,3 Jian Li,4 Rong Ma5
1Office of Hospital Infection Control, People’s Hospital of Rizhao, Rizhao 276826, People’s Republic of China; 2Department of Hemodialysis, People’s Hospital of Rizhao, Rizhao 276826, People’s Republic of China; 3Department of Clinical Laboratory, People’s Hospital of Rizhao, Rizhao 276826, People’s Republic of China; 4Department of Interventional Radiology, People’s Hospital of Rizhao, Rizhao 276826, People’s Republic of China; 5Department of General Medicine, People’s Hospital of Rizhao, Rizhao 276826, People’s Republic of China
Correspondence: Rong Ma Email [email protected]
Background: Hepatocellular carcinoma (HCC) is a common malignant tumor in the world. Circular RNA hsa_circ_0008274 (circUGGT2) is reported to be upregulated in HCC tissues. Notwithstanding, the role and regulatory mechanism of circUGGT2 in HCC are indistinct.
Methods: Quantitative real-time polymerase chain reaction (qRT-PCR) was implemented to examine the levels of circUGGT2, microRNA (miR)-526b-5p, and ras-related protein Rab-1A (RAB1A) mRNA in HCC tissues and cells. Cell proliferation and colony formation were assessed with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) or colony formation assays. The levels of cyclin D1, proliferating cell nuclear antigen (PCNA), and RAB1A were detected with Western blotting. Cell cycle progression, migration, and invasion were evaluated by using flow cytometry or transwell assays. The relationship between circUGGT2 or RAB1A and miR-526b-5p was verified via dual-luciferase reporter and/or RNA pull-down assays. Xenograft assay was executed to confirm the role of circUGGT2 in vivo.
Results: We observed that circUGGT2 and RAB1A were upregulated while miR-526b-5p was downregulated in HCC tissues and cells. CircUGGT2 silencing suppressed tumor growth in vivo and curbed proliferation, colony formation, cell cycle progression, migration, and invasion of HCC cells in vitro. Mechanically, circUGGT2 regulated RAB1A expression via competitively binding to miR-526b-5p. Also, the inhibitory influence of circUGGT2 silencing on the malignancy of HCC cells was overturned by miR-526b-5p inhibitor. Furthermore, RAB1A overexpression reversed the suppressive influence of miR-526b-5p mimic on the malignancy of HCC cells.
Conclusion: CircUGGT2 silencing inhibited HCC development via modulating the miR-526b-5p/RAB1A axis, providing a possible target for HCC treatment.
Keywords: HCC, circUGGT2, miR-526b-5p, RAB1A
Introduction
Liver cancer is the fourth leading cause of cancer-related deaths worldwide, with approximately 782,000 deaths and 841,000 new cases each year.1 Hepatocellular carcinoma (HCC) is the main histological subtype of liver cancer.2 Also, HCC has high invasiveness, poor prognosis, and limited treatment in the clinic.3 Although significant progress has been made in the treatment of HCC, the 5-year survival rate of HCC patients is still low.4 Therefore, the current research aimed to reveal the latent molecular mechanism of HCC.
Circular RNAs (circRNAs) are usually derived from exons of pre-mRNAs in lariat-circulating or back-spacing manner.5 CircRNAs are mainly distributed in the cytoplasm, where they take part in a series of biological processes by interacting with microRNAs (miRs).6 Increasing researches have revealed that circRNAs are associated with the initiation and advancement of diverse tumors.7 For instance, circRNA circBPTF accelerated bladder cancer recurrence and advancement through sponging miR-31-5p and regulating RAB27A expression.8 CircRNA UDP-glucose glycoprotein glucosyltransferase 2 (circUGGT2), also termed as hsa_circ_0008274, had been revealed to be upregulated in HCC tissues (GSE94508).9 Also, it was reported that circUGGT2 modulated papillary thyroid carcinoma development through regulation of the AMPK and mTOR pathways.10 However, the role and latent regulatory mechanism of circUGGT2 in HCC are unclear.
MiRs are another subtype of non-coding RNAs that modulate almost all signaling pathways in cells.11 MiRs have great potential to improve cancer prognosis and diagnosis.12 For example, miR-345-5p had been reported as a promising target for pancreatic cancer treatment.13 MiR-526b was uncovered to exert a repressive role in a series of cancers.14,15 It was reported that miR-526b was related to the malignancy of HCC cells.16 Nevertheless, it is unknown whether miR-526b-5p can be regulated by circUGGT2 in HCC.
