Back to Journals » Cancer Management and Research » Volume 12
CircPVT1 Promoted the Progression of Breast Cancer by Regulating MiR-29a-3p-Mediated AGR2-HIF-1α Pathway
Authors Wang J, Huang K, Shi L, Zhang Q, Zhang S
Received 17 June 2020
Accepted for publication 1 October 2020
Published 12 November 2020 Volume 2020:12 Pages 11477—11490
DOI https://doi.org/10.2147/CMAR.S265579
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Jing Wang,1 Kuo Huang,2 Lang Shi,3 Qingyong Zhang,2 Shengchu Zhang1
1Department ofThyroid and Breast Surgery, The First College of Clinical Medical Science, China Three Gorges University, Yichang, Hubei, 443003, People’s Republic of China; 2Department of Clinical Laboratory, The First College of Clinical Medical Science, China Three Gorges University, Yichang, Hubei, 443003, People’s Republic of China; 3The First College of Clinical Medical Science, China Three Gorges University, Yichang, Hubei 443003, People’s Republic of Chinaggg
Correspondence: Shengchu Zhang
Departments of General Surgery, The First College of Clinical Medical Science, China Three Gorges University, Yichang, Hubei 443003, People’s Republic of China
Tel +86 18871788125
Email [email protected]
Background: Breast cancer (BC) is a great contributor to cancer-related death. Mounting studies have identified that circular RNAs (circRNAs) play vital roles in cancer cell proliferation, apoptosis and invasion. Here, we explored the effect of circPVT1 on BC development as well as its downstream mechanisms.
Methods: qRT-PCR was used to determine the relative expression levels of circPVT1 and miR-29a-3p in BC tissue samples and cell lines. We also analyzed the relevance between pathological indexes and circPVT1 expression level. Human breast cancer cell lines MCF-7 and MDA-MB-231 were taken as cell models. Gain- or loss-of-functional assays of circPVT1 and miR-29a-3p were conducted in BC cell lines to investigate their effects on the cell proliferation, apoptosis, migration and invasion. The protein levels of AGR2, HIF-1α, Bax, Bcl2 and Caspase3 were determined by Western blot. Furthermore, dual-luciferase reporter assay and RNA fluorescence in situ hybridization (FISH) were used to confirm the targeted relationships between circPVT1 and miR-29a-3p, miR-29a-3p and anterior gradient 2 (AGR2).
Results: CircPVT1 was highly expressed while miR-29a-3p was lowly expressed in BC tissues and cell lines. Inhibition of circPVT1 or overexpression of miR-29a-3p remarkably suppressed BC cell proliferation, invasion and migration while promoted cell apoptosis. By contrast, circPVT1 upregulation or miR-29a-3p inhibition led to mitigate malignant behaviours of BC cells. Functionally, circPVT1 bound to miR-29a-3p, and AGR2 was a target gene of miR-29a-3p. Overexpressed circPVT1 promoted AGR2 and HIF-1α expression by repressing miR-29a-3p. More importantly, overexpressing AGR2 enhances HIF-1α expression, accompanied with accelerated proliferation, invasion and migration of BC cells.
Conclusion: CircPVT1 acts as an oncogene in BC via promoting the growth, invasion, migration and inhibiting apoptosis through miR-29a-3p-mediated AGR2-HIF-1α axis.
Keywords: circPVT1, miR-29a-3p, AGR2, breast cancer, proliferation, metastasis
Introduction
Breast cancer (BC), the fifth global fatal disease, is the most prevailing malignant tumor, which also seriously threatens women’s health and life.1,2 Although surgical treatment, radiotherapy, and chemotherapy have been continuously improved, they have also brought heavy side effects to BC patients.3 Hence, it is necessary to clarify the molecular mechanism of BC, thus improving the early diagnosis of BC and providing new molecular therapeutic targets.
CircRNA, as a relatively new non-coding RNA, is generated in the formation of a closed-loop by covalent bonds at the 3ʹ and 5ʹ ends. Due to this closed structure, circRNA has been proved to be extremely stable and highly resistant to RNA degradation pathways, suggesting that circRNA may be more effective for molecular biomarkers of human disease than linear non-coding RNAs.4 In recent years, papers have shown that circRNA makes a great contribution to regulating tumorigenesis and tumor development. Therefore, those abnormally expressed circRNAs afford new ideas for tumor diagnosis and treatment.5 CircPVT1 is located in the known cancer susceptibility region chr8: 128902834–128903244, which is a newly identified circRNA.6 It is derived from the third exon of lncRNA-PVT1 and is identified as an oncogene.7 Previous studies have shown that circPVT1 is upregulated in non-small cell lung cancer and maybe a potential prognostic marker.8 However, the expression characteristics and biological functions of circPVT1 in BC are virtually unknown.
MicroRNAs (miRNAs), belonging to non-coding mRNAs, are about 22 nucleotides in length. Current research indicates that miRNAs specifically binds to the 3ʹ-untranslational region (UTR) of mRNA, thus regulating gene expression at the post-transcriptional level.9 Recently, accumulating papers have shown that miRNAs take part in tumor development. Taking miR-411-5p as an example, its expression is notably decreased in non-small cell lung cancer and significantly associated with patients’ poor prognosis.10 Moreover, miR-29a-3p expression was considerably declined in papillary thyroid carcinoma (PTC) tissues and cells. The functional studies revealed that overexpression of miR-29a-3p limited the growth, proliferation, and invasion of PTC cells,11 suggesting that miR-29a-3p exerts an anti-tumor role in cancer. However, the mechanism of its action in BC awaits further clarification.
