Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 13
Cigarette smoke exposure promotes differentiation of CD4+T cells toward Th17 cells by CD40-CD40L costimulatory pathway in mice
Authors Liang Y, Shen Y, Kuang L, Zhou G, Zhang L, Zhong X, Zhang J, Liu J
Received 1 November 2017
Accepted for publication 12 January 2018
Published 21 March 2018 Volume 2018:13 Pages 959—968
DOI https://doi.org/10.2147/COPD.S155754
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Chunxue Bai
Yi Liang,1 Ying Shen,2 Liangjian Kuang,1 Guang Zhou,1 Longju Zhang,1 Xiaoning Zhong,1 Jianquan Zhang,1 Jifeng Liu1
1Department of Respiratory Medicine, The First Affiliated Hospital of Guangxi Medical University, 2Division of General Practice, General Practice School of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China
Purpose: This study aimed to investigate the impact of cigarette smoke exposure upon CD40–CD40L ligation between bone marrow-derived dendritic cells (BMDCs)and CD4+T cells, and to examine the effects of cigarette smoke exposure upon differentiation of CD4+T cells toward Th17 cells through blockade of CD40-CD40L pathway in mice.
Methods: The study was processed in vivo and in vitro. In vivo, Th17 cells, CD40, interleukin (IL)-17A, and IL-27 in the lung tissues were quantified and compared between mice with and without cigarette smoke exposure. In vitro, Th17 cells, IL-17A, and IL-27 yielded by multiple cell cultivations in which BMDCs from mice with or without cigarette smoke exposure were fostered with CD4+ T cells from healthy mice spleens in the presence of antagonistic CD40 antibody and/or cigarette smoke extract (CSE) were quantified and compared. The flow cytometry was used to detect expressions of Th17 cells and CD40, and the liquid chip was used to detect levels of IL-17A and IL-27.
Results: Both in vivo exposed to cigarette smoke and in vitro to CSE, CD40 expressions noticeably escalated on the surfaces of BMDCs. The presence of Th17 cells, IL-17A, and IL-27 in the lung tissues prominently increased in mice exposed to cigarette smoke. The in vitro culture of CD4+ T cells and BMDCs significantly enhanced the differentiation of CD4+ T cells toward Th17 cells and secretions of IL-17A and IL-27 in the case that BMDCs were produced from mice exposed to cigarette smoke or the culture occurred in the presence of CSE. Usage of antagonistic CD40 antibody evidently reduced the number of Th17 cells, IL-17A, and IL-27 that increased due to cigarette smoke exposure.
Conclusion: The CD40–CD40L ligation is associated with the quantities of Th17 cells and relevant cytokines in the context of cigarette smoke exposure. Reducing the number of Th17 cells via the usage of antagonistic CD40 antibody can be an inspiration for pursuing a novel therapeutic target for immune inflammation in COPD.
Keywords: cigarette smoke extract, BMDC, CD4+IL-17+ T cell, CD40–CD40L pathway, IL-17A, IL-27
Introduction
COPD is a globally common lung disease with high morbidity and mortality, low life quality, and heavy disease burden.1 Profound immune inflammation has been well documented as one of the crucial pathophysiologic mechanisms associated with occurrence and development of COPD. Patients with COPD manifested typical signs of immune inflammation, including noticeable infiltration of inflammation cells in airway and in lung tissue, obviously increased number of myeloid dendritic cells (mDCs) in lavage fluid of brochoalveolus and airway,2–4 and excessive presence of Th1 cells, Th17 cells, and CD8+ T cells.5–7 Th17 cells actively engage in the immune inflammation occurring in COPD, secreting CCL2, recruiting macrophage, releasing metalloprotease,8 and enhancing toxicity of CD8+ T cells through secreting interleukin (IL)-21.9 Besides, the presence of IL-17 mRNA significantly increased in the lung tissues of smokers and COPD patients, suggesting potential involvement of IL-17A in the development of COPD,10 which is mainly secreted by Th17 cells and positively correlated with the number of Th17 cells. However, further mechanism behind the engagement of Th17 cells in the pathogenesis of COPD remains unclear. Among COPD patients with heavy cigarette smoke exposure showed noticeably high expressions of CD40 costimulatory molecules on the surfaces of mDC1 and mDC2.11 The CD40–CD40L cross-talk combining CD40 on the surface of dendritic cell (DC) and CD40L on the surface of activated T cells provides the crucial costimulatory signal for the initiation and regulation of specific immunity.12 CD40–CD40L promotes the activation of DC and increases the secretion of IL-2713 that is yielded by DC and can enhance the proliferation and differentiation of CD8+ T cells.14 In addition to as the main bearer of CD40, DC functions as a powerful antigen-presenting cell (APC) required for optimal activation of naive T cell. It induces proliferation and differentiation of CD4+ T cells and engages in the maintenance of effective immune defense and tolerance. According to the aforementioned literature over roles of CD40–CD40L pathway and DC in human immune response, potential connections amid CD40–CD40L pathway, DC, and physiological activities of Th17 cells as well as relevant cytokines have been strongly suggested and also served as the fundamental hypothesis of our study.