Ras-related protein Rab-1A (RAB1A) is a small GTPase that mediates the transport of vesicles from the endoplasmic reticulum to the Golgi.17 The aberrant expression of RAB1A was proved to be related to tumor advancement.18,19 For example, RAB1A could facilitate migration and invasion of colorectal cancer cells via regulating the mTOR/S6K1 pathway.20 Furthermore, RAB1A expression was associated with tumor T stage and size in lung cancer.21 Also, RAB1A contributed to cell migration and proliferation in HCC cells.22 However, the molecular mechanisms related to RAB1A in HCC progression have not been fully understood.
In this study, we verified that circUGGT2 exerted a promoting influence in HCC. Moreover, circUGGT2 knockdown impeded tumor growth through downregulating RAB1A via sponging miR-526b-5p.
Materials and Methods
Patient-Derived Samples
The research was ratified by the Ethics Committee of People’s Hospital of Rizhao (No. 2018RZ991), and was performed in accordance with the Declaration of Helsinki principles. Forty-eight paired HCC tissues and para-carcinoma tissues were collected from People’s Hospital of Rizhao. Written informed consents were obtained from all enrolled patients. None of these patients accepted preoperative radiotherapy or chemotherapy.
Cell Culture and Transfection
HCC cell lines HuH-7 and SK-HEP-1, and human normal liver cells THLE-2 were bought from Bena Culture Collection (Suzhou, Jiangsu, China). HuH-7 and SK-HEP-1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, HyClone, Logan, UT, USA). THLE-2 cells were cultured in BEGM Bullet Kit (Lonza, Basel, Switzerland). These cells were kept in a moist atmosphere with 5% CO2 at 37°C. The used medium was supplemented with fetal bovine serum (FBS, 10%, Solarbio, Beijing, China) and streptomycin/penicillin (1%, Solarbio).
The designated oligonucleotides or vectors were transfected into HCC cells using Lipofectamine 3000 reagent (Life Technologies, Grand Island, NY, USA) when reached 8090% confluence. Small interference (si) RNA targeting circUGGT2 (si-circUGGT2) and corresponding negative control (si-NC), as well as miR-526b-5p mimic and inhibitor (miR-526b-5p and anti-miR-526b-5p) and their matching negative controls (miR-NC and anti-miR-NC) were obtained from GenePharma (Shanghai, China). The overexpression vectors of circUGGT2 (circUGGT2) and RAB1A (pcDNA-RAB1A) were constructed through cloning the full-length sequence of circUGGT2 or RAB1A into the empty pLCDH-ciR vector (vector) (Geneseed, Guangzhou, China) or pcDNA3.1 vector (pcDNA) (Life Technologies), respectively.
RNA Preparation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
The nuclear RNA and cytoplasmic RNA were isolated from HCC cells with the PARIS Kit (Life Technologies). Total RNA was isolated from tissue samples and cells using the miRNeasy Mini Kit (Qiagen, San Diego, CA USA). Complementary DNA (cDNA) was synthesized with the M-MLV Reverse Transcriptase (Promega, Madison, WI, USA) or MicroRNA Reverse Transcription Kit (Life Technologies). QRT-PCR was executed by using the CFX96 Real-time PCR Detection System (Bio-Rad, Hercules, CA, USA) with the SYBR Green PCR Master Mix (Bio-Rad). The relative expression of circUGGT2, UGGT2, miR-526b-5p, and RAB1A mRNA were figured with the 2−ΔΔCt method, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or U6 small nuclear RNA (snRNA) was served as an internal control. The primers for miR-526b-5p (MIRAP00513) were bought from Merck (Darmstadt, Germany) and the other primers were listed as below: GAPDH (F:5ʹ-GACTCCACTCACGGCAAATTCA-3ʹ; R:5ʹ-TCGCTCCTGGAAGATGGTGAT-3ʹ), circUGGT2 (F:5ʹ-TGGGTGGAGTATGATGCTGA-3ʹ; R:5ʹ-CACACCAGGTTTCACACCAC-3ʹ), UGGT2 (F:5ʹ-CCTTCGCAATCTTGGGATCAA-3ʹ; R:5ʹ-GCCGGATCAATAAACAGAACCA-3ʹ), RAB1A (F:5ʹ-AGATTAAAAAGCGAATGGGTCCC-3ʹ; R:5ʹ-GCTTGACTGGAGTGCTCTGAAT-3ʹ), and U6 snRNA (F:5ʹ-GCTCGCTTCGGCAGCACA-3ʹ; R:5ʹ-GAGGTATTCGCACCAGAGGA-3ʹ).
