Back to Journals » OncoTargets and Therapy » Volume 14
Circ_0006168 Promotes the Migration, Invasion and Proliferation of Esophageal Squamous Cell Carcinoma Cells via miR-516b-5p-Dependent Regulation of XBP1
Authors Huang Y, Jiang L, Wei G
Received 20 November 2020
Accepted for publication 22 March 2021
Published 12 April 2021 Volume 2021:14 Pages 2475—2488
DOI https://doi.org/10.2147/OTT.S293180
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Yunhe Huang,* Lei Jiang,* Guangxia Wei
Department of Thoracic Surgery, The First Affiliated Hospital of Nanchang University, Nanchang, 330006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guangxia Wei
Department of Thoracic Surgery, The First Affiliated Hospital of Nanchang University, No. 17, Yongwaizheng Street, Donghu District, Nanchang, Jiangxi, People’s Republic of China
Email [email protected]
Background: Circular RNAs (circRNAs) exert important roles in carcinogenesis. Here, we aimed to uncover the working mechanism of circ_0006168 in esophageal squamous cell carcinoma (ESCC) development.
Methods: Western blot assay and real-time quantitative polymerase chain reaction (RT-qPCR) were used to determine protein and RNA expression, respectively. Wound healing assay and transwell migration assay were performed to assess cell migration ability, whereas cell invasion ability was evaluated by transwell invasion assay. 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and colony formation assay were utilized to analyze cell proliferation ability. Xenograft tumor model was utilized to assess the role of X-box binding protein 1 (XBP1) in xenograft tumor growth in vivo. Dual-luciferase reporter assay, RNA immunoprecipitation (RIP) assay and RNA pull down assay were used to verify intermolecular interactions.
Results: XBP1 silencing suppressed the migration, invasion and proliferation of ESCC cells in vitro and restrained the xenograft tumor growth in vivo. MicroRNA-516b-5p (miR-516b-5p) interacted with the 3ʹ untranslated region (3ʹUTR) of XBP1 in ESCC cells. MiR-516b-5p overexpression inhibited the proliferation and motility of ESCC cells. MiR-516b-5p was a molecular target of circ_0006168 in ESCC cells. The interference of circ_0006168 restrained the motility and proliferation of ESCC cells. Circ_0006168 acted as miR-516b-5p sponge to up-regulate XBP1 expression in ESCC cells. MiR-516b-5p silencing or the accumulation of XBP1 largely rescued the proliferation ability and motility in circ_0006168-silenced ESCC cells.
Conclusion: In conclusion, circ_0006168 contributed to ESCC development through promoting the proliferation and motility of ESCC cells via mediating miR-516b-5p/XBP1 axis.
Keywords: esophageal squamous cell carcinoma, circ_0006168, miR-516b-5p, XBP1
Introduction
Esophageal squamous cell carcinoma (ESCC) is a common subtype of esophageal cancer (EC) with high incidence.1,2 ESCC patients are always diagnosed at late stage due to the lack of obvious early symptoms and diagnostic methods,3 which results in poor outcome.4 Furthermore, the molecular-targeted strategies for patients with ESCC are unsatisfactory due to the unclear molecular mechanism in ESCC development. Hence, identifying novel biomarkers for early diagnosis and therapy of ESCC is urgently needed to improve the prognosis of ESCC patients.
Circular RNAs (circRNAs) are stable non-coding RNA (ncRNA) molecules that are resistant to exonuclease due to their covalently closed circular structure.5 CircRNAs could act as microRNA (miRNA) sponges to regulate cellular phenotypes.6 For instance, circ_0006168 accelerated the malignant behaviors of ESCC cells through sponging miR-100.7 Xie et al claimed that circ_0006168 facilitated EC progression through targeting miR-384/RBBP7 signaling.8 However, the molecular mechanism behind the oncogenic role of circ_0006168 still needs to be illustrated. Based on bioinformatic analysis, miR-516b-5p was a potential target of circ_0006168. MiR–516b restrained the progression of several malignancies,9–11 including ESCC. In the current study, the interaction between miR-516b-5p and circ_0006168 and their functional association in ESCC progression were explored.
MicroRNAs (miRNAs) regulate protein coding through binding to messenger RNAs (mRNAs) to regulate cell cycle progression, cell apoptosis and motility.12,13 The target interaction between miR-516b–5p and XBP1 was predicted by bioinformatic database. XBP1 is an important transcription factor that is implicated in the process of unfolded protein response (UPR).14 Recently, XBP1 was found to play an oncogenic role in many malignancies.15–17 Xia et al demonstrated that XBP1 accelerated the proliferation and motility in ESCC cells via up-regulating MMP9.18 Jin et al proved that TUG1 contributed to ESCC development through sponging miR-498 and up-regulating XBP1.19 Here, the interaction between XBP1 and miR-516b-5p was tested, and their functional relevance in regulating ESCC progression was analyzed.
