Back to Journals » Cancer Management and Research » Volume 11
Chrysin inhibits sphere formation in SMMC-7721 cells via modulation of SHP-1/STAT3 signaling pathway
Authors Zhang Y, Chen F, Xiao X, Pan W, Yuan Q, Cao J
Received 6 November 2018
Accepted for publication 27 February 2019
Published 10 April 2019 Volume 2019:11 Pages 2977—2985
DOI https://doi.org/10.2147/CMAR.S193647
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Yanqin Zhang,1,2 Feng Chen,1 Xinghua Xiao,1 Weinan Pan,2 Qing Yuan,3 Jianguo Cao3
1Department of Pharmacy, Xiangya Hospital of Central South University, Changsha, 410008, People’s Republic of China; 2Department of Pharmacy, Hunan Food and Drug Vocational College, Changsha, 410208, People’s Republic of China; 3Department of Pharmaceutical Science, Medical College, Hunan Normal University, Changsha 410013, People’s Republic of China
Background: Chrysin is a natural flavonoid which has been identified as a candidate anti-cancer agent due to its inhibitory effect on a variety of cancer cells, including targeted inhibition of sphere formation in hepatocellular carcinoma (HCC) cell lines. However, the mechanism by which chrysin modulates HCC spheres remains unclear.
Materials and methods: In this study, we investigate the effect of chrysin on the regulation of SHP-1 and its downstream signal molecule STAT3 to explain the mechanism by which chrysin inhibits sphere formation of HCC cell lines.
Results: Here, we found that SHP-1 protein expression was markedly down-regulated in the spheres from both SMMC-7721 and MHCC97H cells. Chrysin significantly inhibited sphere formation and upregulated the expression of SHP-1 protein in both SMMC-7721 and MHCC97H cells, as well as reduced p-STAT3 and Twist1 expressions in SMMC-7721 cells. Furthermore, knockdown of SHP-1 in SMMC-7721 cells resulted in the induction of p-STAT3 and Twist1 protein expression and antagonizing the inhibitory effect of chrysin on sphere formation in SMMC-7721 cells.
Conclusion: Overall, the study findings demonstrated that chrysin acts as a candidate for the treatment of HCC through modulating SHP-1/STAT3 signaling pathway.
Keywords: chrysin, hepatocellular carcinoma, SHP-1, STAT3, sphere formation
Introduction
Hepatocellular carcinoma (HCC) is one of the most common digestive system cancers, ranking as the fifth most common cause of cancer-related death worldwide.1 Remarkably, HCC is the third for cancer mortality in China.2 Despite advances in both diagnosis and treatment, the incidence and mortality of HCC continues to rise, thought to be caused by cancer stem cells (CSCs) that have been reported to possess capabilities for self-renewal, invasion, and tumorgenicity.3,4 Therefore, further investigation of biologic properties for CSCs may help to develop a new therapeutic approach for HCC patients.
Signal transducer and activator of transcription 3 (STAT3) is an oncogenic transcription factor and its phosphorylation has been observed in various malignancies, including prostate, liver, and colorectal cancers.5,6,7 STAT3 activation is involved in multiple cellular progress, such as proliferation, apoptosis, and metastasis.8,9 It is worth noting that STAT3 plays an important role in inducing characteristics of CSCs.10,11 Src homology region 2 domain containing phosphatase 1 (SHP-1) belongs to a family of non-receptor protein tyrosine phosphatases (PTPs) and expresses highly in normal lymphoid cells, but is diminished in several of cancer cell lines.12 Several evidences showed SHP-1 acts as a tumor suppressor.13–15 and has been reported to catalyze dephosphorylation of STAT3 at the tyrosine 705 (Tyr705) residue and to directly cause silencing of STAT3.16,17 Accordingly, a loss of SHP-1 can result in activation of STAT3, therefore SHP-1 adjustment to STAT3 activation may be an appealing anti-cancer strategy.