Actinomycin D (ActD) Treatment and RNase R Digestion
HCC cells were cultured in DMEM containing ActD (2 μg/mL, Sigma, St Louis, MO, USA) for 0, 4, 8, 12, or 24 h to impede RNA transcription. Total RNA of HCC cells were incubated with 3 U/μg RNase R (Geneseed) for 15 min at 37°C. Subsequently, the levels of circUGGT2 and UGGT2 were analyzed with qRT-PCR.
Cell Proliferation Assay
The proliferation of HCC cells was analyzed with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) assay. In short, HCC cells (1×103 cells/well) were seeded on 96-well plates and kept for 24 h, 48 h, or 72 h. Next, the MTT solution (0.5 mg/mL, Sigma) was added to each well. Subsequently, the crystals were dissolved using dimethyl sulfoxide (150 μL, Sigma). Then, the optical density at 570 nm was measured with a microplate reader (Bio-Rad).
Cell Colony Formation Assay
The transfected HCC cells (1×103 cells/well) were seeded on 6-well plates and cultured for 14 days. After fixation with paraformaldehyde (4%, Sigma), the cells were stained with crystal violet (0.5%, Beyotime, Shanghai, China). Next, the colonies (>50 cells) were counted and photographed via the light microscope (Olympus, Tokyo, Japan).
Western Blotting
The RIPA lysis buffer (Beyotime) was employed to isolate total protein form tissue samples and cells. Total protein concentration was quantified with the BCA Protein Assay Kit (Pierce, Rockford, IL, USA). For Western blotting analysis, total protein was separated with the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Next, the separated proteins were transferred to the polyvinylidene difluoride (PVDF) membranes (Beyotime). Subsequently, the membranes were immersed in Tris Buffered Saline Tween (TBST) Buffer containing 5% skim milk. Next, the membranes were incubated with primary antibodies, including anti-cyclin D1 (#2922, 1:1000), anti-proliferating cell nuclear antigen (PCNA) (#2586, 1:2000), anti-RAB1A (#13,099, 1:1000), and anti-GAPDH (#51,332, 1:1000). Thereafter, the membranes were incubated with a secondary antibody (#7076 or #7074). The immunoblots were visualized with enhanced chemiluminescence solution (Beyotime). GAPDH was deemed as a loading control. All antibodies were bought from Cell Signaling Technology (Boston, MA, USA)
Cell Cycle Analysis
After transfection for 48 h, HCC cells (1×106 cells/mL) were harvested and washed with PBS. Then, the cells were fixed with ethanol (70%) at 4°C overnight. Next, the cells were washed PBS, and then treated with RNase A (0.1 mg/mL, Sigma) and propidium iodide (0.05 mg/mL, Sigma) at 37°C for 30 min. Subsequently, the distribution of the cells was evaluated by using a FACScan flow cytometry (BD Biosciences, San Jose, CA, USA) with CellQuest software.
Cell Migration and Invasion Assay
Cell migration was evaluated with an 8 μm pore membrane filter (Costar, Cambridge, MA, USA). The lower chamber was replenished with DMEM containing FBS (10%), and the upper chamber was supplemented with serum-free DMEM containing HCC cells (1×105 cells). After 24 h incubation, the cells on the lower surface were fixed with paraformaldehyde (4%, Sigma) and then stained with crystal violet (0.5%, Beyotime). For invasion assay, the upper chamber was covered with Matrigel (BD Biosciences). The cells were counted with a light microscope (Olympus) at 100 × magnification.
RNA Pull-Down Assay
The biotinylated-circUGGT2 probe (circUGGT2 probe) and Oligo probe were obtained from Sigma. In brief, the circUGGT2 probe was incubated with C-1 magnetic beads (Life Technologies) to generate the probe-coated beads. Thereafter, the lysates of HCC cells were incubated with the probe-coated beads. The bound RNA was extracted with TRIzol reagent (Sigma). The levels of circUGGT2 and miR-526-5p were detected with qRT-PCR.
Dual-Luciferase Reporter Assay
The binding sites between circUGGT2 and miR-526-5p were predicted by using circBank, starBase, and circinteractome databases. The binding sites of miR-526b-5p in RAB1A were predicted with the starBase v2.0 database. The sequences of wild type circUGGT2 (circUGGT2 WT), mutant circUGGT2 (circUGGT2 MUT), wild type 3ʹ untranslated regions (UTR) of RAB1A (RAB1A 3ʹ-UTR-WT), and mutant 3ʹUTR of RAB1A (RAB1A 3ʹ-UTR-MUT) were synthesized and inserted into the pmirGLO luciferase vectors (Promega) to construct luciferase reporter vectors, respectively. Then, the luciferase reporter vectors were cotransfected into HCC cells with miR-NC or miR-526b-5p. The luciferase activities were analyzed with the luciferase reporter assay kit (Promega) by normalizing the firefly luminescence to Renilla luminescence.
