Back to Journals » Infection and Drug Resistance » Volume 16
Chronic Splenic Melioidosis in a Patient with Fever of Unknown Origin Diagnosed by Metagenomics Next-Generation Sequencing: An Emerging Cause and Literature Review
Authors Sheng Z, Li J, Chen C, Xie J, Xu Y, Zhou H, Xie Q
Received 30 January 2023
Accepted for publication 20 April 2023
Published 23 April 2023 Volume 2023:16 Pages 2403—2408
DOI https://doi.org/10.2147/IDR.S406358
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Zike Sheng,1 Junjie Li,2 Chihua Chen,3 Jing Xie,4 Yumin Xu,5 Huijuan Zhou,1 Qing Xie1
1Department of Infectious Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Pulmonary and Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 3Department of Radiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 4Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 5Department of Infection Control/Department of Nursing, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
Correspondence: Huijuan Zhou, Department of Infectious Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Building 36, 197 Ruijin Er Road, Shanghai, 200025, People’s Republic of China, Tel +86-21-64370045-680419, Fax +86-21-64454930, Email [email protected]
Introduction: Human melioidosis is an emerging infectious disease in tropical areas of China, and chronic melioidosis can be a rare cause of fever of unknown origin (FUO). Timely diagnosis may improve the prognosis of melioidosis.
Case Presentation: We report a case of melioidosis with splenic abscesses caused by Burkholderia pseudomallei in a 57-year-old man, who presented with FUO. Positron emission tomography/computed tomography (PET/CT) revealed multiple hypermetabolic lesions in the spleen. The spleen biopsy was conducted and metagenomics next-generation sequencing (mNGS) of the spleen specimen identified the presence of B. pseudomallei, confirming the diagnosis of melioidosis. Antimicrobial treatment was initiated with intravenous meropenem, followed by oral faropenem. During the follow-up, the patient was in good condition except having a low-grade fever occasionally. A splenectomy was performed, and subsequent culture and mNGS of the spleen pus were both positive for B. pseudomallei. Histopathological characteristics of chronic splenic melioidosis were noted.
Conclusion: Melioidosis is a serious endemic disease, and it is critical to raise awareness about this disease.
Keywords: melioidosis, Burkholderia pseudomallei, metagenomics next-generation sequencing, splenectomy
Introduction
Burkholderia pseudomallei is a Gram‑negative, aerobic, non-fermentative bacillus, which naturally inhabits the soil and water in endemic areas. B. pseudomallei causes melioidosis in humans and animals.1 Human melioidosis is a life-threatening and relapsing infection transmitted by inhalation, inoculation, and ingestion of B. pseudomallei, whereas human-to-human and animal-to-human transmissions are rarely reported.1–3 Melioidosis can result in poor pregnancy outcomes, but there is no proof of specific infection of the fetus with B. pseudomallei.4
This potentially fatal disease mimics several diseases due to multi-system involvement and various clinical manifestations, ranging from acute pneumonia to chronic relapsing infection. In subacute and chronic infections, melioidosis can masquerade as tuberculosis or malignancy. Risk factors for human melioidosis include diabetes mellitus (major), excessive alcohol consumption, exposure to soil or water, chronic pulmonary disease, chronic renal failure, liver disease, male gender, malignancy, and immunocompromised status.3
In this report, we described a Chinese patient with fever of unknown origin (FUO), who was diagnosed as chronic splenic melioidosis without involvement of other sites, through metagenomics next-generation sequencing (mNGS) and culture of the spleen specimen.