In the current study, the expression pattern of XBP1 in ESCC cell lines and tissues was firstly analyzed. Loss-of-function experiments were then performed to analyze the biological functions of XBP1 in ESCC cells. Bioinformatic software and verification experiments were utilized to establish circRNA-miRNA-mRNA axis, and their functional association was tested by rescue experiments.
Materials and Methods
Clinical Samples
Thirty pairs of ESCC tissue samples and adjacent non-tumor tissues were collected from 30 ESCC patients at the First Affiliated Hospital of Nanchang University. These tissue samples were stored at −80°C within 30 min after surgical resection. Clinical experiments were performed with the approval of the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University and in accordance with the Declaration of Helsinki. Written informed consent was signed by every participant before the study. The association between clinicopathological features of ESCC patients and the expression level of circ_0006168 is shown in Table 1. RT-qPCR, Western blot assay and immunohistochemistry (IHC) assay were performed to determine RNA and protein expression. IHC assay was performed using the antibody against XBP1 (ab37152; Abcam, Cambridge, MA, USA) at the dilution of 1:300.20
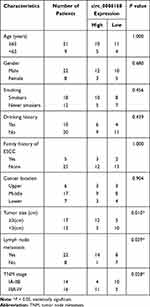 |
Table 1 Correlation Between Clinicopathological Features of ESCC Patients and the Expression Level of circ_0006168 |
Cell Lines
ESCC cell lines (Eca109, KYSE150, KYSE180 and KYSE450) and human esophageal epithelial cell line HET-1A were purchased from the Shanghai Institute of Chinese Academy of Sciences Cell Collection (Shanghai, China). All these five cell lines were cultured with Roswell Park Memorial Institute-1640 medium (RPMI-1460; Gibco, Carlsbad, CA, USA) added with 10% fetal bovine serum (FBS; Gibco), 1% 100 units/mL penicillin (Gibco) and 1% 100 μg/mL streptomycin (Gibco). All cell lines were maintained at 37°C incubator with 5% CO2.
Western Blot Assay
ESCC cells were collected and lysed using whole cell lysis buffer (Abcam). Protein quantification was conducted with the commercial bicinchoninic acid (BCA) protein assay kit (Invitrogen, Carlsbad, CA, USA). Proteins were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred onto the polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA). After blocking, the membrane was incubated with primary antibodies, including anti-XBP1 (ab37152; Abcam) and anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH; ab9485; Abcam). After incubating with secondary antibody (Abcam) for 2 h, protein signals were determined using the commercial enhanced chemiluminescence kit (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom).
Cell Transfection
XBP1 specific small interfering RNA (si-XBP1), circ_0006168 specific siRNA (si-circ_0006168), negative control siRNA (si-NC), short hairpin RNA targeting XBP1 (sh-XBP1), shRNA NC (sh-NC), XBP1 overexpression plasmid (XBP1), empty pcDNA vector (pcDNA), circ_0006168 overexpression plasmid (circ_0006168), empty pLCDH-cir vector (vector), miR-516b-5p mimics (miR-516b-5p), miRNA NC (miR-NC), miR-516b-5p inhibitor (inhibitor-miR-516b-5p) and inhibitor-NC were purchased from GenePharma (Shanghai, China). Lipofectamine 3000 (Invitrogen) was used for transfection.
Wound Healing Assay
Cell monolayer in 6-well plates was scratched with the 200 μL pipette tip, and cell debris was removed through washing with phosphate buffer saline (PBS) buffer (Sangon Biotech, Shanghai, China). After creating the wound for 0 h and 24 h, cell images were obtained at the magnification of 40 ×.
Transwell Assays
ESCC cells were inoculated into the upper chambers with (in transwell invasion assay) or without Matrigel (BD Biosciences, San Jose, CA, USA; in transwell migration assay). The upper chambers were added with serum-free medium, while the lower chambers were added with culture medium plus 10% FBS (Gibco). After 24-h inoculation, ESCC cells that penetrated the membrane were fixed, stained and counted (Magnification: 100 ×).
3-(4,5-Dimethyl-Thiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
In brief, ESCC cells were inoculated into 96-well plates and transfected with plasmid or RNA for 0 h, 24 h, 48 h or 72 h. These transfected ESCC cells were incubated with 10 μL MTT reagent (Beyotime, Shanghai, China) for 4 h prior to incubation with 100 μL dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO, USA). The absorbance at 490 nm was measured, and cell proliferation curve was generated.