Chrysin (5, 7-dihydroxyflavone) is a natural flavone widely found propolis and honey. Chrysin is well documented to possess multiple biological activities, such as antioxidant, anti-inflammatory, especially anti-cancer effects.18–20 Recently, a number of studies have certified that chrysin played the inhibitory effects in drug resistance, invasion, proliferation, and apoptosis in cancer.21–,24 Additionally Lirdprapamongkol et al. found that chrysin overcame TRAIL resistance of cancer cells mainly as blocking STAT3.25 Our recent study demonstrated that chrysin inhibited the sphere formation capability of SKOV3-derived ovarian cancer stem-like cells (CSLCs),26 and 8-Bromo-7-methoxychrysin, a synthetic analog of chrysin,27 reduced sphere-forming rate of the spheres originated from SMMC-7721 cells by blocking STAT3/Twist axis.28 However, the mechanisms underlying the regulation of STAT3 activation by chrysin are also unclear. In the present study, we explored whether chrysin can inhibit sphere formation in SMMC-7721 cells through modulation of the SHP-1/STAT3 signaling pathway.
Materials and methods
Regents and cell culture
Chrysin was obtained from Sigma–Aldrich (St. Louis, MO, USA) and diluted with dimethyl sulfoxide to a stock concentration of 10 mmol·L−1. Other media used for cell culture were from GIBCO, Life Technologies (Grand Island, NY, USA).
HCC cell lines SMMC-7721 cell and MHCC97H cell were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China), and cultured in DMEM medium contained 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin at 37°C and 5% CO2.
Sphere formation assay
SMMC-7721 cells and MHCC97H cells were harvested from normal culture and plated into ultra-low attachment six-well plates (Corning Inc., Corning, NY, USA, 5000 cells/well). These plates were incubated with serum-free DMEM/F12 medium containing 20 ng/mL of hrbFGF and hrEGF, 5 μg/mL insulin, 0.4% BSA, 0.2% B27, and 100 U/mL penicillin and streptomycin at 37°C with 5% CO2. After incubation for 6 days, the spheres that exceed 20 cells were counted, and the sphere formation efficiency was calculated as (number of spheres formed/number of cells seeded) ×100%.
Western blot
Western blot was performed as described previously.7 For total protein extraction, cells were incubated in RIPA buffer containing 1% PMSF on ice for 30 mins. Samples were then separated by SDS-PAGE. Anti-STAT3 (Abcam, Cambridge, MA, USA, cat no: ab119352, dilute at 1:1000), p-STAT3 (Abcam, Cambridge, MA, USA, cat no: ab76315, dilute at 1:1000), SHP-1 (Cell signaling, USA, cat no: 3759, dilute at 1:1000), TWIST1 (Abcam, Cambridge, MA, USA, cat no: 46702, dilute at 1:1000), were used as primary antibodies and β-actin (Cell signaling, USA, cat no:4970, dilute at 1:1000) was used as a control.
SHP-1 knockdown
The siRNA components used in experiments, control (sc-37007) and SHP-1 (sc-44101 primers: sense-GCAGGAGUCCGAGGAUACATT, antisense-UGUACCUCGGACUCCUGCTT)) were purchased from Santa Cruz Biotechnology (Santa Cruz, MO, USA). These siRNA were subsequently transfected into cells using the Lipofectamine2000 reagent (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s instructions.
Statistical analysis
All experimental data are presented as mean ± SD. All quantitative results were entered into SPSS 16.0 (SPSS Inc, Chicago, IL, USA) to perform statistical analysis. Student’s t-test and one-way ANOVA were used for data analysis. p<0.05 was considered statistically significant. All experiments were repeated three times.
Results
Expressions of SHP-1 in spheres as well as a monolayer of HCC cell lines
It has been reported that SHP-1 plays an important role in cancer progression.12 In order to assess SHP-1 protein expression in HCC-derived spheres, expressions of SHP-1 in both the spheres and monolayer of SMMC-7721 cell line or MHCC97H cell line were analyzed using western blot. We find that SHP-1 protein expression is down-regulated in the spheres compared to the monolayer of both SMMC-7721 (Figure 1A) and MHCC97H cell line (Figure 1B), suggesting that SHP-1 may be a potential target for inhibition of sphere formation in HCC cells.