Case Presentation
On November 29, 2021, a 57-year-old-man with FUO for three years was referred to our department, with the maximum body temperature of 38.9 °C. The fever did not subside with antipyretic drugs. He had no cough, but had a weight loss of 8 kg over the previous three years. He went to the local hospital many times, but the cause of fever was not established. He had a history of diabetes mellitus for 20 years, and his fasting plasma glucose levels were not well controlled. He also had a history of smoking for 20 years, and smoked about 20 cigarettes daily. He denied excessive alcohol consumption. He had lived in Hainan province, China for 12 years, and worked in orchard planting management. Before moving to Hainan province, he had lived in Zhejiang province for 45 years. He underwent a 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) examination on November 8, 2021, at a local hospital, and the results demonstrated multiple hypermetabolic lesions (the maximum standard uptake value, SUVmax was 7.7) in the spleen. The lymph nodes in splenic hilum showed slight hypermetabolism, suggestive of primary lymphoma of the spleen and involved lymph nodes. There was no evidence of abnormal FDG uptake elsewhere in the body.
On admission, his temperature was 36.5 °C. A blood test revealed a white blood cell count of 6.05 × 109/L, with 59.1% neutrophils, 31.1% lymphocytes, and 8.8% monocytes, hemoglobin of 110 g/L, and platelets of 99 × 109/L. Serum C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were elevated at 20 mg/L and 80 mm/h, respectively. His fasting plasma glucose level was 9.15 mmol/L. The results of renal and liver function tests were normal. T-SPOT TB test was negative. The serum 1.3-β-D-glucan tests and Aspergillus galactomannan tests for fungi were also negative. Several blood cultures were negative for bacteria. The results of several virus serology tests, such as human immunodeficiency virus, hepatitis B, hepatitis C, Epstein-Barr virus, and cytomegalovirus, were negative.
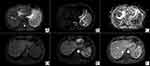
Chest computed tomography (CT) scan showed multiple pulmonary micronodules (3–4 mm in diameter) in bilateral lungs. Ultrasound of the bilateral cervical, supraclavicular, axillary, and inguinal regions revealed no enlarged lymph nodes. Abdominal ultrasound revealed hypoechoic area within the enlarged spleen, and no ascites. Abdominal magnetic resonance imaging (MRI) enhancement scan showed splenomegaly and multiple small hypodense lesions within the spleen (Figure 1A–F). Spleen biopsy guided by CT was performed, and the examination of the puncture fluid showed a few scattered lymphocytes, without evidence of malignancy. Culture of spleen biopsy specimen grew no microorganisms, while mNGS of splenic puncture fluid showed B. pseudomallei, which confirmed splenic melioidosis.
Thus, treatment with intravenous meropenem 1 g every eight hours was given for three weeks, and then the patient was discharged with a stable condition. After discharge, the treatment was changed to oral faropenem for two months. Meanwhile, insulin was used to treat the diabetes mellitus, and his fasting plasma glucose level was normal. During the follow-up, the patient was in good condition except having a low-grade fever occasionally, and a blood test revealed hemoglobin 104 g/L, and platelets of 86 × 109/L, with a normal white blood cell count. Although the clinical condition of the patient was generally stable, he refused to continue with oral medication to treat splenic melioidosis.
The patient was re-admitted to our hospital and strongly requested a splenectomy. Hence, a splenectomy was performed, and the enlarged spleen (around 15 cm in length) was observed. The liver, colon and small intestine showed no obvious abnormalities. The histopathological findings of the spleen indicated chronic inflammation with significant necrosis, and no evidence of malignancy (Figure 2A–C). Culture of pus from the spleen indicated Gram-negative bacilli, and was identified as B. pseudomallei by gene amplification and Sanger sequencing of 16S rRNA with primers 27F/1492R.5,6 Also, mNGS of the pus showed B. pseudomallei, with 1783 unique reads. After the splenectomy, antibiotics were not used. During the 13-month follow-up, the patient had no fever, and the hemoglobin and platelets reverted to normal levels.
Discussion
The diagnosis of FUO is challenging because of numerous causes. Fever is the most common clinical presentation of melioidosis, in 66–95.5% of cases.7,8 Melioidosis caused by B. pseudomallei can cause FUO, and should be included in the differential diagnosis of FUO, especially for patients with FUO who are from endemic regions.