Colony Formation Assay
ESCC cells were inoculated into 6-well plates at 300 cells per well. After 14-d culture at 37°C incubator with 5% CO2/95% air, the colonies were fixed with methanol (Sangon Biotech) for 15 min followed by staining using 0.1% crystal violet (Sangon Biotech) for 15 min. The number of colonies was counted.
Xenograft Tumor Model
Animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Nanchang University and in accordance with the National Guide for the Care and Use of Laboratory Animals. BALB/c nude mice from Vital River Laboratory Animal Technology (Beijing, China) were split into two groups (n=6). The mice in sh-XBP1 group were subcutaneously injected with Eca109 or KYSE150 cells (6 × 106 cells) stably transfected with sh-XBP1 in their right dorsal flank, while mice in sh-NC group were subcutaneously injected with Eca109 or KYSE150 cells (6 × 106 cells) stably transfected with sh-NC. The volume of tumors was monitored every week, and tumors were weighed after 4-week inoculation.
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
TRIzol reagent (Invitrogen) was used to isolate RNA samples from ESCC tissues and cell lines. The complementary DNA (cDNA) for circ_0006168, CCR4-NOT transcription complex subunit 6 like (CNOT6L) and XBP1 was synthesized using All-in-OneTM Synthesis Kit (FulenGen, Guangzhou, China). The cDNA for miR-516b-5p was obtained using miScript reverse transcription kit (Qiagen, Hilden, Germany). SYBR Green (Applied Biosystems, Foster City, CA, USA) was subsequently used to conduct PCR amplification reaction. U6 was used as the internal reference for miR-516b-5p while GAPDH was used as the internal reference for circ_0006168, CNOT6L and XBP1. The primers were listed in Table 2. The fold change was calculated using the 2−ΔΔCt method.
 |
Table 2 Specific Primers in qRT-PCR Assay |
Dual-Luciferase Reporter Assay
Dual-luciferase reporter assay was applied to test the interaction between miR-516b-5p and circ_0006168 or XBP1 mRNA 3ʹ untranslated region (3ʹUTR). Partial wild-type sequence of circ_0006168 or XBP1 mRNA 3ʹUTR was inserted into the psiCHECK2 vector (Promega, Madison, WI, USA) to obtain circ_0006168-wt or XBP1-wt. Meanwhile, circ_0006168-mut or XBP1-mut luciferase plasmid was constructed through inserting the mutant sequence of circ_0006168 or XBP1 3ʹUTR into the psiCHECK2 vector (Promega). ESCC cells were inoculated into 24-well plates and co-transfected with luciferase plasmid and miR-516b-5p or miR-NC. After transfection for 48 h, luciferase intensities were detected using the Dual-luciferase Reporter assay system (Promega).
RNA Immunoprecipitation (RIP) Assay
RIP assay was performed using the EZMagna RIP kit (Merck, Darmstadt, Germany). ESCC cells were disrupted using RIP lysis buffer and then incubated with magnetic beads that pre-coated with Argonaute 2 (Ago2) antibody or Immunoglobulin G (IgG) antibody. After digesting with protease K, the levels of circ_0006168, miR-516b-5p and XBP1 mRNA were measured by RT-qPCR.
RNA Pull Down Assay
Cell lysate (2 μg) was incubated with biotinylated miR-516b-5p (bio-miR-516b-5p; 100 pmol) or its negative control (bio-NC; 100 pmol). 100 μL streptavidin agarose beads were added to incubate for 1 h. The levels of circ_0006168 and XBP1 mRNA were measured by RT-qPCR.
RNase R Digestion
A total of 2 μg RNA was incubated with 6 units of RNase R (Geneseed Biotech, Guangzhou, China) for 20 min at 37°C. The expression of circ_0006168 and CNOT6L was measured by RT-qPCR.
Statistical Analysis
The data from three independent experiments were represented as mean ± standard deviation. The differences in two groups or multiple groups were analyzed by Student’s t-test or one-way analysis of variance (ANOVA) followed by Tukey’s test. P values <0.05 were regarded as statistically significant.