We conducted an animal study containing an in vivo experiment and an in vitro experiment to investigate the hypothetical effects of CD40–CD40L and DC over the differentiation of CD4+ T cells toward Th17 cell. First, a mouse model of cigarette smoke exposure was performed to imitate lung immune inflammation in vivo15,16 and quantities of CD40 and Th17 cells along with relevant cytokines in the mice lungs were evaluated. Second, in vitro experiment, CSE was used to continue the environment of cigarette smoke exposure and bone marrow-derived dendritic cells (BMDCs) of mice with or without cigarette smoke exposure were cultured with CD4+ T cells of healthy mice in the presence of antagonistic CD40 antibody. Then, Th17 and relevant cytokines yielded from the culture of BMDCs and CD4+ T cells were examined.
Methods
Ethics
The Laboratory Animal Ethics Committee of Guangxi Medical University approved all the in vivo and in vitro experiments of this study. “Regulation on the Administration of Experimental Animals (2017 edition)” and “Guideline on the Good Treatment of Experimental Animals (2006 edition)”, both issued by the Ministry of Science and Technology of our country, had been followed to deal with all animals involved in this study.
Animals
The research subjects used in this study were 90 male Balb/c mice, aged 6–8 weeks, weight 20–22 g, specific pathogen free, and purchased from the Animal Experiment Center of Guangxi Medical University (Nanning, China).
Cigarette smoke exposure
Of the 90 healthy Balb/c mice, 60 mice were randomly assigned into two groups by random number table, such as the control group (A group) and the cigarette smoke exposure group (B group) (n=30). Two identical glass boxes marked as “No 1” and “No 2”, respectively, were self-made in the size of 90×55×40 cm, and two holes at the diameter of 2 cm were made on each one for ventilation. The A group was put inside No 1 glass box and allowed to move freely inside for 30 minutes 1 day, 5 days per week up to 4 weeks. The B group was placed into No 2 box and exposed whole bodies to cigarette smoke produced by 10 Marlboro cigarettes (unfiltered cigarette: 12 mg of tar, 0.9 mg of nicotine, and 12 mg of carbon monoxide; Longyan Tobacco Co, Ltd, Nanning, China) on fire for 30 minutes a day, 5 days per week up to 4 weeks. Mice tolerated cigarette smoke exposure without evidence of toxicity (carboxyhemoglobin levels 10% and no weight loss). The rest 30 healthy Balb/c mice were used to attain spleen T lymphocytes.
Preparation of lung mononuclear cells and production of lung homogenate
Mice of both the A group and the B group underwent cervical dislocation and, then, were positioned with the back on the operating board and sterilized three times with iodophor. Both lungs were perfused through right ventricle by 10 mL of phosphate buffer solution (PBS) containing 0.6 M ethylene diamine tetraacetic acid (EDTA) in order to fend off any remaining circulating mononuclear cells. The left lower lung and the right upper lung were removed from the mice chest cavity, rinsed with PBS at 4°C, diced into pieces in 1–2 mm of diameter, and digested in Roswell Park Memorial Institute (RPMI) 1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) including 5% of fetal calf serum and 330 U/mL of collagenase IV in a constant temperature oscillator for 30 minutes. After digestion, lung tissues were filtered through a 200 mesh stainless steel filter and centrifuged at low temperature and 1,000 rpm for 5 minutes. Residual red blood cells from lungs were lysed with 15 mL of red blood cell (RBC) lysis solution. The cell suspension was resuspended twice with 2 mL of ice-cold PBS and centrifuged at low temperature for 5 minutes at 1,000 rpm to attain lung mononuclear cells. Th17 cells and CD40 expressed on the surfaces of lung mononuclear cells were quantified by the flow cytometry.