In this research, we measured circPVT1 expression in clinical samples of BC patients and carried out experiments both in vitro and in vivo to investigate its role on BC development. Our data proved that circPVT1 expression was remarkably raised in BC tissues and cells, while gain and loss experiments confirmed that circPVT1 aggravates BC cell proliferation, migration, and invasion, suggesting its carcinogenic effect on BC progression. Further investigation of its downstream mechanism revealed that circPVT1 regulates miR-29a-3p expression as a competitive endogenous RNA (ceRNA), so as to increase AGR2 and HIF-1α expression. All in all, this study explored the regulation axis of circPVT1/miR-29a-3p/AGR2/HIF-1α in BC development, which provides a new target for its treatment.
Materials and Methods
Tissue Samples
From January 2018 to January 2019, the cancer tissues of 40 BC patients who underwent breast cancer resection in the First College of Clinical Medical Science of China Three Gorges University were selected. The forty patients did not receive adjuvant treatment such as chemotherapy or radiotherapy before the operation. The adjacent normal tissues of the same patient (at least 3 cm away from the surgical resection margin) were used as normal control, and no cancer cells were found in adjacent normal tissues in the postoperative pathological examination. All specimens were immediately stored in liquid nitrogen at −196°C until RNA was extracted. The ethics committee of China Three Gorges University approved our study, and all participators signed informed consents.
Cell Culture
Human breast cancer cell lines (MDA-MB-231 and MCF7) were purchased from the Cell Center in the Chinese Academy of Sciences (Shanghai, China). The above cells were cultured in DMEM-F12 (Thermo Fisher Scientific, MA, USA) with 10% fetal bovine serum (FBS, Thermo Fisher Scientific, MA, USA) and 1% penicillin/tomycin (Invitrogen, CA, USA) in an incubator (37°C, 5% volume fraction of CO2). In the logarithmic growth phase, cells underwent 0.25% trypsinization (Thermo Fisher HyClone, Utah, USA) and passage.
Cell Transfection
In line with the supplier’s instructions, MDA-MB-231 and MCF7 cells underwent transfection using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.). The cells were inoculated in a 12-well plate and cultured in DMEM-F12 containing 10% FBS without antibiotics. After pasted to the wall the next day, the old culture solution was discarded for transfection. Each liposome hole was added with, overexpression plasmid of circPVT1 (pcDNA-circPVT1), AGR2 (pcDNA-AGR2) and their pcDNA empty vectors (vector), short hairpin RNA (shRNA) against circPVT1 (sh-circPVT1) and its negative control (sh-NC), miRNA control (miR-NC), miR-29a-3p mimics, and miR-29a-3p inhibitors were purchased from GenePharma Co., Ltd. (Shanghai, China). qRT-PCR was performed to measure transfection efficiency. Finally, the cells were incubated for 24 hours (37°C, 5% CO2) and were subjected to further analysis.
Real-Time Polymerase Chain Reaction (RT-PCR)
We used TRIzol reagent (Invitrogen, Shanghai, China) to extract total RNA in BC tissues and cells. RNA samples were reversely transcribed to cDNA by MMLV reverse transcriptase and stored at −20°C for later use. RNA got reverse transcription using the stem-loop method and ordinary reverse transcription method, respectively. When the first-strand cDNA was obtained, SYBR premix EXTAQ II (TaKaRa, Dalian, China) was used in the ABI7500 real-time PCR system (Applied Biosystems, SanFrancisco, CA, United States) to conduct quantitative real-time polymerase chain reaction (RT-PCR) in accordance with the manufacturer’s instructions. PCR procedures were as follows: pre-denaturation at 95°C, 10 min; 95°C, 15 s; 60 °C, 15 s; 45 cycles, obtaining fluorescence signal at a temperature of 60°C. GAPDH was as the standardized endogenous control for circPVT1 and AGR2, and U6 was the endogenous control for miR-29a-3p. The results of gene expression were counted via the 2 (-ΔΔCt) method. Each experiment was repeated three times.
CCK-8 Assay
CCK-8 assay was carried out to assess the cell viability. Stably transfected cells were inoculated with 1×103 cells/well into a 96-well plate for a 24-hour incubation. With the addition of 10 μL CCK-8 (Beyotime Biotechnology, Shanghai, China) solution into each well, the cell went through one-hour incubation at 37°C. Then, the absorbance was measured on a spectrophotometer at 450 nm (Bio-Rad, CA, USA).
Transwell Assay
Transwell assay was conducted to examine cell migration and invasion. BC Cells (2×104 cells/well) were added to the Transwell upper chamber with an 8 μm pore size membrane, and 600 μL of a culture solution containing 20% FBS was added to the lower chamber and cultured at 37°C. After 24 hours, cells in the upper chamber were abandoned, and those in the lower chamber were secured with 4% paraformaldehyde, stained with 0.1% crystal violet, photographed and counted after drying. Cell invasion experiment: The upper chamber should be coated with Matrigel matrix gel in advance of cell seeding. The rest steps were the same as the migration experiment.
Dual-Luciferase Reporter Gene Assay
All luciferase reporter vectors (circPVT1-WT, circPVT1-MT1, circPVT1-MT2, circPVT1-MT1+MT2, AGR2-WT, AGR2-MT) were constructed by Promega (Promega, Madison, WI, USA). MDA-MB-231 (5×104) was inoculated into 48-well plates and cultured to 70% confluence. circPVT1-WT and AGR2-WT were the wild type (WT) vectors that contained the binding sites with miR-29a-3p, while circPVT1-MT and AGR2-MT were mutant type (WT) vectors that did not contain the binding sites with miR-29a-3p. Then Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) was used to co-transfect the luciferase reporter vectors with miR-29a-3p mimics or negative control into MDA-MB-231. After 48 hours of transfection, the luciferase activity of the cells was detected. All experiments were carried out in triplicate and conducted for three times.