Melioidosis is endemic in Southeast Asia and northern Australia.7 Recently, increasing number of melioidosis cases have been reported in southern China, particularly in Hainan province,8–11 where the first human melioidosis in mainland China was reported in 1989 and is currently the main endemic area of melioidosis in China.12 The annual incidence of melioidosis is increasing in China and worldwide.7,12,13
Melioidosis is a severe emerging infectious disease, with a high mortality rate of up to 70%.7 The clinical presentation of melioidosis can be acute (about 85%), subacute, and chronic (over two months, usually less than 12%) processes.1,6,7 Multiple organs and tissues of the body can be involved. The most common clinical manifestations are pulmonary melioidosis, bacteremia, and septic shock.7,9,12,13 Other presentations such as abscess formation (prostate, spleen, liver, and kidney), genitourinary, skin and soft tissue, osteomyelitis and septic arthritis, neurological involvement, suppurative parotitis, mycotic aneurysm, and ocular involvement, are also reported.6–8,10,14–16 Melioidosis only involving the spleen is relatively uncommon, as described in this report.17,18
The diagnosis of melioidosis remains challenging, particularly in non-endemic areas with laboratories having no experience with B. pseudomallei. Culture-based method is the gold standard for the diagnosis of melioidosis. As a new potential diagnostic test, mNGS shows good performance in detection of pathogens, especially in critically ill patients and those with an unclear diagnosis. Because infectious diseases are one of the main causes of FUO, mNGS, an unbiased method for pathogen detection, is a promising diagnostic tool for the diagnosis of patients with FUO.19,20 B. pseudomallei infection can cause FUO.6,21 The present report highlighted the role of mNGS in patients with FUO caused by B. pseudomallei infection.
B. pseudomallei is difficult-to-treat since it is intrinsically resistant to several antibiotics, such as penicillin, ampicillin, first and second-generation cephalosporins, macrolides, rifampicin, polymyxin, and aminoglycosides.7 Limited antimicrobial agents are available for treatment, including ceftazidime, carbapenems (imipenem and meropenem), piperacillin/tazobactam, amoxicillin/clavulanate, doxycycline, and SMZ-TMP.8 Intravenous carbapenems and ceftazidime are preferred for the initial intensive therapy, and subsequently oral SMZ-TMP, doxycycline, and amoxicillin/clavulanic acid should be used for at least three months for the eradication therapy.7 In the present case, due to a lack of experience with melioidosis, improper antibiotics and inadequate course of the eradication therapy may have mainly contributed to the failure of treatment. In addition to appropriate antimicrobial therapy, drainage and debridement of the lesions with abscess formation are also vital to treat melioidosis.
18F-FDG PET/CT is important in the management of patients with FUO.22–24 As a noninvasive and sensitive whole-body examination, 18F-FDG PET/CT can detect potential lesions of FUO. It is a useful tool to find potential lesions of FUO, which is critical to obtain histopathological results and then final diagnosis. Further, 18F-FDG PET/CT can detect occult and asymptomatic lesions of melioidosis. Previous studies have reported 18F-FDG PET/CT in the management of B. pseudomallei infection.25–29 Given the possible involvement of multiple sites, this imaging technique may have a promising role in diagnosis and assessing the dissemination of infection.
Conclusions
Clinicians, especially in non-endemic areas, should consider that melioidosis caused by B. pseudomallei can cause FUO. This is the first report, to the best of our knowledge, to provide pathological features of chronic splenic melioidosis. Prompt diagnosis and effective treatment can improve the prognosis. We believe that this case report will be helpful to raise awareness of chronic melioidosis.
Abbreviations
FUO, fever of unknown origin; mNGS, metagenomics next-generation sequencing; 18F-FDG, 18F-fluorodeoxyglucose; PET/CT, positron emission tomography/computed tomography; SUVmax, the maximum standard uptake value; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; CT, computed tomography; MRI, magnetic resonance imaging; SMZ-TMP, trimethoprim-sulfamethoxazole.