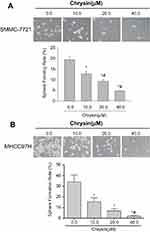
Effect of chrysin on sphere formation in HCC cell lines
To evaluate whether chrysin suppressed the self-renewal capability of HCC cells, SMMC-7721 cells and MHCC97H cells were treated with chrysin (0.0, 10.0, 20.0, 40.0 μM) for 24 hrs and then cultured using sphere-forming culture. Figure 2 indicates that chrysin dose-dependently decreased the sphere formation rate in both SMMC-7721 cells and MHCC97H cells.
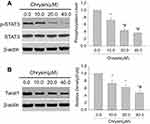
Effects of chrysin on SHP-1 expression in HCC cells
To investigate the underlining mechanism of chrysin, SMMC-7721 cells and MHCC97H cells were treated with or without chrysin for 24 hrs or 48 hrs, and then the SHP-1 expression was detected by western blot. Interestingly, Figure 3 shows that SHP-1 expressions are elevated with increasing drug concentration in both SMMC-7721 cells and MHCC97H cells.
Effect of chrysin on the expressions of p-STAT3 and Twist1 in SMMC-7721 cells
Previous study demonstrated that 8-Bromo-7-methoxychrysin could inhibit the stemness of CSLCs derived from SMMC-7721 cells by blocking the STAT3/Twist axis.28 Many studies have found that SHP-1 tumor suppression may result from its direct downregulating of p-STAT3 Tyr705. To explain the potential mechanism by which chrysin inhibits sphere formation of HCC cells, the expression levels of p-STAT3 and Twist1 in SMMC-7721 cells treated with or without chrysin were determined. We observe drastically down-regulated p-STAT3 (Figure 4A) and Twist1 (Figure 4B) expression in chrysin-treated SMMC-7721 cells. These results suggest that chrysin-associated inhibition of sphere formation was related to the downregulation of STAT3 signaling in SMMC-7721 cells.
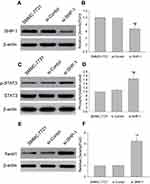
Effect of knocking down of SHP-1 on levels of p-STAT3 and Twist1 in SMMC-7721 cells
Next, in order to understand the relationship between SHP-1 and STAT3/Twist1 signal axis, we knocked-down expression of SHP-1 in SMMC-7721 cells using transfection with SHP-1 siRNA, and then detected the protein expressions of SHP-1, p-STAT3, and Twist1 by western blot analysis. Indeed, SHP-1-knockdown cells show relatively lower SHP-1 protein expression (Figure 5A and B) but display an increase in p-STAT3 (Figure 5C and D) and Twist1 (Figure 5E and F) expressions, compared with the untreated cells or the control siRNA-transfected cells. Together, these results demonstrate that SHP-1 affects STAT3 activation and Twist1 protein expression.
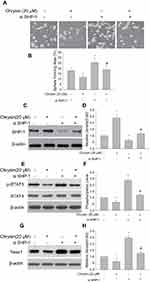
SHP-1 siRNA transfection reverses the inhibitory effect of chrysin on sphere formation and SHP-1/STAT3 signaling in SMMC-7721 cells
In order to further demonstrate that the inhibitory effect of chrysin on sphere forming capability of SMMC-7721 cells involved the SHP-1/STAT3 signaling pathway, SMMC-7721 cells were treated with SHP-1 siRNA or chrysin alone and in combination. It is noteworthy that SHP-1 knockdown abolishes the decrease in sphere-formation induced by chrysin treatment (Figure 6A and B). Notably, SHP-1 knockdown also abrogate upregulation of SHP-1 protein expression (Figure 6C and D) and downregulate the expressions of p-STAT3 (Figure 6E and F) and Twist1 (Figure 6G and H) in response to chrysin treatment. These results suggest that SHP-1/STAT3 is crucial for chrysin-induced sphere formation inhibition in SMMC-7721 cells.