The left upper lung was fetched out of the mice chest, washed with ice-cold PBS, weighed on a scale, immersed in ice-cold PBS, and diced into pieces. Smashed lung tissues were placed into glass homogenate tubes and grinded in a homogenizer. A total of 10% of lung homogenate were centrifuged at low temperature for 10 minutes at 3,000 rpm and restored in a refrigerator at −80°C. Levels of IL-17A and IL-27 were quantified in the lung homogenate by the liquid chip.
Preparation of BMDCs
The thigh bones and tibias of mice from both the A group and the B group were taken out. The bone marrow cell suspension was collected by repeatedly rinsing the bone marrow cavities, centrifuged at 1,500 rpm for 10 minutes, then overlying mouse monocyte lymphocyte separation in twice volume, and centrifuged at 2,000 rpm for 20 minutes. The myeloid mononuclear cell belt was sucked out, washed with PBS twice, and suspended in the RPMI 1640 complete medium containing 150 mL/L of fetal calf serum (FCS), 40 ng/mL of recombinant mouse granulocyte-macrophage colony-stimulating factor (rmGM-CSF) (PeproTech, Rocky Hill, CT, USA), and 10 ng/mL of recombinant mouse interleukin 4 (rmIL-4) (PeproTech). The myeloid mononuclear cell suspension was adjusted to 106/L, distributed in six-pore plates, and fostered in a 50 mL/L CO2 incubator at 37°C for 6 days. At the second, third, and fifth day of the fostering, fresh RPMI 1640 complete medium renewed half of the old medium of each pore in order to keep the optimal concentration including 150 mL/L of fetal calf serum, 40 ng/mL of rmGM-CSF, and 10 ng/mL of rmIL-4. Adherent cells and semiadherent cells were collected at the sixth day of the fostering and cultured with fluorescein isothiocyanate (FITC)-labeled anti-mouse CD11c Monoclonal Antibody (R&D Systems, Inc., Minneapolis, MN, USA). The positive incidence of CD11c+ cells detected by the flow cytometry exceeded 70%.
Cigarette smoke extract (CSE) production
CSE was produced through the process reported by Li et al.17 A total of 15 burning cigarettes (unfiltered cigarette: 12 mg of tar, 0.9 mg of nicotine, and 12 mg of carbon monoxide) were put inside a glass 50 mL syringe that was connected by a rubber tube with a plastic 50 mL syringe containing 10 mL of PBS. CSE was collected by repeated suctions of the plastic 50 mL syringe, purified through a sterile strainer, and measured for the concentration by ultraviolet spectrophotometry.
