Back to Journals » Cancer Management and Research » Volume 11
CIP2A overexpression in Taiwanese oral cancer patients
Authors Velmurugan BK , Wang HK, Chung CM , Lee CH, Huang LR , Yeh KT, Lin SH
Received 10 January 2019
Accepted for publication 5 March 2019
Published 5 April 2019 Volume 2019:11 Pages 2589—2594
DOI https://doi.org/10.2147/CMAR.S201154
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Bharath Kumar Velmurugan,1 Hsin-Kai Wang,2,3 Chia-Min Chung,4,5 Chien-Hsun Lee,6 Lan-Ru Huang,7 Kun-Tu Yeh,6,7* Shu-Hui Lin6,8*
1Faculty of Applied Sciences, Ton Duc Thang University, Ho Chi Minh City, Vietnam; 2Public Health Bureau, Tainan City Government, Tainan City, Taiwan; 3Jenteh Junior College of Medicine, Nursing and Management, Taiwan; 4Graduate Institute of BioMedical Sciences, China Medical University, Taichung, Taiwan; 5Environment-Omics-Diseases Research Center, China Medical University Hospital, Taichung, Taiwan; 6Department of Surgical Pathology, Changhua Christian Hospital, Changhua, Taiwan; 7School of Medicine, Chung Shan Medical University, Taichung, Taiwan; 8Department of Medical Laboratory Science and Biotechnology, Central Taiwan University of Science and Technology, Taichung, Taiwan
*These authors contributed equally to this work
Introduction: Oral cancer is a prevalent form of cancer worldwide, particularly in Taiwan, and mechanisms involved in oral squamous cell carcinoma (OSCC) progression remain relatively unknown. Cancerous inhibitor of protein phosphatase 2A (CIP2A), an oncoprotein, is aberrantly expressed in many human malignant tumors including oral cancer. However, the expression and role played by CIP2A in oral cancer pathogenesis remain obscure.
Methods: In this study, immunohistochemistry was used to analyze CIP2A expression between OSCC tissues and their adjacent noncancerous tissues. Furthermore, associations between CIP2A expression and histopathological parameters were investigated.
Results: In this study, we showed that CIP2A was overexpressed in most of the OSCC tissues. High CIP2A expression was significantly associated with moderate/poor tumor differentiation (P=0.02). No significant association was found between CIP2A expression and other clinical parameters. Kaplan–Meier analysis revealed that high CIP2A expression showed poorer survival rates than those with low CIP2A expression (P=0.047). Multivariate Cox regression analysis indicated that CIP2A expression, N stage, American Joint Committee on Cancer stage and clinical therapy were independent prognostic factors for survival.
Conclusion: Thus, our study suggests that CIP2A is an independent prognostic marker for OSCC and a novel target for OSCC treatment.
Keywords: CIP2A, OSCC: prognosis, survival, Taiwan
Introduction
Oral squamous cell carcinoma (OSCC), the most commonly occurring malignancy of the head and neck region, is a serious growing problem in many parts of the globe. This is the fourth most commonly occurring cancer affecting Taiwanese men. Despite so many advances in treatment and diagnostic aids, the 5-year survival rate in patients with OSCC remains ∼50%.1 Survival rates directly depend on the stage at which the disease is diagnosed.2 Thus, survival and morbidity rates will be exceptionally improved if the disease is identified in initial stages. For that, we need a good prognostic marker to better understand the disease and its outcome.
Cancerous inhibitor of PP2A (CIP2A), also recognized as KIAA1524 and p90, is located in the 3ql3.13 of chromosomes.3 CIP2A is abundantly expressed in various cancers like gastric cancer,4 breast cancer,5 lung cancer6 and head and neck carcinomas.7 Importantly, overexpression of CIP2A in head and neck squamous cell carcinoma (HNSCC) correlates with poor prognosis7,8 and is linked to poor overall 5-year survival in HNSCC.8 Bockelman et al studied CIP2A expression in 73 OSCC patients with stage T1N0M0 and T2N0M0 and identified CIP2A expression as an independent prognostic marker in early-stage OSCC patients.7 Katz et al examined the expression of CIP2A in oral cancer cell lines and oral dysplasia and OSCC tissues.9 Thus, it is difficult to conclude the role of CIP2A in OSCC.