Discussion
Here, we demonstrated, for the first time, that chrysin could inhibit sphere formation in HCC cells by suppressing STAT3 activation through upregulation of SHP-1 expression. This novel mechanism of chrysin suggests new insights for the design of HCC-associated targeted therapy.
Various studies have shown that chrysin exhibits anti-tumor activities in solid tumors. For example, inhibition of proliferation and promotion of apoptosis in human ovarian cancer cells via modulation of mitochondrial dysfunction,29 suppression of prostate cancer cell proliferation via cell cycle arrest,30 and induction of apoptosis in HCC cell lines.31,32 Similarly, we found that chrysin significantly inhibited sphere formation in SMMC-7721 cells. Given that sphere formation capability reflects the self-renewal properties of CSCs,33 chrysin might possess the potential to target inhibition of CSCs in HCC.
To the best of our knowledge, this is the first time that chrysin induced the expression of SHP-1 protein expression, indicating that SHP-1 may be a pivotal target molecule for the antitumor activity of chrysin. SHP-1 is known to be a negative regulator of STAT3 that participates in tumor progression.34 Reports have shown that chrysin suppressed angiogenesis and overcomed TRAIL resistance in HCC via STAT3 singling.25,35 It was also reported that SHP-1 functions as a suppressor of TGF-β1-triggered EMT and metastasis via targeting p-STAT3 in HCC.13SPH-1/STAT3 signaling pathway was also involved in inducing autophagy in HCC cell lines.36 And it was critically associated with the radiosensitivity of HCC cells. In this study, the first time, we determined that the inhibitory effects of chrysin on HCC involved in the up-regulation of SHP-1 protein via suppressing STAT3 being activated.
Our work demonstrated that SHP-1 expressions in spheres derived from SMMC-7721 cells, and MHCC97H cells are higher than in their parental cells. Chrysin induces the expression of SHP-1 and reduces STAT3 phosphorylation and Twist1 expression. Remarkably, we observe that SPH-1 siRNA transfection significantly abrogated the inhibitiory effect of chrysin on sphere formation. Together, these findings strongly indicate that SHP-1 is a gene that can repress self-renewal, and its activity can be mechanistically enhanced by chrysin. Finally, the elevated expression of SPH-1 is known to be an important inhibitor of p-STAT3 and Twist1 and may affect tumor development directly or indirectly.
In summary, our findings demonstrate that chrysin effectively inhibit sphere formation in HCC cells. Additionally, SHP-1 is shown to act as a key molecular mechanism of chrysin-inhibited self-renewal capability in HCC cells through its tyrosine phosphatase activity that negatively targets p-STAT3. Suppression of SHP-1/STAT3 signaling axis, therefore, might serve as a powerful potential therapeutic target for human HCC.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (No. 81301894, 81302249).
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi:10.3322/caac.21254
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Dawood S, Austin L, Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology (Williston Park). 2014;28(12):1101–7, 10.
4. Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J Clin Invest. 2013;123(5):1911–1918. doi:10.1172/JCI66024
5. De Simone V, Franze E, Ronchetti G, et al. Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34(27):3493–3503. doi:10.1038/onc.2014.286
6. Don-Doncow N, Escobar Z, Johansson M, et al. Galiellalactone is a direct inhibitor of the transcription factor STAT3 in prostate cancer cells. J Biol Chem. 2014;289(23):15969–15978. doi:10.1074/jbc.M114.564252
7. Cui Y, Sun S, Ren K, et al. Reversal of liver cancer-associated stellate cell-induced stem-like characteristics in SMMC-7721 cells by 8-bromo-7-methoxychrysin via inhibiting STAT3 activation. Oncol Rep. 2016;35(5):2952–2962. doi:10.3892/or.2016.4637
8. Kundu J, Choi BY, Jeong CH, Kundu JK, Chun KS. Thymoquinone induces apoptosis in human colon cancer HCT116 cells through inactivation of STAT3 by blocking JAK2- and srcmediated phosphorylation of EGF receptor tyrosine kinase. Oncol Rep. 2014;32(2):821–828. doi:10.3892/or.2014.3223
9. Rokavec M, Oner MG, Li H, et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014;124(4):1853–1867. doi:10.1172/JCI73531
10. Schroeder A, Herrmann A, Cherryholmes G, et al. Loss of androgen receptor expression promotes a stem-like cell phenotype in prostate cancer through STAT3 signaling. Cancer Res. 2014;74(4):1227–1237. doi:10.1158/0008-5472.CAN-13-0594
11. Zhang H, Cai K, Wang J, et al. MiR-7, inhibited indirectly by lincRNA HOTAIR, directly inhibits SETDB1 and reverses the EMT of breast cancer stem cells by downregulating the STAT3 pathway. Stem Cells. 2014;32(11):2858–2868. doi:10.1002/stem.1795
12. Wu C, Sun M, Liu L, Zhou GW. The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene. 2003;306:1–12.
13. Fan LC, Shiau CW, Tai WT, et al. SHP-1 is a negative regulator of epithelial-mesenchymal transition in hepatocellular carcinoma. Oncogene. 2015;34(41):5252–5263. doi:10.1038/onc.2014.445
14. Sun Z, Pan X, Zou Z, Ding Q, Wu G, Peng G. Increased SHP-1 expression results in radioresistance, inhibition of cellular senescence, and cell cycle redistribution in nasopharyngeal carcinoma cells. Radiat Oncol. 2015;10:152. doi:10.1186/s13014-015-0445-1
15. Lz W, Ding K, Zr W, et al. SHP-1 acts as a tumor suppressor in hepatocarcinogenesis and HCC progression. Cancer Res. 2018;78(16):4680–4691.doi:10.1158/0008-5472.
16. Liu CY, Tseng LM, Su JC, et al. Novel sorafenib analogues induce apoptosis through SHP-1 dependent STAT3 inactivation in human breast cancer cells. Breast Cancer Res. 2013;15(4):R63. doi:10.1186/bcr3457
17. Tai WT, Shiau CW, Chen PJ, et al. Discovery of novel Src homology region 2 domain-containing phosphatase 1 agonists from sorafenib for the treatment of hepatocellular carcinoma. Hepatology. 2014;59(1):190–201. doi:10.1002/hep.26640
18. Chen YH, Yang ZS, Wen CC, et al. Evaluation of the structure-activity relationship of flavonoids as antioxidants and toxicants of zebrafish larvae. Food Chem. 2012;134(2):717–724. doi:10.1016/j.foodchem.2012.02.166
19. Gresa-Arribas N, Serratosa J, Saura J, Sola C. Inhibition of CCAAT/enhancer binding protein delta expression by chrysin in microglial cells results in anti-inflammatory and neuroprotective effects. J Neurochem. 2010;115(2):526–536. doi:10.1111/j.1471-4159.2010.06952.x
20. Kasala ER, Bodduluru LN, Madana RM, Gogoi R, Barua CC. Chemopreventive and therapeutic potential of chrysin in cancer: mechanistic perspectives. Toxicol Lett. 2015;233(2):214–225. doi:10.1016/j.toxlet.2015.01.008
21. Kasala ER, Bodduluru LN, Barua CC, Gogoi R. Chrysin and its emerging role in cancer drug resistance. Chem Biol Interact. 2015;5(236):7–8. doi:10.1016/j.cbi.2015.04.017
22. Xia Y, Lian S, Khoi PN, et al. Chrysin inhibits cell invasion by inhibition of recepteur d’origine nantais via suppressing early growth response-1 and NF-kappaB transcription factor activities in gastric cancer cells. Int J Oncol. 2015;46(4):1835–1843. doi:10.3892/ijo.2015.2847
23. Jia WZ, Zhao JC, Sun XL, Yao ZG, Wu HL, Xi ZQ. Additive anticancer effects of chrysin and low dose cisplatin in human malignant glioma cell (U87) proliferation and evaluation of the mechanistic pathway. JBUON. 2015;20(5):1327–1336.