Results
XBP1 Interference Restrains Cell Migration and Invasion Abilities of ESCC Cells
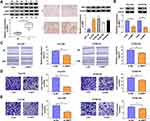
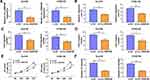
The expression characteristic of XBP1 in ESCC tissues and cell lines was initially explored. As shown in Figure 1A, XBP1 was highly expressed in ESCC tissues in comparison with that in para-cancerous normal tissues as verified by Western blot assay and IHC assay. Compared with human esophageal epithelial cell line HET-1A, XBP1 was observed to be notably up-regulated in four ESCC cell lines (Figure 1A), especially in Eca109 and KYSE150 cell lines. Thus, Eca109 and KYSE150 cell lines were chosen for loss-of-function experiments. High silencing efficiency of si-XBP1 was verified in both ESCC cell lines (Figure 1B). With the silencing of XBP1, the rate of wound healing was markedly reduced (Figure 1C), suggesting that XBP1 interference suppressed the migration ability of ESCC cells. Apart from wound healing assay, cell migration ability was further analyzed by transwell migration assay. The number of migrated ESCC cells was reduced in XBP1-silenced group compared with si-NC group (Figure 1D), which further demonstrated that XBP1 knockdown restrained the migration of ESCC cells. Based on the results of transwell invasion assay, we found that XBP1 interference notably decreased the number of invaded ESCC cells (Figure 1E), suggesting that XBP1 silencing markedly inhibited the invasion ability of ESCC cells. Taken together, XBP1 interference inhibited the migration and invasion of ESCC cells.
XBP1 Silencing Suppresses Cell Proliferation Ability of ESCC Cells in vitro and in vivo
The effect of XBP1 silencing on the proliferation ability of ESCC cells was explored through MTT assay and colony formation assay. XBP1 interference significantly restrained the proliferation ability of ESCC cells (Figure 2A). With the silencing of XBP1, the number of colonies was notably decreased compared with that in si-NC group (Figure 2B). The results of MTT assay and colony formation assay together demonstrated that XBP1 silencing suppressed the proliferation ability of ESCC cells in vitro. Eca109 and KYSE150 cell lines stably transfected with sh-XBP1 or sh-NC were established to explore the role of XBP1 in xenograft tumor growth in vivo. As shown in Figure 2C and D, XBP1 silencing significantly reduced tumor volume and weight, suggesting that XBP1 silencing suppressed ESCC progression in vivo. Overall, XBP1 interference restrained the proliferation of ESCC cells in vitro and in vivo.
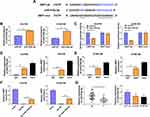
XBP1 is a Target of miR-516b-5p in ESCC Cells
XBP1 was predicted as a downstream target of miR-516b-5p via TargetScan software based on their complementary sites shown in Figure 3A. High overexpression efficiency of miR-516b-5p mimics was verified in both ESCC cell lines (Figure 3B). The wild-type or mutant type 3ʹUTR fragment of XBP1 mRNA, including the putative or mutant binding sites with miR-516b-5p, was amplified and inserted into the luciferase plasmid, termed as XBP1-wt or XBP1-mut. Next, we tested the interaction between miR-516b-5p and XBP1 mRNA 3ʹUTR through co-transfecting ESCC cells with luciferase plasmid (XBP1-wt or XBP1-mut) and miR-516b-5p or miR-NC. MiR-516b-5p accumulation caused significant reduction in luciferase activity of wild-type reporter plasmid (XBP1-wt) (Figure 3C). MiR-516b-5p overexpression had almost no effect on the luciferase activity of mutant reporter plasmid (XBP1-mut) (Figure 3C), suggesting that miR-516b-5p interacted with XBP1 3ʹUTR in ESCC cells. RIP assay and RNA pull down assay were conducted to further verify the interaction between XBP1 and miR-516b-5p. MiR-516b-5p and XBP1 were simultaneously enriched when using Ago2 antibody (Figure 3D and E), suggesting that there was spatial interaction between miR-516b-5p and XBP1 in RNA-induced silencing complex (RISC). As shown in Figure 3F, XBP1 was enriched using biotinylated miR-516b-5p, suggesting the interaction between miR-516b-5p and XBP1. MiR-516b-5p was down-regulated in ESCC tissue samples than that in adjacent normal tissues (Figure 3G). The level of miR-516b-5p was notably reduced in two ESCC cell lines compared with that in HET-1A cell line (Figure 3G). Taken together, miR-516b-5p interacted with XBP1 3ʹUTR, and miR-516b-5p was notably down-regulated in ESCC tissues and cell lines.
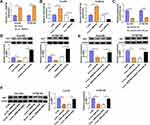
MiR-516b-5p Accumulation Inhibits the Malignant Behaviors of ESCC Cells
Gain-of-function experiments were performed to analyze the biological role of miR-516b-5p in ESCC cells. With the accumulation of miR-516b-5p, cell migration rate was significantly reduced compared with that in miR-NC group (Figure 4A and Supplementary Figure 1A). Through performing transwell assays, we found that the numbers of migrated and invaded ESCC cells were significantly reduced in miR-516b-5p-overexpressed group (Figure 4B and C, Supplementary Figure 1B and C), demonstrating that miR-516b-5p overexpression suppressed the migration and invasion of ESCC cells. As verified by MTT assay, we found that miR-516b-5p overexpression notably hampered the proliferation of ESCC cells (Figure 4D). MiR-516b-5p overexpression also reduced the number of colonies in ESCC cells (Figure 4E and Supplementary Figure 1D). These findings suggested that miR-516b-5p overexpression inhibited the motility and proliferation of ESCC cells.