The aim of the present study was to address the significance of CIP2A expression in oral cancer. We examined protein expression levels of CIP2A in tissue samples by immunohistochemical staining and further analyzed the clinical significance of CIP2A in a cohort of OSCC patients. Our data support the perception that aberrant CIP2A expression is an important oncogenic event in OSCC.
Materials and methods
Participants and clinical tissues
Tissue samples of patients (from January 2000 to December 2008) with OSCC were obtained from the Department of Pathology at Changhua Christian Hospital, Taiwan, of which 133 OSCC specimens were used for immunohistochemistry (IHC) staining. Staging was classified according to the sixth edition of TNM staging system of AJCC (American Joint Committee on Cancer). The main treatment was tumor removal and radical neck dissection, including postoperation irradiation as well as selective patients treated with 5-fluorouracil (5-FU) and cisplatin chemotherapy. This study was approved by the Ethics Committees of Changhua Christian Hospital (Changhua, Taiwan) to use decoded tissue samples, and we adhered to the guidelines approved by (CCH IRB No. 170143).
Tissue microarray and immunohistochemical staining
Formalin-fixed, paraffin-embedded block of cancer tissues were used in this experiment. Tissue microarrays and immunohistochemical staining methods have been described previously.10 The sections were incubated CIP2A antibody in room temperature for 20 mins. After washing three times with PBS, the sections were incubated with appropriate peroxidase-labeled secondary antibodies for 30 mins at room temperature. The sections were washed three times with PBS and then labeled by diaminobenzidine and counterstained with Mayer’s hematoxylin, dehydrated and mounted.
Two experienced pathologists (Kun-Tu Yeh and Chang Wei-Hsiang) independently assessed the results of immunohistochemical staining, and a final agreement was obtained for each score at a discussion microscope. The staining intensities were scored −, 1+ and 2+ for negative, low and high signals, respectively.
Statistical analysis
Results were expressed as the means ± SD of at least three independent experiments. Statistical analysis was performed using version 9.4 of the SAS software package (SAS Institute, Inc.; Cary, NC, USA). Data comparisons were made using Student’s t test between control and treated groups, when P-values <0.05 was considered statistically significant. The associations of clinicopathologic factor and CIP2A expression were examined by logistic regression with adjustment for age and gender.
Cox proportional hazards regression model was utilized for multivariate analyses to screen independent risk factors for prognosis of OSCC patients. HRs and CIs were subsequently calculated. Survival outcomes were summarized by the Kaplan–Meier method. P-values were obtained from log-rank tests for the homogeneity of Kaplan–Meier curves between high and low CIP2A expression.
Results
Patient characteristics
Table 1 summarizes the demographic and clinicopathologic characteristics for OSCC patients. A majority of cancer patients were men (94%), with the tumors mainly occurring at age of <60 years (66.6%). Fifty (37.6%) OSCC patients were diagnosed with III/IV tumor stage and 21 (15.8%) patients with N2/N3 lymph node metastasis, without distant nodal metastasis patients. Of those, 65 (59.4%) and 112 (84.3%) patients were, respectively, determined to be III/IV tumor stages and moderate/poor tumor differentiation.
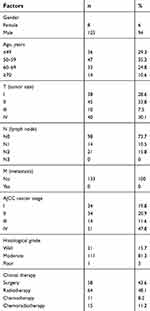 | Table 1 Demographic and characteristics among oral cancer patients |
CIP2A is overexpressed in OSCC tissues and correlated with survival of OSCC patients
First, CIP2A expression was evaluated in clinical samples including tumor part and non-tumor part using immunohistochemical staining, and the staining intensity was scored from – to 2+. In this experiment, we used nontumor part CIP2A expression as a standard to compare CIP2A expression in OSCC tissue. Based on CIP2A expression, OSCC patients were categorized into two groups: low expression group (− and 1+) if the staining intensity was lower than the staining intensity of the non-tumor tissues. The staining was scored as “2+” if the staining intensity in the tumor part is higher than the staining intensity of non-tumor tissues. Figure 1A shows that CIP2A protein was located in the cytoplasm of oral cancer tissues. Based on the IHC scoring, 58 (43.6%) cases showed low CIP2A expression and 75 (56.3%) cases exhibited high expression. Overall, CIP2A was highly expressed in tumor cells compared with non-tumor tissues.