Western Blot
After the cells were processed in different factors, the old culture solution was discarded, and the cells were trypsinized with the lysate of RIPA (containing 1% PMSF) (Beyotime Biotechnology, Shanghai, China) and collected by centrifugation to extract the total protein. After total protein was extracted, a BCA protein concentration kit (Beyotime Biotechnology, Shanghai, China) was used to quantify protein concentration. Twenty microgram total protein in each group was subjected to SDS-PAGE for the separation of proteins. When the electrophoresis was finished, the isolated protein was transferred to the PVDF membranes at 200 mA for 90 min. Then, the membranes were blocked with a 10% skim milk powder solution for 2 h, the AGR2 monoclonal antibody (Abcam, ab76473; 1:1000), HIF-1A monoclonal antibody (Abcam, ab179483; 1:1000), Cleaved Caspase-3 polyclonal antibody (Abcam, ab2302; 1:1000), Bcl2 polyclonal antibody (Abcam, ab182858; 1:1000), Bax monoclonal antibody (Abcam, ab32503; 1:1000) were used for incubation overnight at 4°C. Next, TBST was applied to wash the membranes for three times (15 min each time), and the membranes were incubated with the secondary antibody for 2 h at room temperature and washed three times with TBST, and protein blots were exposed using an ECL chemiluminescence solution (Beyotime Biotechnology, Shanghai, China).
Cell Apoptosis Assay
The stable transfected MDA-MB-231 and MCF7 cells were cultured in DMEM-F12 medium supplemented with 10% FBS, respectively. After a 48-hour culture, the cells were collected, and the Annexin V-FITC assay was used for the determination of cell apoptosis using the PE Annexin V apoptosis detection kit (BD Pharmingen). BD FACSCalibur™ flow cytometer (Becton Dickinson, Mountain View, CA, USA) was used for flow cytometric analysis. The data were collected from three repeated experiments.
Xenograft Tumor Assay
In vivo experiment on nude mice was conducted to test the role of circPVT1 on BC cell growth. Twenty male BALB/c nude mice (4 weeks old) were provided by the Experimental animal center of Three Gorges University. The mice were kept in specific-pathogen free (SPF) conditions and free for food and water. Each nude mouse was subcutaneously injected with a total number of 1×108 MCF7 cells that were transfected with vectors or circPVT1 overexpressing plasmids. From the 2nd week of injection, the length and width of the formed tumors were tested every week and the tumor volume were calculated as: volume = 0.5 × length × width2. At the 5th week, the mice were sacrificed, and the tumors were excised and weighed. Our animal study has been approved by the Animal Care and Use Committee of Three Gorges University. All experimental procedures were performed in accordance with the approval and guidelines from the Institutional Animal Care and Use Committee of Three Gorges University.
RNA FISH
The co-location of circPVT1 and miR-29a-3p in BC cells were examined by RNA FISH assay according to previous study.12 We obtained the DNA oligo probes of circPVT1 (labeled with FAM) and miR-29a-3p (labeled by Cy5) from GenePharma (Shanghai, China). MCF7 cells were isolated and cultured in 24-well plates (each well contained about 1×105 cells) and then subjected to cellular immunofluorescence. 4, 6-diamidino- 2-phenylindole (DAPI) (Beyotime Biotechnology) was used for nuclei staining. The Leica SP5 confocal microscope (Leica Microsystems, Mannheim, Germany) was used to observe and photograph cells.
Statistical Methods
SPSS17.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for analysis. T-test was used for comparison between two groups, and analysis of variance was used for comparison of mean between multiple groups. Measurement data were expressed as mean ± standard deviation (x±s). Enumeration data were presented in four tables (or percentages), and differences between the two groups were evaluated by χ2. P < 0.05 was considered statistically significant.
Results
CircPVT1 Was Upregulated in BC Tissues and MiR-29a-3p Was Downregulated in BC Tissues
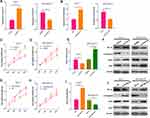
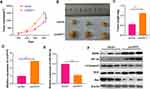
To explore the function of circPVT1 and miR-29a-3p in BC, we first examined the expression of circPVT1 and miR-29a-3p in BC tissues. RT-PCR results illustrated that circPVT1 was remarkably increased in BC tissues (compared with adjacent normal tissues), while miR-29a-3p levels were considerably decreased (Figure 1A and B). Furthermore, we compared the expression of circPVT1 and miR-29a-3p in BC tissues and normal samples using the Starbase database (http://starbase.sysu.edu.cn/index.php). Interestingly, circPVT1 was upregulated while miR-29a-3p was lower both compared with normal samples (Figure 1C and D). Further analysis of the overall survival through Kaplan–Meier analysis (http://kmplot.com/analysis/) demonstrated that BC patients with low miR-29a levels had worse survival and less median survival time (Figure 1E). Moreover, Pearson χ2 test was employed to assess the correlation between circPVT1 and clinicopathological features. The statistics represented that overexpressed circPVT1 was greatly correlated with positive lymph node examination and tumor size (Table 1). Collectively, those data suggested that circPVT1 and miR-29a-3p are both involved in BC development.