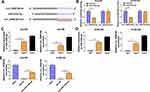
Circ_0006168 Interacts with miR-516b-5p in ESCC Cells
MiR-516b-5p-circRNAs interactions were explored using bioinformatic Circinteractome software, and miR-516b-5p was predicted to be a possible target of circ_0006168. The putative binding sequence between circ_0006168 and miR-516b-5p is shown in Figure 5A. There was a significant reduction in luciferase activity in circ_0006168-wt and miR-516b-5p co-transfected group compared with that in circ_0006168-wt and miR-NC co-transfected group (Figure 5B). Meanwhile, luciferase activity was unaffected in circ_0006168-mut group with the addition of miR-NC or miR-516b-5p (Figure 5B), demonstrating that miR-516b-5p interacted with circ_0006168 in ESCC cells. Compared with IgG antibody group, miR-516b-5p and circ_0006168 were both enriched in Ago2 antibody group (Figure 5C and D), suggesting that miR-516b-5p interacted with circ_0006168 in RISC. Moreover, the interaction between miR-516b-5p and circ_0006168 was confirmed by RNA pull down assay (Figure 5E). Taken together, miR-516b-5p was a target of circ_0006168 in ESCC cells.
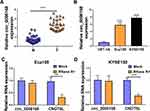
Circ_0006168 is Highly Expressed in ESCC Tissues and Cell Lines
Circ_0006168 expression was up-regulated in ESCC tissues than that in adjacent non-tumor tissues (Figure 6A). High expression of circ_0006168 in tumor tissues was correlated with large tumor size, positive lymph node metastasis and advanced TNM stage in ESCC patients (Table 1). Compared with HET-1A cell line, circ_0006168 level was significantly up-regulated in both ESCC cell lines (Figure 6B). Subsequently, the stability of circ_0006168 was tested using exonuclease RNase R. Circ_0006168 was resistant to RNase R, whereas RNase R digestion markedly reduced the level of its linear counterpart CNOT6L (Figure 6C and D). Taken together, circ_0006168 was a stable circular transcript, and it was aberrantly up-regulated in ESCC tissues and cell lines.
Circ_0006168 Knockdown Hampers the Malignant Phenotypes of ESCC Cells in vitro
As verified by RT-qPCR, the interference efficiency of si-circ_0006168 was high in two ESCC cell lines (Figure 7A). Through performing wound healing assay and transwell migration assay, we found that circ_0006168 silencing notably suppressed the migration ability of ESCC cells (Figure 7B and C, Supplementary Figure 2A and B). As shown in Figure 7D and Supplementary Figure 2C, with the silencing of circ_0006168, the number of invaded ESCC cells was reduced, demonstrating that circ_0006168 knockdown suppressed the invasion ability of ESCC cells. The proliferation capacity of ESCC cells was blocked in circ_0006168-silenced group compared with si-NC group (Figure 7E). With the interference of circ_0006168, the number of colonies was decreased compared with that in si-NC group (Figure 7F and Supplementary Figure 2D). Taken together, circ_0006168 interference blocked the migration, invasion and proliferation of ESCC cells.
Circ_0006168 Acts as miR-516b-5p Sponge to Up-Regulate XBP1 in ESCC Cells
The overexpression efficiency of circ_0006168 plasmid was high in ESCC cells (Figure 8A). A negative regulatory relationship between circ_0006168 and miR-516b-5p was confirmed by RT-qPCR assay (Figure 8B). RT-qPCR assay verified the high silencing efficiency of inhibitor-miR-516b-5p in ESCC cells (Figure 8C). The negative regulatory relationship between miR-516b-5p and XBP1 in ESCC cells was verified by Western blot assay (Figure 8D). Circ_0006168 interference decreased the protein level of XBP1 in ESCC cells, and the addition of inhibitor-miR-516b-5p largely recovered the protein expression of XBP1 in ESCC cells (Figure 8E). These findings demonstrated that circ_0006168 silencing reduced the expression of XBP1 in ESCC cells partly through up-regulating miR-516b-5p. Circ_0006168 silencing reduced XBP1 protein level, while the addition of XBP1 plasmid largely regained the expression of XBP1 in ESCC cells (Figure 8F). Taken together, circ_0006168 positively regulated the expression of XBP1 partly through sponging miR-516b-5p in ESCC cells.