In order to understand the possible roles of CIP2A in the OSCC development, we examined the association between CIP2A expression and clinicopathological characteristics (Table 2). The results show that CIP2A expression was significantly associated with moderate/poor tumor differentiation (P=0.02). However, no significant association was found between CIP2A expression and other clinical parameters, such as TNM stage and AJCC cancer stage. Kaplan–Meier analysis was performed to evaluate the associations of CIP2A expression with survival of the patients. Results showed that patients with higher CIP2A expression had lower survival rate than those with lower CIP2A expression (Figure 1B, P=0.047).
 | Table 2 Clinicopathologic factors associated with CIP2A expression |
Multivariate analysis to analyze prognostic value of CIP2A expression
The effects of clinicopathologic factors and CIP2A expression on mortality in the OSCC patient cohort are shown in Table 3. Adjusted for the effects of age and gender, nodal metastasis, AJCC stage and chemotherapy/radiotherapy were significantly associated with a higher mortality risk (adjusted HR [aHR], 2.5; 95% CI, 1.7–3.7; P = 0.0051; aHR, 3.7; 95% CI, 2.2–6.0; P = 0.0127; aHR, 2.9; 95% CI, 2.0–4.1; P<0.0001, respectively). To further understand whether CIP2A expression is an independent prognostic factor for OSCC patients, variables such as age, gender and histological grade were adjusted. Compared with high CIP2A expression, OSCC patients with low CIP2A expression had a 1.4-fold higher mortality risk (95% CI, 0.8−2.5; P = 0.0239). These results clearly demonstrate that CIP2A expression is an independent predictor for OSCC patients.
 | Table 3 The effect of clinicopathologic factor and CIP2A expression on mortality density and adjusted hazard ratio (aHR) among OSCC patients |
Discussion
In this study, we demonstrated that CIP2A expression in oral cancer tissues was significantly increased compared with nontumoral tissues. Normal epithelium showed weak or partly moderate staining, whereas tumor cells showed variable distribution and intensity of CIP2A. As determined by IHC, a total of 58 cases showed low CIP2A expression (43.6%) and 75 cases showed high CIP2A expression (56.3%). This finding indicates that CIP2A was highly expressed in tumor tissues than nontumor tissues, which is consistent with previous studies.4,5,7,9,11–15
Increased CIP2A expression confers poor clinical outcome in several types of cancer. In this study, the relationship between CIP2A expression levels and certain clinicopathological parameters of oral cancer was evaluated. High CIP2A expression is associated with late pathologic stage and metastatic lymph node status in various cancers types.4,5,14,16–18 In this study, clinicopathological analyses revealed a significant association between CIP2A expression and histological grade; this result is consistent with Bockelman et al (2007) and our study.7 Dong et al (2011) found that non-small-cell lung carcinoma (NSCLC) patients with CIP2A-positive expression showed reduced survival rate when compared to CIP2A-negative NSCLC.12 CIP2A overexpression is associated with shorter overall survival in patients with breast cancer, renal cancer5,17 and oral cancer and nasopharyngeal cancer.7,19 Similarly, in our study, high CIP2A expression was significantly correlated with poor prognosis in OSCC patients (P=0.047).
Dong et al (2010) have demonstrated that CIP2A expression was a significant prognostic factor for NSCLC patients.12 Multivariate analysis indicated that CIP2A expression was an independent risk factor for T1N0M0 and T2N0M0 OSCC patients.7 Consistent with these findings, the present report showed that CIP2A is an independent prognostic factor in oral cancer. In conclusion, CIP2A is an oncogenic protein12 in oral cancer and may serve as prognostic marker for overall survival.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–316. doi:10.1016/j.oraloncology.2008.06.002
2. Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44(1):10–22. doi:10.1016/j.oraloncology.2007.06.011
3. Xu ST, Wen WJ, Zhou J, Liu JX, Gu GJ. Clinicopathological significance of expression of CIP2A and c-myc in human gallbladder carcinoma. Int J Clin Exp Patho. 2017;10(5):5868–5874.