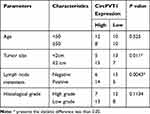 |
Table 1 Association Between CircPVT1 Expression and Clinicopathological Features |
 |
Figure 1 Expression of circPVT1 and miR-29a-3p in BC tissues. (A–B) RT-PCR was used to detect the expression of circPVT1 and miR-29a-3p in BC tissues and compared normal tissues, ***P<0.001. (C and D) The expression of circPVT1 and miR-29a-3p in BC tissues was analysed by Starbase database (http://starbase.sysu.edu.cn/index.php). (E) The Kaplan–Meier plotter (http://kmplot.com/analysis/index.php?p=background) was used to assess different level of miR-29a on the survival of BC patients. |
The Effects of CircPVT1 and MiR-29a-3p in BC Cell Proliferation and Apoptosis
To further explore the effects of circPVT1 and miR-29a-3p on BC progression, gain- and loss-of-function assays were performed (Figure 2A and B). What’s more, altered BC cell proliferation and apoptosis were examined via CCK-8 assay and flow cytometry. Results of CCK-8 showed that overexpression of circPVT1 accelerated the proliferation of BC cells while downregulation of circPVT1 inhibited BC cells proliferation (Figure 2C–D). In addition, overexpression of circPVT1 mitigated apoptosis rate and the protein level of cleaved Caspase3, Bax, and promoted Bcl2 expression. However, circPVT1 inhibition attenuated apoptotic levels (Figure 2E–F). On the other hand, upregulating miR-29a-3p repressed cell proliferation and increased cell apoptosis, while downregulating miR-29a-3p had the opposite effects (Figure 2G–J). What’s more, we measured the protein level of HIF-1α. The data showed that HIF-1α level was markedly enhanced with circPVT1 overexpression and miR-29a-3p inhibition, but significantly repressed by circPVT1 knockdown and miR-29a-3p upregulation (Figure 2F and J). Hence, the above results verified that circPVT1 exerts an oncogenic role in BC and miR-29a-3p is an anti-tumor gene.
The Overexpression of CircPVT1 and Downregulation of MiR-29a-3p Enhanced BC Cell Invasion and Migration
It is well known that the metastasis of malignant tumors is an important factor affecting patients’ prognosis. Here, we examined BC cells’ migration and invasion by Transwell assay. It turned out that the number of migrated and invaded BC cells remarkably increased after overexpression of circPVT1, while knockdown of circPVT1 considerably suppressed cell migration and invasion (Figure 3A and B). Additionally, the effect of miR-29a-3p on BC cells’ metastasis was detected. The results manifested that overexpression of miR-29a-3p notably mitigated cell migration and invasion, while down-expression of miR-29a-3p had the opposite effects (Figure 3C and D). Therefore, we further confirmed that both of circPVT1 and miR-29a-3p could modulate BC development via affecting BC cell invasion and migration.
CircPVT1 Can Target MiR-29a-3p as a miRNA Sponge
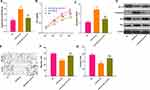
Aiming to further explore the downstream mechanisms of circPVT1, we browsed the bioinformatics database Starbase (http://starbase.sysu.edu.cn/index.php) to search for potential target genes of circPVT1. As a result, it was found that circPVT1 contains two binding sites with miR-29a-3p (Figure 4A). Further, we used linear regression to analyze the levels of circPVT1 and miR-29a-3p in BC tissues. The results revealed that there was a negative correlation between the two genes (P < 0.001, Figure 4B). Next, the relationship between circPVT1 and miR-29a-3p was identified using the luciferase reporter gene assay. The results demonstrated that the miR-29a-3p mimics reduced the luciferase activity of circPVT1-WT, but had no obvious effect on the activity of circPVT1-MT luciferase (Figure 4C). Additionally, the results of RNA FISH indicated that circPVT1 and miR-29a-3p were both mainly distributed in the cytoplasm of MCF7 cells, and they also have direct interaction in the cytoplasm (Figure 4D). What’s more, qRT-PCR experiment results showed that the level of miR-29a-3p was downregulated when circPVT1 was upregulated, while enhanced with circPVT1 knockdown (Figure 4E). These results represented that circPVT1 can sponge miR-29a-3p and inhibit its expression.
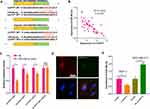 |
Figure 4 CircPVT1 can target miR-29a-3p. (A) Using the bioinformatics database Starbase (http://starbase.sysu.edu.cn/index.php) to look for potential target genes for circPVT1, circPVT1 contains a potential binding site to miR-29a-3p. (B) The correlation of circPVT1 and miR-29a-3p expression in BC tissues was analysed by using linear regression analysis. (C) Further luciferase experiments were used to verify the relationship between the two molecules. (D) RNA-FISH was used to detect the location of circPVT1 and miR-29a-3p in MCF7 cells. (E) qRT-PCR was utilized to detect the level of miR-29a-3p in BC cell lines after overexpression and knockdown of circPVT1. NS. P>0.05, *P<0.05, **P<0.01, ***P<0.001. N=3. |
Overexpression of CircPVT1 Attenuated the Inhibitory Effect of MiR-29a-3p on BC Cell Proliferation and Metastasis
To further explore the effect of circPVT1 and miR-29a-3p on BC, we performed rescue experiment. RT-PCR results revealed that miR-29a-3p expression was decreased following the overexpression of circPVT1 (vs miR-29a-3p group) (Figure 5A). Besides, the proliferation, apoptosis, migration and invasion of BC cells were detected. We found that the proliferative, migrative and invasive levels of BC cells in the miR-29a-3p+circPVT1 group were increased while the apoptosis rate was less compared with those in the miR-29a-3p group (Figure 5B–G). The above findings indicated that overexpression of circPVT1 attenuated the inhibitory effect of miR-29a-3p on BC cells.