Circ_0006168 Silencing-Mediated Effects are Largely Overturned by the Silencing of miR-516b-5p or the Overexpression of XBP1 in ESCC Cells
We performed rescue experiments to explore the functional relevance among circ_0006168, miR-516b-5p and XBP1 in ESCC cells. Circ_0006168 silencing-mediated suppressive effects on cell migration and invasion were largely counteracted by the silencing of miR-516b-5p or the overexpression of XBP1 via wound healing assay and transwell assays (Figure 9A–C, Supplementary Figure 3A–C). Circ_0006168 knockdown suppressed the proliferation ability of ESCC cells, and this inhibitory effect was partly attenuated by the interference of miR-516b-5p or the overexpression of XBP1 (Figure 9D). Cell colony formation ability was suppressed with the silencing of circ_0006168, and the addition of inhibitor-miR-516b-5p or XBP1 plasmid largely recovered cell colony formation ability (Figure 9E, Supplementary Figure 3D). Overall, circ_0006168 silencing suppressed ESCC progression partly through up-regulating miR-516b-5p and reducing XBP1 expression.
Discussion
Dysregulated circRNAs are found to be closely associated with the pathogenesis of many malignancies,21 including ESCC.22,23 For example, Pan et al found that circ_0006948 was highly expressed in ESCC, and it accelerated ESCC progression through sponging miR-490-3p and up-regulating HMGA2.24 Circ_0000337 was abnormally up-regulated in ESCC, and high level of circ_0000337 accelerated the proliferation and motility of ESCC cells.25 We focused on the biological role of circ_0006168 in ESCC progression. Previous studies have identified circ_0006168 as a carcinogenic factor. Shi et al demonstrated that circ_0006168 aggravated ESCC development through enhancing mTOR expression via sponging miR-100.7 Xie et al claimed that circ_0006168 contributed to ESCC development through targeting miR-384/RBBP7/S6K/S6 signaling cascade.8 Here, we further investigated the working mechanism of circ_0006168 in ESCC progression. We found that circ_0006168 abundance was elevated in ESCC tissues and cell lines. Circ_0006168 silencing significantly hampered the migration, invasion and proliferation of ESCC cells, and the pro-tumor role of circ_0006168 in ESCC was in line with former articles.7,8
CircRNAs possess abundant miRNA binding sites, and they modulate the expression of downstream genes through competitively binding to miRNAs, which is also called competitive endogenous RNA (ceRNA) mechanism.26 Based on the previous studies, circ_0006168 played an oncogenic role in ESCC through sponging miR-1007 and miR-384.8 Here, miR-516b-5p was identified as a novel miRNA target of circ_0006168 in our study. MiR-516b-5p functioned as a tumor suppressor in lung cancer and ESCC.11,27,28 MiR-516b was reported to restrain the malignant potential of ESCC cells by directly regulating CCNG1.11 We found that miR-516b-5p overexpression blocked the migration, invasion and proliferation of ESCC cells, and the anti-tumor role of miR-516b-5p was in agreement with the former study.11 The results of rescue experiments revealed that circ_0006168 silencing-induced effects in ESCC cells were largely diminished by the knockdown of miR-516b-5p, suggesting that circ_0006168 silencing suppressed ESCC progression largely through up-regulating miR-516b-5p. Hereto, circ_0006168 was found to exert its pro-tumor role in ESCC through sponging miR-100,7 miR-3848 and miR-516b-5p, which provided novel insight in targeting circ_0006168-mediated carcinogenic effects in ESCC.
MiRNAs induce the translational repression or degradation of mRNAs through targeting their 3ʹUTR, leading to the changes in cell biological behaviors. For instance, miR-133b hampered the proliferation ability and motility of ESCC cells through down-regulating EGFR.29 Liu et al found that miR-601 suppressed ESCC development through targeting HDAC6.30 In our study, we identified XBP1 as a target of miR-516b-5p for the first time. XBP1 was identified as a pivotal regulator for cell fate determination during endoplasmic reticulum stress.31 Recently, XBP1 was confirmed to play an oncogenic role in multiple malignancies, including breast cancer,15 oral squamous cell carcinoma,32 hepatocellular carcinoma33 and ESCC.18,19 For instance, Xia et al found that XBP1 facilitated the proliferation ability and invasion ability of ESCC cells through inducing MMP9 expression.18 Jin et al demonstrated that XBP1 acted as the downstream molecule of TUG1/miR-498 axis to contribute to ESCC development.19 We found that XBP1 interference resulted in notable suppression in cell proliferation, migration and invasion abilities of ESCC cells, and the pro-tumor role of XBP1 in ESCC was consistent with former studies.18,19 Through performing rescue experiments, we found that XBP1 overexpression reversed circ_0006168 silencing-mediated inhibitory effects in the malignant behaviors of ESCC cells, suggesting that circ_0006168 silencing suppressed the migration, invasion and proliferation of ESCC cells largely through down-regulating XBP1. Circ_0006168 acted as a ceRNA to up-regulate the expression of XBP1 via absorbing miR-516b-5p in ESCC cells.