Xenograft Assay
Lentivirus carrying short hairpin RNA targeting circUGGT2 (sh-circUGGT2) and corresponding negative control (sh-NC) were bought from GenePharma. For stable knockdown of circUGGT2, HuH-7 cells were infected with lentivirus particles and then selected with puromycin (2 μg/mL). The protocols of xenograft assay were authorized by the Animal Ethics Committee of People’s Hospital of Rizhao. Animal studies were performed in compliance with the ARRIVE guidelines and the Basel Declaration. All animals received humane care according to the National Institutes of Health (USA) guidelines. For xenograft assay, the HuH-7 cells (2×106 cells/0.2 mL PBS) transfected with sh-circUGGT2 or sh-NC were subcutaneously injected into the right flank of 12 BALB/c nude mice (4-week-old, Experimental Animal Center, Shanghai, China) (6 mice/group). All nude were fed under Specific Pathogen Free conditions. Tumor volume was measured once a week with a caliper. Until day 35, the mice were sacrificed by cervical dislocation under isoflurane (5%) to take tumor tissue for subsequent analysis. Tumors volume was calculated according to the following equation: Volume = (length ×width2)/2.
Statistical Analysis
All experiments in vitro were repeated at least 3 times. Data were exhibited as mean ± standard deviation. The normal distribution was determined with a Kolmogorov–Smirnov test. The variance homogeneity was analyzed with the F-test. Statistical analysis was implemented with GraphPad Prism 6 software (GraphPad, San Diego, CA, USA). Differences were deemed significant if P < 0.05. Paired or unpaired Student’s t-test was utilized to compare the difference between 2 groups. For 3 groups or above, the differences were determined by one-way variance analysis (ANOVA) with Turkey’s post hoc test.
Results
CircUGGT2 Expression Was Elevated in HCC Tissues and Cells
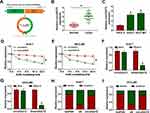
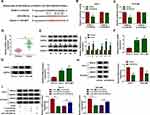
Previous study (microarray data: GSE94508) displayed that circUGGT2 was upregulated in HCC tissues.9 The sequence length of circUGGT2 is 244b, which is formed by the reverse splicing of the UGGT2 gene (exon37 and exon38) (Figure 1A). To explore the biological function of circUGGT2 in HCC, we examined the level of circUGGT2 in 48 paired HCC tissues and para-carcinoma tissues. QRT-PCR results manifested that circUGGT2 expression was elevated in HCC tissues in contrast to the para-carcinoma tissues (Figure 1B). Furthermore, circUGGT2 was highly expressed in HCC cells (HuH-7 and SK-HEP-1) compared to the THLE-2 cells (Figure 1C). We observed that circUGGT2 did not change in HuH-7 and SK-HEP-1 cells treated with ActD for 24 h, while the linear UGGT2 mRNA was significantly reduced (Figure 1D and 1E). Moreover, circUGGT2 was resistant to RNase R digestion in HuH-7 and SK-HEP-1 cells relative to the linear UGGT2 mRNA (Figure 1F and G). Also, circUGGT2 was mainly located at the cytoplasm of HuH-7 and SK-HEP-1 cells (Figure 1H and I). These data indicated that high circUGGT2 expression might be involved in HCC development.
CircUGGT2 Silencing Inhibited Proliferation, Colony Formation, Cell Cycle Progression, Migration, and Invasion of HCC Cells
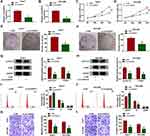
Given that the upregulation of circUGGT2 in HCC tissues and cells, we further investigated the role of circUGGT2 in HCC progression through loss-of-function experiments. We observed that circUGGT2 expression was apparently decreased in HuH-7 and SK-HEP-1 cells after si-circUGGT2 transfection (Figure 2A and B). MTT assay indicated that circUGGT2 knockdown impeded cell proliferation in HuH-7 and SK-HEP-1 cells (Figure 2C and D). Cell colony formation assay presented that the colonial number of circUGGT2-silenced HuH-7 and SK-HEP-1 cells was reduced when compared to the control group (Figure 2E and F). Moreover, the levels of cyclin D1 and PCNA were downregulated in circUGGT2-silenced HuH-7 and SK-HEP-1 cells (Figure 2G and H). Flow cytometry assay manifested that the silence of circUGGT2 elevated the number of HuH-7 and SK-HEP-1 cells in G0/G1 stage and reduced the number of HuH-7 and SK-HEP-1 cells in S stage (Figure 2I and J). Transwell assay exhibited that circUGGT2 knockdown curbed cell migration and invasion in HuH-7 and SK-HEP-1 cells (Figure 2K and L). These findings suggested that circUGGT2 knockdown repressed the malignant behaviors of HCC cells.