The in vitro cultures of CD4+ T cells and BMDCs
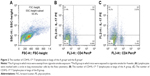
Spleens were fetched out from the rest 30 healthy mice, treated with RPMI 1640 medium, diced into pieces, and filtered by 200 mesh stainless steel filter. The spleen cells’ suspension was added with PBS, centrifuged at 1,500 rpm for 10 minutes, overlying mouse monocyte lymphocyte separation in twice volume, and centrifuged at 2,000 rpm for 20 minutes. The spleen mononuclear cells were sucked out of centrifuge tubes, rinsed with PBS twice, and centrifuged at 1,000 rpm for 5 minutes. The mononuclear cells were purified by CD4+ T-negative immunomagnetic beads (Thermo Fisher Scientific) and examined for purity by the flow cytometry. The CD4+ T cells were put into 96-well plates, 200 μL unit in each well, and fostered with BMDCs in 24-well plates in the following seven groups: 1) the CD4+ T group; 2) the CD4+ T + CSE group in which CD4+ T cells were cultured in the presence of 10 mL/L CSE; 3) the CD4+ T + BMDC group in which CD4+ T cells were cultured with BMDCs of healthy mice; 4) the CD4+ T + smoke BMDC group in which CD4+ T cells were cultured with BMDCs of mice exposed to the cigarette smoke for 4 weeks; 5) the CD4+ T + BMDC + CSE group in which CD4+ T cells were cultured with BMDCs of healthy mice along with 10 mL/L CSE; 6) the CD4+ T + smoke BMDC + CD40 antibody group in which CD4+ T cells were cultured with BMDCs of mice exposed to the cigarette smoke for 4 weeks along with 10 μg/mL of antagonistic CD40 antibodies (BD Pharmingen, San Diego, CA, USA); and 7) the CD4+ T + BMDC + CSE + CD40 antibody group in which CD4+ T cells were cultured with BMDCs of healthy mice in the presence of 10 μg/mL of antagonistic CD40 antibodies18 and 10 mL/L CSE (Figure 1). Each well was inoculated 106/mL of cells and fostered in serum-free mediums for 24 hours.
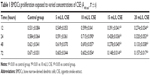
A range of concentrations of CSE, including 5, 10, 15, and 20 mL/L, were used to sift out the optimal one, to which BMDCs were exposed and showed more active proliferation measured by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay than to other three concentrations. All absorbance values of BMDCs detected at 490 nm at 12, 24, 48, and 72 hours culminated with 10 mL/L CSE in comparison with other three concentrations of CSE (Table 1). Hence, the 10 mL/L CSE was applied to the cultures of CD4+ T cells and BMDCs in vitro. Before these in vitro cultures started, parts of BMDCs produced from the A group were cultured with 10 mL/L CSE in 24-well plates for 24 h. Then, the flow cytometry was used to evaluate CD40 expression on the surfaces of BMDCs that were sampled from ones prepared from the A group, from the B group, and from the A group and cultured with CSE. Th17 cells produced by cultivations of CD4+ T cells and BMDCs were quantified by the flow cytometry, and IL-17A and IL-27 were quantified by the liquid chip.
Flow cytometry
Cells were stained with FITC-labeled CD11c+ mAb for the detection of expression of CD11c+, with PE-labeled CD40 mAb (R&D Systems, Inc.) for the quantification of CD40, with peridinin chlorophyll protein (PercP)-labeled antirat CD4 mouse mAb (R&D System, Inc.) for purification of CD4+ T cells, and with PE-labeled antirat IL-17 mAb (R&D System, Inc.) for Th17 cells count according to each manufacturer’s instruction. The stained cells were analyzed on a FACSCalibur Four Color Fluorescence Flow Cytometer (BD Pharmingen).
Liquid chip
The concentrations of IL-17A and IL-27 in mice lung homogenate and in the above seven groups of cultivations of CD4+ T cells and BMDCs were measured by Luminex liquid phase chips (ProcartaPlex Mouse-Il-27 and IL-17A-Simplex 96; eBioscience, Dummer, Germany) through a Luminex 200 standard high-throughput detection technology platform (LianKe Bio, Hangzhou, China).
Statistical analysis
The SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. All data were expressed as x– ± s. The independent sample t-test was used to examine the difference between two groups. Repeated measures analysis of variance with post hoc Bonferroni procedure were used for comparisons of multiple groups over normally distributed samples. In the case of sample with heteroscedasticity, nonparametric Kruskall–Wallis test and Mann–Whitney test were used for measuring differences. The differences were considered to be statistically significant when P≤0.05.
Results
In vivo: Th17 cell, IL-17A, IL-27, and CD40 on the surface of lung mononuclear cell
Mice exposed to cigarette smoke for 4 weeks indicated significantly higher quantity of CD4+IL-17+ T cells (2.81±0.58) (Figure 2A–C) and levels of IL-17A (484.00±74.08) and IL-27 (25.30±3.74) in the lung than that of the controls (Th17 cell: 1.12±0.36, P<0.05, IL-17A: 220.31±83.09 and IL-27: 7.22±1.18, P<0.01). As well, CD40 expressions on surfaces of lung mononuclear cells of mice with cigarette smoke exposure noticeably increased (43.67±7.03) in comparison with that of those exempt from smoke exposure (15.86±3.84, P<0.01; Figure 3A and B).