Our study demonstrated that circ_0006168 aggravated ESCC progression through acting as miRNA sponge to absorb miR-516b-5p to up-regulate XBP1 expression. Circ_0006168/miR-516b-5p/XBP1 signaling axis might be a novel treatment target for ESCC.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Takeno S, Noguchi T, Takahashi Y, Fumoto S, Shibata T, Kawahara K. Assessment of clinical outcome in patients with esophageal squamous cell carcinoma using TNM classification score and molecular biological classification. Ann Surg Oncol. 2007;14(4):1431–1438. doi:10.1245/s10434-006-9286-3
3. Zhang Y, Zhang Y, Zhang L. Expression of cancer-testis antigens in esophageal cancer and their progress in immunotherapy. J Cancer Res Clin Oncol. 2019;145(2):281–291. doi:10.1007/s00432-019-02840-3
4. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412. doi:10.1016/S0140-6736(12)60643-6
5. Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12(4):381–388. doi:10.1080/15476286.2015.1020271
6. Panda AC. Circular RNAs act as miRNA sponges. Adv Exp Med Biol. 2018;1087:67–79.
7. Shi Y, Guo Z, Fang N, et al. hsa_circ_0006168 sponges miR-100 and regulates mTOR to promote the proliferation, migration and invasion of esophageal squamous cell carcinoma. Biomed Pharmacother. 2019;117:109151. doi:10.1016/j.biopha.2019.109151
8. Xie ZF, Li HT, Xie SH, Ma M. Circular RNA hsa_circ_0006168 contributes to cell proliferation, migration and invasion in esophageal cancer by regulating miR-384/RBBP7 axis via activation of S6K/S6 pathway. Eur Rev Med Pharmacol Sci. 2020;24(1):151–163. doi:10.26355/eurrev_202001_19906
9. Fang G, Ye BL, Hu BR, Ruan XJ, Shi YX. CircRNA_100290 promotes colorectal cancer progression through miR-516b-induced downregulation of FZD4 expression and Wnt/β-catenin signaling. Biochem Biophys Res Commun. 2018;504(1):184–189. doi:10.1016/j.bbrc.2018.08.152
10. Zhu J, Zhang Y, Yang X, Jin L. Clinical significance and tumor-suppressive function of miR-516b in nonsmall cell lung cancer. Cancer Biother Radiopharm. 2017;32(4):115–123. doi:10.1089/cbr.2016.2163
11. Zhao Y, Wang Y, Xing G. miR-516b functions as a tumor suppressor by directly modulating CCNG1 expression in esophageal squamous cell carcinoma. Biomed Pharmacother. 2018;106:1650–1660. doi:10.1016/j.biopha.2018.07.074
12. Sun FB, Lin Y, Li SJ, Gao J, Han B, Zhang CS. MiR-210 knockdown promotes the development of pancreatic cancer via upregulating E2F3 expression. Eur Rev Med Pharmacol Sci. 2018;22(24):8640–8648. doi:10.26355/eurrev_201812_16628
13. Lu L, Yue S, Jiang L, et al. Myeloid Notch1 deficiency activates the RhoA/ROCK pathway and aggravates hepatocellular damage in mouse ischemic livers. Hepatology. 2018;67(3):1041–1055. doi:10.1002/hep.29593
14. Andres SA, Wittliff JL. Relationships of ESR1 and XBP1 expression in human breast carcinoma and stromal cells isolated by laser capture microdissection compared to intact breast cancer tissue. Endocrine. 2011;40(2):212–221. doi:10.1007/s12020-011-9522-x
15. Chen X, Iliopoulos D, Zhang Q, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature. 2014;508(7494):103–107. doi:10.1038/nature13119
16. Zhu H, Abulimiti M, Liu H, Su XJ, Liu CH, Pei HP. RITA enhances irradiation-induced apoptosis in p53-defective cervical cancer cells via upregulation of IRE1α/XBP1 signaling. Oncol Rep. 2015;34(3):1279–1288. doi:10.3892/or.2015.4083
17. Bae J, Samur M, Munshi A, et al. Heteroclitic XBP1 peptides evoke tumor-specific memory cytotoxic T lymphocytes against breast cancer, colon cancer, and pancreatic cancer cells. Oncoimmunology. 2014;3(12):e970914. doi:10.4161/21624011.2014.970914
18. Xia T, Tong S, Fan K, et al. XBP1 induces MMP-9 expression to promote proliferation and invasion in human esophageal squamous cell carcinoma. Am J Cancer Res. 2016;6(9):2031–2040.