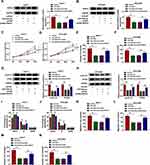
CircUGGT2 Served as a Sponge for miR-526b-5p in HCC Cells
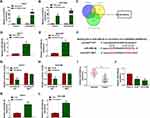
Next, we explored the molecular mechanism of circUGGT2 in HCC. RNA pull-down assay indicated that circUGGT2 was overtly pulled down by circUGGT2 probe in circUGGT2-overexpressed HuH-7 and SK-HEP-1 cells (Figure 3A and B). We predicted miRs that possessed the possible binding sites for circUGGT2 through circBank, starBase, and circinteractome databases. Venn diagram analysis showed that miR-526b-5p had the potential binding sites for circUGGT2 (Figure 3C). RNA pull-down assay exhibited that miR-526b-5p was markedly pulled down by circUGGT2 probe in contrast to the Oligo probe (Figure 3D and E). The potential binding sites between circUGGT2 and miR-526b-5p are exhibited in Figure 3F. Dual-luciferase reporter assay indicated that miR-526b-5p mimic could decrease the luciferase activity of the luciferase reporter vectors with circUGGT2 WT relative to the control group, while there was no marked change in the luciferase reporter vectors with circUGGT2 MUT (Figure 3G and H). Furthermore, miR-526b-5p expression was overtly decreased in HCC tissues and cells compared to their corresponding negative controls (Figure 3I and J). Also, circUGGT2 inhibition could elevate miR-526b-5p expression in HuH-7 and SK-HEP-1 cells (Figure 3K and L). These results indicated that circUGGT2 acted as a sponge for miR-526b-5p in HCC cells.
MiR-526b-5p Inhibitor Reversed circUGGT2 Knockdown-Mediated Effects on Proliferation, Colony Formation, Cell Cycle Progression, Migration, and Invasion of HCC Cells
Based on the above results, we further explored whether miR-526b-5p was involved in the progression of HCC regulated by circUGGT2. The results presented that circUGGT2 silencing increased miR-526b-5p expression in HuH-7 and SK-HEP-1 cells, while this tendency was reversed after anti-miR-526b-5p transfection (Figure 4A and B). Moreover, miR-526b-5p inhibitor abolished the suppressive influence of circUGGT2 downregulation on proliferation and colony formation of HuH-7 and SK-HEP-1 cells (Figure 4CF). Furthermore, the downregulation of cyclin D1 and PCNA in HuH-7 and SK-HEP-1 cells caused by circUGGT2 inhibition were overturned after miR-526b-5p silencing (Figure 4G and H). Also, the inhibitory impact of circUGGT2 silencing on cell cycle progression, migration, and invasion of HuH-7 and SK-HEP-1 cells was abrogated by miR-526b-5p knockdown (Figure 4IN). Together, these data indicated that circUGGT2 regulated the malignancy of HCC cells through sponging miR-526b-5p.
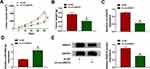
RAB1A Acted as a Target for miR-526b-5p in HCC Cells
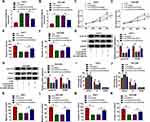
To seek the downstream target of miR-526b-5p in HCC, we predicted the possible targets for miR-526b-5p through the starBase v2.0 database. We found that RAB1A had the latent binding sites for miR-526b-5p (Figure 5A). The forcing expression of miR-526b-5p inhibited the luciferase activity of the luciferase reporter vectors containing RAB1A 3ʹ-UTR-WT in HuH-7 and SK-HEP-1 cells, while the luciferase activity of the luciferase reporter vectors containing RAB1A 3ʹ-UTR-MUT did not change (Figure 5B and C). We also observed that RAB1A mRNA and protein levels were overtly elevated in HCC tissues and cells relative to their matching negative controls (Figure 5DG). Also, miR-526b-5p mimic repressed the levels of RAB1A mRNA and protein in HuH-7 and SK-HEP-1 cells (Figure 5H). In addition, circUGGT2 silencing inhibited the levels of RAB1A mRNA and protein in HuH-7 and SK-HEP-1 cells, while this influence was abrogated after miR-526b-5p knockdown (Figure 5I). Collectively, these findings suggested that circUGGT2 regulated RAB1A expression via sponging miR-526b-5p in HCC cells.