Data Sharing Statement
The original contributions presented in the study are included in the report, further inquiries can be directed to the corresponding author.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Review Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. Written informed consent has been obtained from the patient in accordance with the Declaration of Helsinki.
Consent for Publication
The patient of this report had already agreed and signed the consent form.
Acknowledgments
The authors thank Dr. Ruokun Li for his help in revising the MR images and legends.
Funding
This study was supported by funding from the Shanghai Municipal Key Clinical Specialty (Infectious disease, YW20190002 to Q. Xie).
Disclosure
The authors declare that they have no competing interests.
References
1. Wiersinga WJ, Virk HS, Torres AG, et al. Melioidosis. Nat Rev Dis Primers. 2018;4:17107. doi:10.1038/nrdp.2017.107
2. Abbink FC, Orendi JM, de Beaufort AJ. Mother-to-child transmission of Burkholderia pseudomallei. N Engl J Med. 2001;344:1171–1172. doi:10.1056/NEJM200104123441516
3. Aziz A, Currie BJ, Mayo M, Sarovich DS, Price EP. Comparative genomics confirms a rare melioidosis human-to-human transmission event and reveals incorrect phylogenomic reconstruction due to polyclonality. Microb Genom. 2020;6(2):e000326. doi:10.1099/mgen.0.000326
4. Chang CY, Lau NLJ, Currie BJ, Podin Y. Disseminated melioidosis in early pregnancy - an unproven cause of foetal loss. BMC Infect Dis. 2020;20(1):201. doi:10.1186/s12879-020-4937-8
5. Hou Q, Bai X, Li W, et al. Design of primers for evaluation of lactic acid bacteria populations in complex biological samples. Front Microbiol. 2018;9:2045. doi:10.3389/fmicb.2018.02045
6. Liu Y, Zong Z. Prolonged intermittent fever and massive splenomegaly in a miner working in the tropical jungle, China. PLoS Negl Trop Dis. 2020;14:e0008278. doi:10.1371/journal.pntd.0008278
7. Gassiep I, Armstrong M, Norton R. Human melioidosis. Clin Microbiol Rev. 2020;33(2):e00006–19. doi:10.1128/CMR.00006-19
8. Wu H, Wang X, Zhou X, et al. Osteomyelitis and septic arthritis due to Burkholderia pseudomallei: a 10-year retrospective melioidosis study from South China. Front Cell Infect Microbiol. 2021;11:654745. doi:10.3389/fcimb.2021.654745
9. Zhu X, Chen H, Li S, et al. Molecular characteristics of Burkholderia pseudomallei collected from humans in Hainan, China. Front Microbiol. 2020;11:778. doi:10.3389/fmicb.2020.00778
10. Wu H, Wang X, Zhou X, et al. Mycotic aneurysm secondary to melioidosis in China: a series of eight cases and a review of literature. PLoS Negl Trop Dis. 2020;14:e0008525. doi:10.1371/journal.pntd.0008525
11. Wu H, Huang D, Wu B, Pan M, Lu B. Fatal deep venous thrombosis and pulmonary embolism secondary to melioidosis in China: case report and literature review. BMC Infect Dis. 2019;19:984. doi:10.1186/s12879-019-4627-6
12. Zheng X, Xia Q, Xia L, Li W. Endemic melioidosis in Southern China: past and present. Trop Med Infect Dis. 2019;4(1):39. doi:10.3390/tropicalmed4010039
13. Fang Y, Chen H, Li YL, Li Q, Ye ZJ, Mao XH. Melioidosis in Hainan, China: a restrospective study. Trans R Soc Trop Med Hyg. 2015;109:636–642. doi:10.1093/trstmh/trv065