19. Jin G, Yang Y, Tuo G, Wang W, Zhu Z. LncRNA TUG1 promotes tumor growth and metastasis of esophageal squamous cell carcinoma by regulating XBP1 via competitively binding to miR-498. Neoplasma. 2020;67(4):751–761. doi:10.4149/neo_2020_190805N717
20. Gao D, Zhou Z, Huang H. miR-30b-3p inhibits proliferation and invasion of hepatocellular carcinoma cells via suppressing PI3K/Akt pathway. Front Genet. 2019;10:1274. doi:10.3389/fgene.2019.01274
21. Qu S, Liu Z, Yang X, et al. The emerging functions and roles of circular RNAs in cancer. Cancer Lett. 2018;414:301–309. doi:10.1016/j.canlet.2017.11.022
22. Fan L, Cao Q, Liu J, Zhang J, Li B. Circular RNA profiling and its potential for esophageal squamous cell cancer diagnosis and prognosis. Mol Cancer. 2019;18(1):16. doi:10.1186/s12943-018-0936-4
23. Jiang C, Xu D, You Z, Xu K, Tian W. Dysregulated circRNAs and ceRNA network in esophageal squamous cell carcinoma. Front Biosci (Landmark Ed). 2019;24:277–290. doi:10.2741/4717
24. Pan Z, Lin J, Wu D, et al. Hsa_circ_0006948 enhances cancer progression and epithelial-mesenchymal transition through the miR-490-3p/HMGA2 axis in esophageal squamous cell carcinoma. Aging (Albany NY). 2019;11(24):11937–11954. doi:10.18632/aging.102519
25. Song H, Xu D, Shi P, et al. Upregulated circ RNA hsa_circ_0000337 promotes cell proliferation, migration, and invasion of esophageal squamous cell carcinoma. Cancer Manag Res. 2019;11:1997–2006. doi:10.2147/CMAR.S195546
26. Qi X, Zhang DH, Wu N, Xiao JH, Wang X, Ma W. ceRNA in cancer: possible functions and clinical implications. J Med Genet. 2015;52(10):710–718. doi:10.1136/jmedgenet-2015-103334
27. Song H, Li H, Ding X, et al. Long non‑coding RNA FEZF1‑AS1 facilitates non‑small cell lung cancer progression via the ITGA11/miR‑516b‑5p axis. Int J Oncol. 2020;57(6):1333–1347. doi:10.3892/ijo.2020.5142
28. Xu Y, Jiang T, Wu C, Zhang Y. CircAKT3 inhibits glycolysis balance in lung cancer cells by regulating miR-516b-5p/STAT3 to inhibit cisplatin sensitivity. Biotechnol Lett. 2020;42(7):1123–1135. doi:10.1007/s10529-020-02846-9
29. Zeng W, Zhu JF, Liu JY, et al. miR-133b inhibits cell proliferation, migration and invasion of esophageal squamous cell carcinoma by targeting EGFR. Biomed Pharmacother. 2019;111:476–484. doi:10.1016/j.biopha.2018.12.057
30. Liu C, Tian X, Sun HB, Wang ZF, Jiang LF, Li ZX. MiR-601 inhibits the proliferation and metastasis of esophageal squamous cell carcinoma (ESCC) by targeting HDAC6. Eur Rev Med Pharmacol Sci. 2019;23(3):1069–1076. doi:10.26355/eurrev_201902_16995
31. He Y, Sun S, Sha H, et al. Emerging roles for XBP1, a sUPeR transcription factor. Gene Expr. 2010;15(1):13–25. doi:10.3727/105221610X12819686555051
32. Sun Y, Jiang F, Pan Y, et al. XBP1 promotes tumor invasion and is associated with poor prognosis in oral squamous cell carcinoma. Oncol Rep. 2018;40(2):988–998. doi:10.3892/or.2018.6498
33. Zhou T, Lv X, Guo X, et al. RACK1 modulates apoptosis induced by sorafenib in HCC cells by interfering with the IRE1/XBP1 axis. Oncol Rep. 2015;33(6):3006–3014. doi:10.3892/or.2015.3920
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.