4. Khanna A, Bockelman C, Hemmes A, et al. MYC-dependent regulation and prognostic role of CIP2A in gastric cancer. J Natl Cancer Inst. 2009;101(11):793–805. doi:10.1093/jnci/djp103
5. Come C, Laine A, Chanrion M, et al. CIP2A is associated with human breast cancer aggressivity. Clin Cancer Res. 2009;15(16):5092–5100. doi:10.1158/1078-0432.CCR-08-3283
6. Xu P, Xu XL, Huang Q, Zhang ZH, Zhang YB. CIP2A with survivin protein expressions in human non-small-cell lung cancer correlates with prognosis. Med Oncol. 2012;29(3):1643–1647. doi:10.1007/s12032-011-0053-3
7. Bockelman C, Hagstrom J, Makinen LK, et al. High CIP2A immunoreactivity is an independent prognostic indicator in early-stage tongue cancer. Brit J Cancer. 2011;104(12):1890–1895. doi:10.1038/bjc.2011.167
8. Ventela S, Sittig E, Mannermaa L, et al. CIP2A is an Oct4 target gene involved in head and neck squamous cell cancer oncogenicity and radioresistance. Oncotarget. 2015;6(1):144–158. doi:10.18632/oncotarget.2670
9. Katz J, Jakymiw A, Ducksworth MK, et al. CIP2A expression and localization in oral carcinoma and dysplasia. Cancer Biol Ther. 2010;10(7):694–699. doi:10.4161/cbt.10.7.12895
10. Hsu LS, Wu PR, Yeh KT, et al. Positive nuclear expression of KLF8 might be correlated with shorter survival in gastric adenocarcinoma. Ann Diagn Pathol. 2014;18(2):74–77. doi:10.1016/j.anndiagpath.2013.12.001
11. Rantanen T, Kauttu T, Akerla J, et al. CIP2A expression and prognostic role in patients with esophageal adenocarcinoma. Med Oncol. 2013;30:3. doi:10.1007/s12032-013-0684-7
12. Dong QZ, Wang Y, Dong XJ, et al. CIP2A is overexpressed in non-small cell lung cancer and correlates with poor prognosis. Ann Surg Oncol. 2011;18(3):857–865. doi:10.1245/s10434-010-1313-8
13. Junttila MR, Puustinen P, Niemela M, et al. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130(1):51–62. doi:10.1016/j.cell.2007.04.044
14. Cha GQ, Xu JY, Xu XY, et al. High expression of CIP2A protein is associated with tumor aggressiveness in stage I-III NSCLC and correlates with poor prognosis. Onco Targets Ther. 2017;10:5907–5914. doi:10.2147/OTT.S148250
15. He H, Wu G, Li WJ, Cao YC, Liu YF. CIP2A is highly expressed in hepatocellular carcinoma and predicts poor prognosis. Diagn Mol Pathol. 2012;21(3):143–149. doi:10.1097/PDM.0b013e3182-49fd8b
16. Bockelman C, Lassus H, Hemmes A, et al. Prognostic role of CIP2A expression in serous ovarian cancer. Brit J Cancer. 2011;105(7):989–995. doi:10.1038/bjc.2011.346
17. Ren J, Li W, Yan L, et al. Expression of CIP2A in renal cell carcinomas correlates with tumour invasion, metastasis and patients’ survival. Brit J Cancer. 2011;105(12):1905–1911. doi:10.1038/bjc.2011.492
18. Yu GZ, Liu GH, Dong J, Jin YY. Clinical implications of CIP2A protein expression in breast cancer. Med Oncol. 2013;30:2. doi:10.1007/s12032-013-0524-9
19. Liu N, He QM, Chen JW, et al. Overexpression of CIP2A is an independent prognostic indicator in nasopharyngeal carcinoma and its depletion suppresses cell proliferation and tumor growth. Mol Cancer. 2014;13:111.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