AGR2 Was a Functional Target of MiR-29a-3p
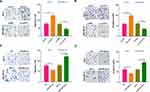
To explore the downstream target of miR-29a-3p in BC, we selected candidate target for miR-29a-3p at TargetScan (http://www.targetscan.org/vert_72/). It turned out that miR-29a-3p binds to the 3ʹUTR region of AGR2 mRNA, suggesting that the AGR2 is likely to be a potential target gene for miR-29a-3p (Figure 6A). Furthermore, the luciferase assay showed that miR-29a-3p mimics reduced the luciferase activity of cells transfected with AGR2-WT, but had no obvious effect on cells transfected with AGR2-MT (Figure 6B). Next, we analyzed the expression of AGR2 in BC tissues through GEPIA database (http://gepia.cancer-pku.cn/). As the data shows, AGR2 was upregulated in BC tissues compared with the normal tissues (Figure 6C). Moreover, we searched another online database The Human Protein Atlas (https://www.proteinatlas.org/), we found that both of AGR2 and HIF-1α were slightly-expressed in normal breast tissues. In the BC tissues, AGR2 protein is markedly enhanced, mainly locating in the cytoplasm and cell membrane of tumor cells, while HIF-1α also has a moderate expression in BC tissues and mainly locates in the cytoplasm (Figure 6D). Next, we detected AGR2 mRNA and protein levels after the selective regulation of circPVT1 and miR-29a-3p. Our data revealed that AGR2 was overexpressed after circPVT1 overexpression or miR-29a-3p downregulation, while downregulated when circPVT1 was lowly expressed or miR-29a-3p was upregulated (Figure 6E, F, H, I). Moreover, forced expression of circPVT1 on miR-29a-3p overexpressed cells also enhanced AGR2 expression (Figure 6G and J). These statistics demonstrated that AGR2 is a target gene of miR-29a-3p and positively regulated by circPVT1 in BC cells.
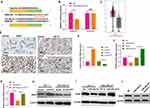 |
Figure 6 AGR2 was a functional target of miR-29a-3p. (A) The target of miR-29a-3p was selected at TargetScan (http://www.targetscan.org/vert_72/), and the results showed that miR-29a-3p can bind to the 3ʹUTR region of AGR2 mRNA. (B) Dual-luciferase activity assay was used to verify the targeting relationship of miR-29a-3p and AGR2 in MCF7 cells, NS. P>0.05, **P<0.01. (C) Expression of AGR2 in BC tissues was analysed through GEPIA (http://gepia.cancer-pku.cn/), BRCA represents breast cancer, T represents tumor tissues and N represents normal tissues. (D) The Human Protein Atlas (https://www.proteinatlas.org/) database was used analyze AGR2 and HIF-1α expression in normal breast tissues and BC tissues. (E–J) The mRNA (E–G) and protein (H–J) expression of AGR2 were detected by Western blot after selective regulation of circPVT1 and miR-29a-3p. NS P>0.05, *P<0.05, **P<0.01, ***P<0.001. N=3. |
MiR-29a-3p Reversed the Promotive Effects of AGR2 on BC Cells
To further explore the bio-functions of miR-29a-3p/AGR2 axis in BC development, an AGR2-overexpressed cell model was constructed and then transfected with miR-29a-3p mimics. The results of qRT-PCR and Western blot showed that miR-29a-3p overexpression restrained AGR2 expression (Figure 7A and B). Moreover, we detected the malignant phenotypes of BC cells. Our data showed that AGR2 overexpression promoted the proliferation, migration, invasion and repressed apoptosis (Figure 7C–F). Interestingly, forced miR-29a-3p expression attenuated the effects induced by AGR2 overexpression (Figure 7C-F). It is worth noting that overexpression of AGR2 enhanced HIF-1α level, while miR-29a-3p attenuated HIF-1α (Figure 7E). As a result, we believed that AGR2 was an oncogene in BC via promoting HIF-1α.
CircPVT1 Overexpression Promoted BC Cells Growth
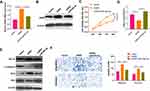
We performed xenograft tumor assay to test the role of circPVT1 on BC cell growth. The results suggested that overexpression of circPVT1 significantly accelerated the growth of BC cells (Figure 8A–C). Next, we detected the expression of circPVT1/miR-29a-3p/AGR2 in the tumor tissues. Our data showed that circPVT1 and AGR2 were significantly overexpression while miR-29a-3p was markedly downregulated after the overexpression of circPVT1 (Figure 8D–F). Moreover, the protein level of c-Caspase3 and Bax were downregulated in the circPVT1 group, while Bcl2 and HIF-1α were upregulated (Figure 8F). Taken together, the data proved that circPVT1 also exerts an oncogenic effect on BC by modulating miR-29a-3p-mediated AGR2/HIF-1α axis.
Discussion
Breast cancer is a malignancy caused by aberrant expression of many genes and proteins. These abnormally expressed molecules have an important effect on BC occurrence and growth.13 In this study, we initially discovered that circPVT1 promotes BC progression, and regulates BC cell proliferation, metastasis and apoptosis by regulating the miR-29a-3p/AGR2/HIF-1α axis.
Accumulating studies have shown that circRNAs may function as a molecular marker for the diagnosis and treatment of diseases, and is instrumental in the occurrence and development of human diseases, especially tumors.5 For example, circ-PIP5K1A is upregulated in colon cancer tissues and can be used as an oncogene to promote cancer progression.14 In addition, circ_0005576 can be used as a biomarker for the diagnosis and prognosis of cervical cancer.15 The progression of BC is accompanied by altered expressions of multiple circRNAs, and these circRNAs are widely involved in tumor cell proliferation, apoptosis, metastasis, and invasion.16 For instance, circ-ABCB10 is upregulated in BC tissues and cells, which can promote the proliferation, migration, and invasion of BC cells by targeting miR-1271.17 Increased expression of circANKS1B in BC tissues is closely related to lymph node metastasis and clinical staging of BC, and is an independent risk factor for patients’ overall survival and accelerates cell metastasis.18
The PVT1 gene encodes a long non-coding RNA locus that has been identified as a candidate oncogene in many types of cancers, including head and neck squamous cell19 and leukemic cells.20 Consistent with its association with various types of cancer, transcription of this gene is regulated by the tumor suppressor p53 through a canonical p53-binding site, and it has been implicated in regulating levels of the proto-oncogene MYC to promote tumorigenesis.21–23 In this research, we further explored the effect of circPVT1 in BC. Our results both in vitro and in vivo showed that circPVT1 was upregulated in BC tissues promoted cell proliferation and metastasis, but inhibited cell apoptosis. These findings manifest that circPVT1 may be an oncogene in BC.