In vitro: CD40 on the surface of BMDCs
CD40 expression obviously escalated on the surfaces of BMDCs that were sampled from ones prepared from the B group (51.61±2.23, P<0.01), and from the A group and fostered with 10 mL/L CSE(44.93±5.71, P<0.01), when compared to that from the A group without exposed to CSE(10.39±2.81). (see Table 2 and Figure 4A–C).
In vitro: Th17 cells, IL-17A, and IL-27
All the CD4+ T + CSE group (Th17: 3.85±0.73, IL-17A: 93.83±14.71), the CD4+ T + BMDC group (Th17: 3.91±0.42, IL-17A: 109.29±16.09), the CD4+ T + smoke BMDC group (Th17: 5.35±1.03, IL-17A: 313.13±49.35), and the CD4+ T + BMDC + CSE group (Th17: 7.78±0.76, IL-17A: 329.36±45.18) exhibited prominent increases in a comparison with the CD4+ T group (Th17:1.39±0.48, IL-17A: 38.42±9.41) with respect to the number of Th17 cells (Table 3) and IL-17A (Table 4). Among these groups, the CD4+ T + BMDC + CSE group achieved the most positive impact upon the number of Th17 cells and IL-17A. Besides no values of IL-27 showed in either the CD4+ T group or the CD4+ T + CSE group, values of IL-27 (Table 4) indicated a similar trend as that of Th17 and IL-17A among the CD4+ T + BMDC group, the CD4+ T + smoke BMDC group, and CD4+ T + BMDC + CSE group. The administration of antagonistic CD40 antibody substantially reversed the rising tendency on the above three indicators in the case of CSE or BMDCs from cigarette smoke-exposed mice, resulting in obviously decreased presence of Th17 cells, IL-17A, and IL-27 as revealed either in the CD4+ T + smoke BMDC + CD40 antibody group (Th17: 3.27±0.80; IL-17A 174.24±35.21; IL-27 14.37±4.95) or in the CD4+ T + BMDC + CSE + CD40 antibody group (Th17: 3.88±0.41; IL-17A 141.18±29.27; IL-27 13.75±5.11) (Tables 3 and 4 and Figure 5A–G).
Discussion
Among the studies focusing on the role of CD4+IL-17+ T cells in the pathogenesis and development of COPD, this study is one of the first attempts to explore the impacts that DCs and CD40–CD40L costimulatory pathway generated on CD4+IL-17+ T cells under the circumstance of cigarette smoke exposure. As demonstrated in the “Results” section, regardless of in vivo or in vitro, cigarette smoke exposure always enhanced the differentiation of CD4+ T toward CD4+IL-17+ T cells, significantly expanding the expression of CD4+IL-17+ T cells. Our previous study similarly indicated the excessive presence of CD4+IL-17+ T cells in the lungs of enrolled COPD patients and smokers and further demonstrated that IL-17 cells positively correlated with the size of alveolar space or thickness of pulmonary artery wall or the number of lung CD4+ and CD8+ cells while negatively related to FEV1%pred of patients.10 CD4+IL-17+ T cells, also called Th17 cells and generally identified as one subpopulation of helper T cell, are characterized with promoting the expression of transcription factor ROR-γt and yielding a variety of cytokines mediating immune response.19 Hence, Th17 cells are indispensable to immune response targeting antigens and immune toleration in normal situations. Activated IL-17 triggers recruitments of neutrophils and macrophages, induces releases of CXCL8 and CXCL10 from bronchial epithelial cells and macrophages, and, in consequence, promotes airway chemotaxis of neutrophils and mononuclear cells.20–23 IL-17 is mainly secreted by Th17 cells and IL-27 produced by activated DCs. However, there is evidence showing increased expression of IL-17A/F in the cultivation of lung tissue cells and CSE in healthy population and COPD patients24 and elevated level of IL-27 in sputum and blood plasma of COPD patients,25 which is consistent with our study conducted on mice that indicated significantly elevated levels of IL-17A and IL-27 in vivo or in vitro after cigarette smoke exposure or CSE stimulation.