RAB1A Overexpression Abrogated miR-526b-5p Mimic-Mediated Influence on Proliferation, Colony Formation, Cell Cycle Progression, Migration, and Invasion of HCC Cells
In consideration of the relationship between miR-526b-5p and RAB1A in HCC cells, we further explored whether miR-526b-5p exerted its role via targeting RAB1A in HCC cells. We discovered that miR-526b-5p mimic downregulated the level of RAB1A protein in HuH-7 and SK-HEP-1 cells, while this trend was reversed after pcDNA-RAB1A introduction (Figure 6A and B). Furthermore, RAB1A elevation overturned the repressive influence of miR-526b-5p mimic on proliferation and colony formation of HuH-7 and SK-HEP-1 cells (Figure 6CF). Also, miR-526b-5p overexpression inhibited the levels of cyclin D1 and PCNA in HuH-7 and SK-HEP-1 cells, while this suppression was abolished by forcing RAB1A expression (Figure 6G and H). Additionally, RAB1A overexpression restored the suppressive impact of miR-526b-5p mimic on cell cycle progression, migration, and invasion of HuH-7 and SK-HEP-1 cells (Figure 6IN). Taken together, these results suggested that miR-526b-5p exerted its role via targeting RAB1A in HCC cells.
CircUGGT2 Silencing Inhibited HCC Growth in vivo
Considering that circUGGT2 knockdown impeded the malignancy of HCC cells in vitro, we further verified the role of circUGGT2 in HCC in vivo through xenograft assay. We discovered that circUGGT2 silencing could impede tumor growth and reduce tumor weight in contrast to the control group (Figure 7A and B). Also, circUGGT2 was downregulated while miR-526b-5p was upregulated in mice tumor tissues in the sh-circUGGT2 group compared to the control group (Figure 7C and D). In addition, RAB1A protein was also downregulated in mice tumor tissues in the sh-circUGGT2 group than that in the sh-NC group (Figure 7E). These data indicated that circUGGT2 knockdown could curb HCC growth in vivo.
Discussion
Recently, a series of circRNAs have been verified in human cancers.23,24 For HCC, some circRNAs have been unmasked to play a promoting or suppressive role. Zhang et al proved that circRNA circ_104075 stimulated YAP-dependent tumorigenesis via sponging miR-582-3p in HCC.25 Moreover, circRNA circ_RHOT1 could facilitate HCC progression via initiating NE2F6 expression by recruiting TIP60.26 Another example, circRNA circ_5692 played a inhibitory role in HCC by downregulating DAB2IP via sponging miR-328-5p.27 As far as we know, the role of circUGGT2 in HCC is indistinct. Thus, we explored the role and regulatory of circUGGT2 in HCC progression.
Herein, we verified that circUGGT2 expression was elevated in HCC tissues and cells. Furthermore, the downregulation of circUGGT2 curbed tumor growth in vivo and impeded proliferation, colony formation, cell cycle progression, migration, and invasion of HCC cells in vitro. Previous research reported that circUGGT2 was upregulated in HCC tissues.9 Zhou et al revealed that circUGGT2 was highly expressed in papillary thyroid carcinoma tissues, and circUGGT2 silencing inhibited papillary thyroid carcinoma progression via inactivation of the AMPK/mTOR pathway.10 These data illustrated that circUGGT2 exerted a cancerogenic role in HCC.
Increasing researches have indicated that circRNAs can regulate the development of various tumors via serving as competing endogenous RNAs (ceRNAs). For example, circRNA circ_5692 suppressed HCC progression via elevating DAB2IP expression through competitively binding to miR-328-5p.27 MiR-526b acted as a tumor suppressor in diverse tumors. One report uncovered that miR-526b mimic repressed glycolysis, metastasis, and proliferation of colon cancer cells through downregulating HIF-1α.28 Another study proved that miR-526b overexpression inhibited the malignancy of glioma cells via targeting WEE1.29 Wei et al revealed that circRNA circ_0091518 overexpression inhibited miR-526b expression in HCC cells, thereby accelerating the malignancy of HCC cells.16 Report of Zhao et al revealed that circRNA circ_UHRF1 accelerated the tumorigenesis and epithelial-mesenchymal transformation of oral squamous cell carcinoma via circUHRF1/miR-526b-5p/c-Myc/TGF-β1/ESRP1 feedback loop.30 Herein, we discovered that circUGGT2 modulated RAB1A expression via sponging miR-526b-5p inHCC cells. Furthermore, miR-526b-5p inhibitor overturned circUGGT2 inhibition-mediated influence on the malignancy of HCC cells. Thus, we inferred that circUGGT2 regulated HCC advancement through sponging miR-526b-5p.