New evidence confirms that miRNAs play a crucial part in cancer progression as whether carcinogenic or tumor suppressor genes. For example, miR-421 promotes the progression of osteosarcoma by targeting MCPIP1,24 while miR-133a-5p targets FUS/AR to inhibit AR-positive prostate cancer cell proliferation.25 In addition, studies have confirmed that abnormal expression of miRNA is associated with BC. For example, miR-214-3p expression is dramatically downregulated in BC, which can inhibit the malignant phenotypes of BC by targeting survivin protein.26 Moreover, miR-937 is a cancer-promoting gene in BC that targets apoptotic peptidase activating factor 1 (APAF1) to promote cancer progression.27 As a member of miRNAs, miR-29a-3p can suppress gastric cancer proliferation and metastasis and limit its growth.28 Here, we investigated the expression and function of miR-29a-3p in BC. In contrast to circPVT1, miR-29a-3p had a lower level in BC tissues and also was a favourable predictor of the overall survival of BC patients. Functionally, miR-29a-3p overexpression significantly inhibited the proliferation and metastasis, while promoted apoptosis of BC cells, indicating that miR-29a-3p has an inhibitory effect in BC.
A growing number of reports have found that circRNA can make a difference in competitive endogenous RNA to sponge miRNA, thereby affecting the effect of the targeted mRNA of miRNA. This circRNA-miRNA-mRNA regulatory network has a prominent role in a variety of diseases including tumors.29 For example, circRNA_0025202 inhibits BC growth by regulating the miR-182-5p/FOXO3a axis.30 Circ_0007534 regulates the proliferation and migration of BC cells by regulating MUC19 (mucin 19, MUC19) by targeting miR-593.31 However, the circular RNA PVT1/miR-203/HOXD3 pathway was found to promote the progression of hepatocellular carcinoma.32 In view of the above research basis, we speculate that circPVT1 may be a ceRNA in BC. Using the bioinformatics database, we identified that miR-29a-3p might interact with circPVT1. Further studies demonstrated that cirPVT1, which is mainly located at the cytoplasm of BC cells, sponged miR-29a-3p and inhibited its expression. The rescue experiment revealed that overexpression of circPVT1 significantly reduced the inhibitory effect of miR-29a-3p on BC cells. Thus, circPVT1 exerts its carcinogenic effect on BC by inhibiting miR-29a-3p.
Anterior gradient 2 (AGR2) was located in human chromosome 7p21.1, the corresponding mRNA length contained 1697 bp, and the encoded protein was composed of 175 amino acid residues. This gene codes a member of the disulfide isomerase (PDI) family of endoplasmic reticulum (ER) proteins that catalyze protein folding and thiol-disulfide interchange reactions. In addition, AGR2 is upregulated in various human cancers and reportedly has oncogenic activities. For example, AGR2 promoted CRC tumorigenesis and acting as an independent prognostic factor of poor outcome. Moreover, AGR2 mediated the resistance to 5-Aza-2 ‘-deoxycytidine (5-Aza) treatment.33 Besides, AGR2 knockout induces apoptosis of pancreatic cancer cells and participates in chemotherapy resistance of pancreatic cancer by inhibiting the ERK/Akt axis.34 Additionally, AGR2 is a target of canonical Wnt/β-catenin signaling and is important for the stemness maintenance in colorectal cancer stem cells.35 In previous studies, the expression of AGR2 was also found to be regulated by a variety of miRNAs. MiR-135b-5p enhances the sensitivity of BC cells to doxorubicin by targeting AGR2.36 Here, we first determined that miR-29a-3p may interact with AGR2 through a bioinformatics database. In addition, overexpression of AGR2 significantly enhanced the malignant behaviours of BC cells, which were mitigated by miR-29a-3p. Moreover, circPVT1 promoted AGR2 expression both in vivo and in vitro. The findings revealed that circPVT1 exerts its biological effects in part by regulating AGR2 expression.
Our data also showed that AGR2 overexpression repressed Bax and Caspase3 levels but promoted Bcl2 expression. The results were consistent with AGR2-mediated anti-apoptosis effects on BC cells. However, how did AGR2 modulate cell apoptosis remains unknown. In our further study, we detected the expressions of HIF-1α under selective regulation of circPVT1, miR-29a-3p and AGR2. We found that HIF-1α was promoted with circPVT1 and AGR2 overexpression, while downregulated with miR-29a-3p upregulation. Those results, in our thought, might explain how AGR2 affect the apoptosis-related protein expression. Previous studies have proved that AGR2 promoting cancer progression via enhancing HIF-1α or HIF-2α expression.37–39 Even HIF-1A upregulation led to higher level of AGR2.40 As for HIF-1α, it is a vital transcription factor that regulates cellular and systemic homeostatic response to hypoxia by activating transcription of many genes. Those genes are widely involved in energy metabolism, angiogenesis, apoptosis, and other genes whose protein products increase oxygen delivery or facilitate metabolic adaptation to hypoxia.41–43 Hence, we believed that AGR2 could affect the apoptotic proteins expression via modulating HIF-1α.