DC is the most powerful APC in human body and acts as a bridge connecting innate immunity and acquired immunity. DCs not only capture and present antigens but also activate native T lymphocytes. Cigarette smoke exposure induces proliferation and maturation of DCs in the lung tissues and drives DCs immigrating to the lung lymphoid tissue. Toll-like receptor located in the DCs functions as a pattern recognition receptor, selectively recognizing pathogen, activating downstream signal pathway, strengthening secretions of cytokines from activated immune cells, upregulating major histocompatibility complex (MHC) molecules and costimulatory molecules, and promoting maturation of DC and activation of T cells.26–28 COPD patients with smoking habit demonstrated increased expression levels of CD40 on mDC1 and mDC2. Plasma-soluble CD40L that constitutes another half part of the CD40–CD40L ligation significantly increased in COPD patients. The activation and synergy of CD40/CD40L signal contributed to the development of the emphysema caused by sensitized Fas-mediated apoptosis of alveolar epithelial cells.29 Otherwise, COPD is a lung alien with systemic inflammation that involves not only the lung but also other organs.30 Accordingly in our study, the BMDCs produced from mice exposed to cigarette smoke for 4 weeks exhibited obviously increased expressions of CD40, which echoed with the BMDCs from healthy mice cultured in vitro with CSE and the lung mononuclear cells from mice exposed to cigarette smoke for 4 weeks.
It is well documented that CD40–CD40L costimulatory pathway is required to optimal activation of naive T lymphocytes and proliferation of T lymphocytes,31 which also activates CD80 and CD86 and elevates their count. During the process of T-lymphocyte activation, CD86 combined with CD28 provides synergetic signals necessary for the early activation of T-cell clone and CD80 and CD28 maintain effective proliferation of T-cell clone.32 Our study revealed that the CD40–CD40L costimulatory pathway was clearly associated with the quantities of Th17, IL-17A, and IL-27. As indicated in the “Results” section, BMDCs with high expression of CD40 as a result of cigarette smoke exposure or CSE exposure substantially enhanced the differentiation of CD4+ T cells toward Th17 cells and the secretions of IL-17A and IL-27, giving rise to elevated expression of Th17 cells and levels of IL-17A and IL-27 after being cultured with CD4+ T cells, while the administration of antagonistic CD40 antibody that effectively blocked CD40–CD40L broke the augment effects over Th17 cells, IL-17A, and IL-27 due to cigarette smoke exposure or CSE exposure.
CD40–CD40L promotes the activation of DCs and the secretion of IL-27, activating STAT1 through IL-27-27R axis, inducing expressions of T-bet and eomesodermin (EOMES), and further promoting the expression of CD8+ T cells and secretions of perforin and granzyme B.33 IL-27 was reported to generate a positive impact upon differentiation of T lymphocytes toward Th1 cells34 but a restrictive impact upon differentiation toward Th17 cells, although either of the two opposed impacts were suggested mild in the context of inflammatory response. Hence, IL-27 scarcely inhibits mature Th17 in the process of inflammation.35 In our study, increased level of IL-27 did not deliver specific depression in the presence of Th17 and IL-17. The CD40–CD40L pathway blocked by the antagonistic CD40 antibody reduced levels of IL-27and IL-17A in our study, so blockade of CD40–CD40L ligation might mitigate heated inflammatory responses caused by exposure to cigarette smoke or to CSE. Iezzi et al36 reported that DCs with low expression of CD40 reduced productions of related cytokines and restrained differentiations toward Th17. Martin et al37 found that DCs with deficient presence of CD40 suppressed RelB of nuclear factor-κB (NF-κB) family, ceasing the initiation of immune response and curbing the unfoldment of ongoing immune response. The aforementioned two studies revealed the indispensable role that CD40 has played in the action of antigen presented by DCs.