24. Li X, Huang JM, Wang JN, Xiong XK, Yang XF, Zou F. Combination of chrysin and cisplatin promotes the apoptosis of Hep G2 cells by up-regulating p53. Chem Biol Interact. 2015;232:12–20. doi:10.1016/j.cbi.2015.03.003
25. Lirdprapamongkol K, Sakurai H, Abdelhamed S, et al. Chrysin overcomes TRAIL resistance of cancer cells through Mcl-1 downregulation by inhibiting STAT3 phosphorylation. Int J Oncol. 2013;43(1):329–337. doi:10.3892/ijo.2013.1926
26. Li HZ, Chen YH, Fang YL, et al. Effects of chrysin on sphere formation and CK2alpha expression of ovarian cancer stem-like cells derived from SKOV3 cell line. Zhonghua Yi Xue Za Zhi. 2016;96(25):2013–2016. doi:10.3760/cma.j.issn.0376-2491.2016.25.012
27. Zheng X, Meng WD, Xu YY, Cao JG, Qing FL. Synthesis and anticancer effect of chrysin derivatives. Bioorg Med Chem Lett. 2003;13(5):881–884. doi:10.1016/S0960-894X(02)01081-8
28. Luo Y, Cui Y, Cao X, et al. 8-Bromo-7-methoxychrysin-blocked STAT3/Twist axis inhibits the stemness of cancer stem cell-like cell originated from SMMC-7721 cells. Acta Biochim Biophys Sin (Shanghai). 2017;49(5):458–464. doi:10.1093/abbs/gmx025
29. Lim W, Ryu S, Bazer FW, Kim SM, Song G. Chrysin attenuates progression of ovarian cancer cells by regulating signaling cascades and mitochondrial dysfunction. J Cell Physiol. 2018;233(4):3129–3140. doi:10.1002/jcp.26150
30. Ryu S, Lim W, Bazer FW, Song G. Chrysin induces death of prostate cancer cells by inducing ROS and ER stress. J Cell Physiol. 2017;232(12):3786–3797. doi:10.1002/jcp.25861
31. Xu D, Jin J, Yu H, et al. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J Exp Clin Cancer Res. 2017;36(1):44. doi:10.1186/s13046-017-0514-4
32. Zhang Q, Ma S, Liu B, Liu J, Zhu R, Li M. Chrysin induces cell apoptosis via activation of the p53/Bcl-2/caspase-9 pathway in hepatocellular carcinoma cells. Exp Ther Med. 2016;12(1):469–474. doi:10.3892/etm.2016.3282
33. Abbaszadegan MR, Bagheri V, Razavi MS, Momtazi AA, Sahebkar A, Gholamin M. Isolation, identification, and characterization of cancer stem cells: a review. J Cell Physiol. 2017;232(8):2008–2018. doi:10.1002/jcp.25759
34. Su JC, Chiang HC, Tseng PH, et al. RFX-1-dependent activation of SHP-1 inhibits STAT3 signaling in hepatocellular carcinoma cells. Carcinogenesis. 2014;35(12):2807–2814. doi:10.1093/carcin/bgu210
35. Lin CM, Shyu KG, Wang BW, Chang H, Chen YH, Chiu JH. Chrysin suppresses IL-6-induced angiogenesis via down-regulation of JAK1/STAT3 and VEGF: an in vitro and in ovo approach. J Agric Food Chem. 2010;58(11):7082–7087. doi:10.1021/jf100421w
36. Su JC, Tseng PH, Hsu CY, et al. RFX1-dependent activation of SHP-1 induces autophagy by a novel obatoclax derivative in hepatocellular carcinoma cells. Oncotarget. 2014;5(13):4909–4919. doi:10.18632/oncotarget.2054
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.