Collectively, our study demonstrated that circPVT1 is upregulated in BC tissues. The functional studies have demonstrated that circPVT1 accelerates BC development by modulating the miR-29a-3p/AGR2/HIF-1α axis. Taken together, this circPVT1/miR-29a-3p/AGR2 axis may be a valuable and promising target for BC treatment.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request, and any data intended for sharing is deidentified.
Ethics Statement
Our study was approved by the Ethics Review Board of China Three Gorges University.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Donepudi MS, Kondapalli K, Amos SJ. Venkanteshan P.Breast cancer statistics and markers. J Cancer Res Ther. 2014;10(3):506–511.
3. Kraschnewski J, Schmitz KH. Exercise in the Prevention and Treatment of Breast Cancer: what Clinicians Need to Tell Their Patients. Curr Sports Med Rep. 2017;16(4):263–267. doi:10.1249/JSR.0000000000000388
4. Yavropoulou MP, Poulios C, Michalopoulos N, et al. A Role for Circular Non-Coding RNAs in the Pathogenesis of Sporadic Parathyroid Adenomas and the Impact of Gender-Specific Epigenetic Regulation. Cells. 2018;8(1):1. doi:10.3390/cells8010015
5. Meng S. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16(1):94.
6. Panda AC, et al. Identification of senescence-associated circular RNAs (SAC-RNAs) reveals senescence suppressor CircPVT1. Nucleic Acids Res. 2017;45(7):4021–4035.
7. Riquelme E, et al. Frequent co-amplification and cooperation between C-MYC and PVT1 oncogenes promote malignant pleural mesothelioma. J Thorac Oncol. 2014;9(7):998–1007.
8. Yang YR, et al. Increased expression of the lncRNA PVT1 promotes tumorigenesis in non-small cell lung cancer. Int J Clin Exp Pathol. 2014;7(10):6929–6935.
9. Bottani M, Banfi G. Lombardi G.Circulating miRNAs as Diagnostic and Prognostic Biomarkers in Common Solid Tumors: focus on Lung, Breast, Prostate Cancers, and Osteosarcoma. J Clin Med. 2019;8(10):
10. Xia LH, Yan QH, Sun QD, Gao YP. MiR-411-5p acts as a tumor suppressor in non-small cell lung cancer through targeting PUM1. Eur Rev Med Pharmacol Sci. 2018;22(17):5546–5553.
11. Ma Y. miR-29a-3p inhibits growth, proliferation, and invasion of papillary thyroid carcinoma by suppressing NF-kappaB signaling via direct targeting of OTUB2. Cancer Manag Res. 2019;11:13–23. doi:10.2147/CMAR.S184781
12. Chen D, Ma W, Ke Z, Xie F. CircRNA hsa_circ_100395 regulates miR-1228/TCF21 pathway to inhibit lung cancer progression. Cell Cycle. 2018;17(16):
13. van Schooneveld E, Wildiers H, Vergote I, Vermeulen PB, Dirix LY, Van Laere SJ. Dysregulation of microRNAs in breast cancer and their potential role as prognostic and predictive biomarkers in patient management. Breast Cancer Res. 2015;17(1):21. doi:10.1186/s13058-015-0526-y
14. Zhang Q, Zhang C, Ma JX, Ren H, Sun Y, Xu JZ. Circular RNA PIP5K1A promotes colon cancer development through inhibiting miR-1273a. World J Gastroenterol. 2019;25(35):5300–5309. doi:10.3748/wjg.v25.i35.5300
15. Ma H, Tian T, Liu X, et al. Upregulated circ_0005576 facilitates cervical cancer progression via the miR-153/KIF20A axis. Biomed Pharmacother. 2019;118:109311. doi:10.1016/j.biopha.2019.109311
16. Wang X, Fang L. Advances in circular RNAs and their roles in breast Cancer. J Exp Clin Cancer Res. 2018;37(1):206.
17. Liang HF, Zhang XZ, Liu BG, Jia GT. Circular RNA circ-ABCB10 promotes breast cancer proliferation and progression through sponging miR-1271. Am J Cancer Res. 2017;7(7):1566–1576.
18. Zeng K, He B, Yang BB, et al. The pro-metastasis effect of circANKS1B in breast cancer. Mol Cancer. 2018;17(1):160. doi:10.1186/s12943-018-0914-x
19. Verduci L, Ferraiuolo M, Sacconi A, et al. The oncogenic role of circPVT1 in head and neck squamous cell carcinoma is mediated through the mutant p53/YAP/TEAD transcription-competent complex. Genome Biol. 2017;18(1):237. doi:10.1186/s13059-017-1368-y
20. Zeng C, Yu X, Lai J, et al. Overexpression of the long non-coding RNA PVT1 is correlated with leukemic cell proliferation in acute promyelocytic leukemia. J Hematol Oncol. 2015;8(1):126. doi:10.1186/s13045-015-0223-4
21. Olivero CE, Martínez-Terroba E, Zimmer J, et al. p53 Activates the Long Noncoding RNA Pvt1b to Inhibit Myc and Suppress Tumorigenesis. Mol Cell. 2020;77(4):761–774.e8. doi:10.1016/j.molcel.2019.12.014
22. Barsotti AM, Beckerman R, Laptenko O, Huppi K, Caplen NJ, Prives C. p53-dependent Induction of PVT1 and miR-1204. J Biol Chem. 2012;287(4):2509–2519. doi:10.1074/jbc.M111.322875
23. Rao PH, Zhao S, Zhao Y-J, et al. Coamplification of M yc / P vt1 and homozygous deletion of N lrp1 locus are frequent genetics changes in mouse osteosarcoma. Genes Chromosomes Cancer. 2015;54(12):796–808. doi:10.1002/gcc.22291
24. Ren Z, He M, Shen T, et al. MiR-421 promotes the development of osteosarcoma by regulating MCPIP1 expression. Cancer Biol Ther. 2019;12:1–10.