Conclusion
DCs and CD40–CD40L costimulatory pathway are associated with the development of immune inflammation caused by cigarette smoke exposure. The blockade of CD40–CD40L pathway with antagonistic CD40 antibody effectively restrains differentiations of T lymphocytes toward Th17 cells and, in consequence, substantially cuts down excessive presence of Th17 cell, IL-17A, and IL-27 due to cigarette smoke exposure. Depressing the CD40–CD40L ligation between DCs and T cells can be an inspiration for pursuing a new therapeutic target for immune inflammation in COPD.
Acknowledgments
This study was funded by grants from National Natural Science Foundation of China (grant number 81660007), Youth Foundation of Guangxi Medical University (grant number GXMUYSF-201614), and Talents Highland of Emergency and Medical Rescue of Guangxi Province in China (grant number GXJZ201514). YL and YS are the co-first authors.
Author contributions
XZ made substantial contributions to concept and design of this study, data interpretation, and critical modifications of some important intellectual contents. YL and YS equally made substantial contributions over drafting this article, data acquisition, and data interpretation. LK, GZ, and LZ were significantly involved in data acquisition and data analysis. JZ managed some important modifications over the content. JL substantially contributed to data analysis. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Faner R, Cruz T, Agusti A. Immune response in chronic obstructive pulmonary disease. Expert Rev Clin Immunol. 2013;9(9):821–833. | ||
Lommatzsch M, Bratke K, Knappe T, et al. Acute effects of tobacco smoke on human airway dendritic cells in vivo. Eur Respir J. 2010;35(5):1130–1136. | ||
Vassallo R, Walters PR, Lamont J, Kottom TJ, Yi ES, Limper AH. Cigarette smoke promotes dendritic cell accumulation in COPD; a Lung Tissue Research Consortium study. Respir Res. 2010;26(11):45. | ||
Van Pottelberge GR, Bracke KR, Demedts IK, et al. Selective accumulation of langerhans-type dendritic cells in small airways of patients with COPD. Respir Res. 2010;22(11):35. | ||
Eppert BL, Wortham BW, Flury JL, Borchers MT. Functional characterization of T cell populations in a mouse model of chronic obstructive pulmonary disease. J Immunol. 2013;190(3):1331–1340. | ||
Li XN, Pan X, Qiu D. Imbalances of Th17 and Treg cells and their respective cytokines in COPD patients by disease stage. Int J Clin Exp Med. 2014;7(12):5324–5329. | ||
Li H, Liu Q, Jiang Y, Zhang Y, Zhang Y, Xiao W. Disruption of th17/treg balance in the sputum of patients with chronic obstructive pulmonary disease. Am J Med Sci. 2015;349(5):392–397. | ||
Chen K, Pociask DA, McAleer JP, et al. IL-17RA is reacquired for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS One. 2011;6(5):e20333. | ||
Duan MC, Huang Y, Zhong XN, Tang HJ. Th17 cell enhances CD8 T-cell cytotoxicity via IL-21 production in emphysema mice. Mediators Inflamm. 2012;2012:898053. | ||
Zhang J, Chu S, Zhong X, Lao Q, He Z, Liang Y. Increased expression of CD4+IL-17+ cells in the lung tissue of patients with stable chronic obstructive pulmonary disease and smokers. Int Immunopharmacol. 2013;15(1):58–66. | ||
Freeman CM, Martinez FJ, Han MK, et al. Lung dendritic cell expression of maturation molecules increases with worsening chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(12):1179–1188. | ||
Kawabe T, Naka T, Yoshida K, et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1(3):167–178. | ||
Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and P28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16(6):779–790. | ||
de Groot R, van Beelen AJ, Bakdash G, Taanman-Kueter EW, de Jong EC, Kapsenberg ML. Viral dsRNA-activated human dendritic cells produce IL-27, which selectively promotes cytotoxicity in naive CD8+ T cells. J Leukoc Biol. 2012;92(3):605–610. | ||