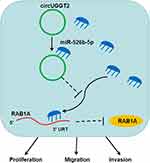
RAB1A was verified as an oncogene in diverse tumors. Xu et al claimed that RAB1A facilitated triple-negative breast cancer progression via activating the mTORC1 pathway.31 Also, RAB1A downregulation inhibited the development of ovarian cancer32 and colorectal cancer.33 Zhang et al revealed that miR-634 curbed HCC development via downregulating RAB1A.22 In this study, RAB1A acted as a target for miR-526b-5p in HCC cells. RAB1A elevation overturned the suppressive influence of miR-526b-5p mimic on the malignancy of HCC cells. Thus, we concluded that circUGGT2 regulated HCC progression via regulating RAB1A expression by sponging miR-526b-5p (Figure 8). Regrettably, the complete mechanism related to the circUGGT2/miR-526b-5p/RAB1A axis is still unclear, and this can be further explored in the future.
In summary, circUGGT2 played a carcinogenic role in HCC. Downregulation of circUGGT2 curbed HCC development via regulating the miR-526b-5p/RAB1A axis indicating that circUGGT2 might be a latent target for HCC treatment.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no financial or non-financial conflicts of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur j Cancer. 2012;48(5):599–641. doi:10.1016/j.ejca.2011.12.021
3. Zhang P-F, Wei C-Y, Huang X-Y, et al. Circular RNA circTRIM33-12 acts as the sponge of MicroRNA-191 to suppress hepatocellular carcinoma progression. Mol Cancer. 2019;18(1):105. doi:10.1186/s12943-019-1031-1
4. Oliveri RS, Wetterslev J, Gluud C. Hepatocellular carcinoma. Lancet. 2012;380(9840):
5. Fu L, Jiang Z, Li T, Hu Y, Guo J. Circular RNAs in hepatocellular carcinoma: functions and implications. Cancer Med. 2018;7:3101–3109. doi:10.1002/cam4.1574
6. Han B, Chao J, Yao H. Circular RNA and its mechanisms in disease: from the bench to the clinic. Pharmacol Ther. 2018;187:31–44. doi:10.1016/j.pharmthera.2018.01.010
7. Qu S, Yang X, Li X, et al. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015;365(2):141–148. doi:10.1016/j.canlet.2015.06.003
8. Bi J, Liu H, Cai Z, et al. Circ-BPTF promotes bladder cancer progression and recurrence through the miR-31-5p/RAB27A axis. Aging. 2018;10(8):1964–1976. doi:10.18632/aging.101520
9. Qiu L, Wang T, Ge Q, et al. Circular RNA signature in hepatocellular carcinoma. J Cancer. 2019;10(15):3361–3372. doi:10.7150/jca.31243
10. Zhou GK, Zhang GY, Yuan ZN, Pei R, Liu DM. Has_circ_0008274 promotes cell proliferation and invasion involving AMPK/mTOR signaling pathway in papillary thyroid carcinoma. Eur Rev Med Pharmacol Sci. 2018;22(24):8772–8780. doi:10.26355/eurrev_201812_16644
11. Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi:10.1146/annurev-pathol-012513-104715
12. Kwan JYY, Psarianos P, Bruce JP, Yip KW, Liu -F-F. The complexity of microRNAs in human cancer. J Radiat Res. 2016;57(Suppl 1):i106i111. doi:10.1093/jrr/rrw009
13. Mou T, Xie F, Zhong P, et al. MiR-345-5p functions as a tumor suppressor in pancreatic cancer by directly targeting CCL8. Biomed Pharmacother. 2019;111:
14. Liu YQ, Cong YZ, Jiang J, et al. MiR-526b suppresses cell proliferation, cell invasion and epithelial-mesenchymal transition in breast cancer by targeting Twist1. Eur Rev Med Pharmacol Sci. 2020;24(6):3113–3121. doi:10.26355/eurrev_202003_20678
15. Yan M, Gao H, Lv Z, et al. Circular RNA PVT1 promotes metastasis via regulating of miR-526b/FOXC2 signals in OS cells. J Cell Mol Med. 2020. doi:10.1111/jcmm.15215
16. Wei X, Zheng W, Tian P, et al. Oncogenic hsa_circ_0091581 promotes the malignancy of HCC cell through blocking miR-526b from degrading c-MYC mRNA. Cell Cycle. 2020;19(7):817–824. doi:10.1080/15384101.2020.1731945
17. Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91(1):119–149. doi:10.1152/physrev.00059.2009
18. Wang J, Xing H, Nikzad AA, et al. Long noncoding RNA MNX1 antisense RNA 1 exerts oncogenic functions in bladder cancer by regulating miR-218-5p/RAB1A Axis. J Pharmacol Exp Ther. 2020;372(3):237–247. doi:10.1124/jpet.119.262949
19. Thomas JD, Zhang Y-J, Wei Y-H, et al. Rab1A is an mTORC1 activator and a colorectal oncogene. Cancer Cell. 2014;26(5):754–769. doi:10.1016/j.ccell.2014.09.008
20. Cheng Z, Shao X, Xu M, et al. Rab1A promotes proliferation and migration abilities via regulation of the HER2/AKT-independent mTOR/S6K1 pathway in colorectal cancer. Oncol Rep. 2019;41(5):2717–2728. doi:10.3892/or.2019.7071
21. Wang X, Liu F, Qin X, et al. Expression of Rab1A is upregulated in human lung cancer and associated with tumor size and T stage. Aging. 2016;8(11):2790–2798. doi:10.18632/aging.101087
22. Zhang CZ, Cao Y, Fu J, Yun J-P, Zhang M-F. miR-634 exhibits anti-tumor activities toward hepatocellular carcinoma via Rab1A and DHX33. Molecular Oncol. 2016;10(10):1532–1541. doi:10.1016/j.molonc.2016.09.001
23. Xiong -D-D, Feng Z-B, Lai Z-F, et al. High throughput circRNA sequencing analysis reveals novel insights into the mechanism of nitidine chloride against hepatocellular carcinoma. Cell Death Dis. 2019;10(9):658. doi:10.1038/s41419-019-1890-9
24. Li X-N, Wang Z-J, Ye C-X, Zhao B-C. RNA sequencing reveals the expression profiles of circRNA and indicates that circDDX17 acts as a tumor suppressor in colorectal cancer. J Experimental Clin Cancer Res. 2018;37(1):325. doi:10.1186/s13046-018-1006-x
25. Zhang X, Xu Y, Qian Z, et al. circRNA_104075 stimulates YAP-dependent tumorigenesis through the regulation of HNF4a and may serve as a diagnostic marker in hepatocellular carcinoma. Cell Death Dis. 2018;9(11):1091. doi:10.1038/s41419-018-1132-6
26. Wang L, Long H, Zheng Q, Bo X, Xiao X, Li B. Circular RNA circRHOT1 promotes hepatocellular carcinoma progression by initiation of NR2F6 expression. Mol Cancer. 2019;18(1):119. doi:10.1186/s12943-019-1046-7
27. Liu Z, Yu Y, Huang Z, et al. CircRNA-5692 inhibits the progression of hepatocellular carcinoma by sponging miR-328-5p to enhance DAB2IP expression. Cell Death Dis. 2019;10(12):900. doi:10.1038/s41419-019-2089-9
28. Zhang R, Zhao J, Xu J, Wang J, Jia J. miR-526b-3p functions as a tumor suppressor in colon cancer by regulating HIF-1α. Am J Transl Res. 2016;8(6):2783–2789.
29. Wu M, Li X, Liu Q, Xie Y, Yuan J, Wanggou S. miR-526b-3p serves as a prognostic factor and regulates the proliferation, invasion, and migration of glioma through targeting WEE1. Cancer Manag Res. 2019;11:
30. Zhao W, Cui Y, Liu L, et al. Splicing factor derived circular RNA circUHRF1 accelerates oral squamous cell carcinoma tumorigenesis via feedback loop. Cell Death Differ. 2020;27(3):919–933. doi:10.1038/s41418-019-0423-5
31. Xu H, Qian M, Zhao B, et al. Inhibition of RAB1A suppresses epithelial-mesenchymal transition and proliferation of triple-negative breast cancer cells. Oncol Rep. 2017;37(3):1619–1626. doi:10.3892/or.2017.5404
32. Zha JF, Chen DX. MiR-655-3p inhibited proliferation and migration of ovarian cancer cells by targeting RAB1A. Eur Rev Med Pharmacol Sci. 2019;23(9):3627–3634. doi:10.26355/eurrev_201905_17786
33. Pei F-L, Cao M-Z, Li Y-F. Circ_0000218 plays a carcinogenic role in colorectal cancer progression by regulating miR-139-3p/RAB1A axis. J Biochem. 2020;167(1):55–65. doi:10.1093/jb/mvz078
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.