25. Zheng L, Kang Y, Zhang L, Zou W. MiR-133a-5p inhibits androgen receptor (AR)-induced proliferation in prostate cancer cells via targeting FUsed in Sarcoma (FUS) and AR. Cancer Biol Ther. 2019;17:1–9.
26. Han LC, Wang H, Niu FL, Yan JY, Cai HF. Effect miR-214-3p on proliferation and apoptosis of breast cancer cells by targeting survivin protein. Eur Rev Med Pharmacol Sci. 2019;23(17):7469–7474.
27. Fang H, Jiang W, Jing Z, Mu X, Xiong Z. <p>miR-937 regulates the proliferation and apoptosis via targeting APAF1 in breast cancer. Onco Targets Ther. 2019;12:5687–5699. doi:10.2147/OTT.S207091
28. Zhao Z, Wang L, Song W, et al. Reduced miR-29a-3p expression is linked to the cell proliferation and cell migration in gastric cancer. World J Surg Oncol. 2015;13(1):101. doi:10.1186/s12957-015-0513-x
29. Rong D, Sun H, Li Z, et al. An emerging function of circRNA-miRNAs-mRNA axis in human diseases. Oncotarget. 2017;8(42):73271–73281. doi:10.18632/oncotarget.19154
30. Sang Y, Chen B, Song X, et al. circRNA_0025202 Regulates Tamoxifen Sensitivity and Tumor Progression via Regulating the miR-182-5p/FOXO3a Axis in Breast Cancer. Mol Ther. 2019;27(9):1638–1652. doi:10.1016/j.ymthe.2019.05.011
31. Song L, Xiao Y. Downregulation of hsa_circ_0007534 suppresses breast cancer cell proliferation and invasion by targeting miR-593/MUC19 signal pathway. Biochem Biophys Res Commun. 2018;503(4):2603–2610. doi:10.1016/j.bbrc.2018.08.007
32. Zhu Y, Liu Y, Xiao B, et al. The circular RNA PVT1/miR-203/HOXD3 pathway promotes the progression of human hepatocellular carcinoma. Biology Open. 2019;8(9):9. doi:10.1242/bio.043687
33. Li J, Hu J, Luo Z, et al. AGR2 is controlled by DNMT3a-centered signaling module and mediates tumor resistance to 5-Aza in colorectal cancer. Exp Cell Res. 2019;385(1):111644. doi:10.1016/j.yexcr.2019.111644
34. Liu Q-G, Li Y-J, Yao L. Knockdown of AGR2 induces cell apoptosis and reduces chemotherapy resistance of pancreatic cancer cells with the involvement of ERK/AKT axis. Pancreatology. 2018;18(6):678–688. doi:10.1016/j.pan.2018.07.003
35. Dahal Lamichane B, Jung SY, Yun J, et al. AGR2 is a target of canonical Wnt/β-catenin signaling and is important for stemness maintenance in colorectal cancer stem cells. Biochem Biophys Res Commun. 2019;515(4):600–606. doi:10.1016/j.bbrc.2019.05.154
36. Zhang Y, Xia F, Zhang F, Cui Y, Wang Q, Liu H. miR-135b-5p enhances doxorubicin-sensitivity of breast cancer cells through targeting anterior gradient 2. J Exp Clin Cancer Res. 2019;38(1):26. doi:10.1186/s13046-019-1024-3
37. Gong W, Ekmu B, Wang X, Lu Y, Wan L. AGR2-induced glucose metabolism facilitated the progression of endometrial carcinoma via enhancing the MUC1/HIF-1α pathway. Hum Cell. 2018;68(6):790–800. doi:10.1007/s13577-020-00356-4
38. Li Z, Zhu Q, Hu L, Chen H, Wu Z, Li D. Anterior gradient 2 is a binding stabilizer of hypoxia inducible factor-1α that enhances CoCl2 -induced doxorubicin resistance in breast cancer cells. Cancer Sci. 2015;106(8):1041–1049.
39. Pajdzik K, Wilamowski M, Żurawek D, et al. Anterior gradient 2 promotes tumorigenesis through upregulation of CCAAT-enhancer binding protein beta and hypoxia-inducible factor-2α and subsequent secretion of interleukin-6, interleukin-8, and vascular endothelial growth factor in the Caki-1 clear cell renal cell carcinoma cell line. IUBMB Life. 2020;72(8):1807–1818.
40. Hong XY, Wang J, Li Z. AGR2 expression is regulated by HIF-1 and contributes to growth and angiogenesis of glioblastoma. Cell Biochem Biophys. 2013;67(3):1487–1495.
41. Ai Z, Lu Y, Qiu S, Fan Z. Overcoming cisplatin resistance of ovarian cancer cells by targeting HIF-1-regulated cancer metabolism. Cancer Lett. 2016;373(1):36–44.
42. Lee YM, Kim GH, Park EJ, et al. Thymoquinone Selectively Kills Hypoxic Renal Cancer Cells by Suppressing HIF-1α-Mediated Glycolysis. Int J Mol Sci. 2019;20(5):1092.
43. Yeh YH, Hsiao HF, Yeh YC, Chen TW, Li TK. Inflammatory interferon activates HIF-1α-mediated epithelial-to-mesenchymal transition via PI3K/AKT/mTOR pathway. J Exp Clin Cancer Res. 2018;37(1):70.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.