Wang H, Peng W, Weng Y, et al. Imbalance of Th17/Treg cells in mice with chronic cigarette smoke exposure. Int Immunopharmacol. 2012;14(4):504–512. | ||
Kuang LJ, Deng TT, Wang Q, et al. Dendritic cells induce Tc1 cell differentiation via the CD40/CD40L pathway in mice after exposure to cigarette smoke. Am J Physiol Lung Cell Mol Physiol. 2016;311(3):L581–L589. | ||
Li M, Zhong X, He Z, et al. Effect of erythromycin on cigarette-induced histone deacetylase protein expression and nuclear factor-κB activity in human macrophages in vitro. Int Immunopharmacol. 2012;12(4):643–650. | ||
Karimi MH, Ebadi P, Pourfathollah AA, Moazzeni SM. Tolerance induction by CD40 blocking through specific antibody in dendritic cells. Iran J Allergy Asthma Immunol. 2010;9(3):141–147. | ||
Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. | ||
Vanaudenaerde BM, Wuyts WA, Dupont LJ, Van Raemdonck DE, Demedts MM, Verleden GM. Interleukin-17 stimulates release of interleukin-8 by human airway smooth muscle cells in vitro: a potential role for interleukin-17 and airway smooth muscle cells in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2003;22(11):1280–1283. | ||
Wuyts WA, Vanaudenaerde BM, Dupont LJ, Van Raemdonck DE, Demedts MG, Verleden GM. Interleukin-17-induced interleukin-8 release in human airway smooth muscle cells: role for mitogen-activated kinases and nuclear factor-kappa B. J Heart Lung Transplant. 2005;24(7):875–881. | ||
Lindén A, Hoshino H, Laan M. Airway neutrophils and interleukin-17. Eur Respir J. 2000;15(5):973–977. | ||
Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194(4):519–527. | ||
Chang Y, Al-Alwan L, Alshakfa S, et al. Upregulation of IL-17A/F from human lung tissue explants with tobacco smoke exposure: implications for COPD. Respir Res. 2014;15(1):145. | ||
Cao J, Zhang L, Li D, et al. IL-27 is elevated in patients with COPD and patients with pulmonary TB and induces human bronchial epithelial cells to produce CXCL10. Chest. 2012;141(1):121–130. | ||
Parker LC, Prince LR, Sabroe I. Translational mini-review series on Toll-like receptors: networks regulated by Toll-like receptors mediate innate and adaptive immunity. Clin Exp Immunol. 2007;147(2):199–207. | ||
Vermaelen K, Pauwels R. Pulmonary dendritic cells. Am J Respir Crit Care Med. 2005;172(5):530–551. | ||
Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5(12):1219–1226. | ||
Shigeta A, Tada Y, Wang JY, et al. CD40 amplifies Fas-mediated apoptosis: a mechanism contributing to emphysema. Am J Physiol Lung Cell Mol Physiol. 2012;303(2):L141–L151. | ||
Agustí A, Edwards LD, Rennard SI, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7(5):e37483. | ||
Sun Y, Peng D, Lecanda J, et al. In vivo gene transfer of CD40 ligand into colon cancer cells induces local production of cytokines and chemokines, tumor eradication and protective antitumor immunity. Gene Ther. 2000;7(17):1467–1476. | ||
Slavik JM, Hutchcroft JE, Bierer BE. CD80 and CD86 are not equivalent in their ability to induce the tyrosine phosphorylation of CD28. J Biol Chem. 1999;274(5):3116–3124. | ||
Morishima N, Mizoguchi I, Okumura M, et al. A pivotal role for interleukin-27 in CD8+ T cell functions and generation of cytotoxic T lymphocytes. J Biomed Biotechnol. 2010;2010:605483. | ||
Owaki T, Asakawa M, Morishima N, et al. A role for IL-27 in early regulation of Th1 differentiation. J Immunol. 2005;175(4):2191–2200. | ||
El-behi M, Ciric B, Yu S, Zhang GX, Fitzgerald DC, Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol. 2009;183(8):4957–4967. | ||
Iezzi G, Sonderegger I, Ampenberger F, Schmitz N, Marsland BJ, Kopf M. CD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc Natl Acad Sci U S A. 2009;106(3):876–881. | ||
Martin E, O’Sullivan B, Low P, Thomas R. Antigen-specific suppression of a primed immune response by dendritic cells mediated by regulatory T cells secreting interleukin-10. Immunity. 2003;18(1):155–167. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



 ± s)
± s)