14. Pal P, Ray S, Moulick A, Dey S, Jana A, Banerjee K. Liver abscess caused by Burkholderia pseudomallei in a young man: a case report and review of literature. World J Clin Cases. 2014;2:604–607. doi:10.12998/wjcc.v2.i10.604
15. Mabayoje DA, Kenna DTD, Dance DAB, NicFhogartaigh C. Melioidosis manifesting as chronic femoral osteomyelitis in patient from Ghana. Emerg Infect Dis. 2022;28:201–204. doi:10.3201/eid2801.211800
16. Chang CY. Periorbital cellulitis and eyelid abscess as ocular manifestations of melioidosis: a report of three cases in Sarawak, Malaysian Borneo. ID Cases. 2019;19:e00683. doi:10.1016/j.idcr.2019.e00683
17. Yik CC. Ruptured splenic abscess and splenic vein thrombosis secondary to melioidosis: a case report. J Acute Dis. 2020;9:89–92. doi:10.4103/2221-6189.281326
18. Chen H, Hu ZQ, Fang Y, et al. A case report: splenic abscess caused by Burkholderia pseudomallei. Medicine. 2018;97(26):e11208. doi:10.1097/MD.0000000000011208
19. Fu ZF, Zhang HC, Zhang Y, et al. Evaluations of clinical utilization of metagenomic next-generation sequencing in adults with fever of unknown origin. Front Cell Infect Microbiol. 2022;11:745156. doi:10.3389/fcimb.2021.745156
20. Dong Y, Gao Y, Chai Y, Shou S. Use of quantitative metagenomics next-generation sequencing to confirm fever of unknown origin and infectious disease. Front Microbiol. 2022;13:931058. doi:10.3389/fmicb.2022.931058
21. Chin C, Chen YS, Lee SSJ, et al. Fever of unknown origin in Taiwan. Infection. 2006;34(2):75–80. doi:10.1007/s15010-006-5010-2
22. Sheng JF, Sheng ZK, Shen XM, et al. Diagnostic value of fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography in patients with fever of unknown origin. Eur J Intern Med. 2011;22(1):112–116. doi:10.1016/j.ejim.2010.09.015
23. Sheng ZK, Ye J, Li JJ, Zhao K, Sheng JF. Utility of fluorodeoxyglucose positron emission tomography/computed tomography in patients with fever of unknown origin diagnosed as lymphoma. Med Princ Pract. 2014;23(5):437–442. doi:10.1159/000364812
24. Kouijzer IJE, Mulders-Manders CM, Bleeker-Rovers CP, Oyen WJG. Fever of unknown origin: the value of FDG-PET/CT. Semin Nucl Med. 2018;48(2):100–107.
25. Nair SS, Varsha N, Sunil HV. Melioidosis presenting as septic arthritis: the role of F-18 fludeoxyglucose positron emission tomography/computed tomography in diagnosis and management. Indian J Nucl Med. 2021;36:59–61. doi:10.4103/ijnm.IJNM_132_20
26. Kulkarni P, Shelley S, Elangoven IM, Jaykanth A, Ejaz AP, Rao NS. 18-Fluorine-fluorodeoxyglucose positron emission tomography-computed tomography in the evaluation of the great masquerader melioidosis: a case series. Indian J Nucl Med. 2020;35:222–225.
27. Subran B, Ackermann F, Watin-Augouard L, et al. Melioidosis in a European traveler without comorbidities: a case report and literature review. Int J Infect Dis. 2013;17:e781–783. doi:10.1016/j.ijid.2013.01.029
28. Zaw KK, Wasgewatta SL, Kwong KK, Fielding D, Heraganahally SS, Currie BJ. Chronic pulmonary melioidosis masquerading as lung malignancy diagnosed by EBUS guided sheath technique. Respir Med Case Rep. 2019;28:100894. doi:10.1016/j.rmcr.2019.100894
29. Conrad A, Valour F, Ferry T, Ader F, Lyon BJI; Study Group. Multifocal melioidosis with femoral osteomyelitis in a healthy 25-year-old traveller. BMJ Case Rep. 2016;2016:bcr2016216356. doi:10.1136/bcr-2016-216356
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.